The continuously growing natural killer (NK) cell line NK-92 is highly cytotoxic against malignant cells of various origins without affecting normal human cells. Based on this selectivity, the potential of NK-92 cells for adoptive therapy is currently being investigated in phase I clinical studies. To further enhance the antitumoral activity of NK-92 cells and expand the range of tumor entities suitable for NK-92–based therapies, here by transduction with a retroviral vector we have generated genetically modified NK-92 cells expressing a chimeric antigen receptor specific for the tumor-associated ErbB2 (HER2/neu) antigen, which is overexpressed by many tumors of epithelial origin. The chimeric antigen receptor consists of the ErbB2-specific scFv(FRP5) antibody fragment, a flexible hinge region derived from CD8, and transmembrane and intracellular regions of the CD3 ζ chain. Transduced NK-92-scFv(FRP5)-ζ cells express high levels of the fusion protein on the cell surface as determined by fluorescence-activated cell-scanning (FACS) analysis. In europium release assays, no difference in cytotoxic activity of NK-92 and NK-92-scFv(FRP5)-ζ cells toward ErbB2-negative targets was found. However, even at low effector-to-target ratios, NK-92-scFv(FRP5)-ζ cells specifically and efficiently lysed established and primary ErbB2-expressing tumor cells that were completely resistant to cytolytic activity of parental NK-92 cells. These results demonstrate that efficient retargeting of NK-92 cytotoxicity can be achieved and might allow the generation of potent cell-based therapeutics for the treatment of ErbB2-expressing malignancies.
Introduction
Natural killer (NK) cells are a subgroup of lymphocytes that play an essential role in the cellular immune defense against virus-infected and malignant cells. NK cells do not rearrange their immune receptor genes, and the cytotoxicity toward tumor and virus-infected cells is not major histocompatibility complex (MHC) restricted.1 NK cell cytotoxic activity does not require sensitization but is enhanced by activation with a variety of cytokines including interleukin (IL)-2.2,3 In patients with malignant disorders, NK cell function can be impaired in terms of a reduced in vitro proliferative response and reduced cytotoxic activity.4 While NK cell dysfunction might allow persistence and metastasis of tumors,5 there is also evidence that tumor cells themselves may have developed mechanisms to escape NK cell immunosurveillance, for example, by up-regulation of classical (HLA-A, B, C) and nonclassical (HLA-G, E) human lymphocyte antigens (HLA).6 These HLA alleles are able to inhibit NK cell function upon ligation with certain killer cell immunoglobulinlike receptors (KIRs) on the NK cell surface.7
Numerous studies have been conducted with the aim to improve the antitumoral activity of NK cells by activation of endogenous NK cells through systemic application of cytokines8-10 or through adoptive transfer of ex vivo–expanded autologous or donor-derived lymphokine-activated killer cells,11-15 or a combination thereof.11,12,16 The efficacy of such approaches can be limited by impaired function of patient-derived NK cells, which is usually not fully reconstituted through ex vivo expansion and lymphokine activation.4,17 Furthermore, the expansion potential of activated NK (A-NK) cells is limited and poorly standardized between different clinical trials, as are the resulting phenotypes of A-NK cell subpopulations with regard to their therapeutic activity and the risk for adverse reactions. This has prompted investigators to also explore the properties and therapeutic potential of cytotoxic NK cell lines.18
The continuously growing IL-2–dependent NK-92 cell line is highly cytotoxic against a variety of malignant cells in vitro and in humanized mouse models in vivo.18-20 NK-92 cells are similar to A-NK cells with respect to the expression of typical NK cell surface receptors and functional characteristics, but they do not harbor Fc-γRIII (CD16).21 When directly compared to A-NK cells, NK-92 cells display a much higher cytolytic activity against a broad spectrum of tumor targets; in particular, malignant cells of hematologic origin.18-20 This might at least in part be due to the absence of inhibiting NK cell receptors. NK-92 cells were shown to lack almost all inhibitory KIRs, with the exception of KIR2DL4,22,23 which, through binding to HLA antigens on target cells, inhibit NK cell cytolytic activity.24Although a large variety of malignant cells are effectively killed by NK-92 cells, no toxicity against nonmalignant allogeneic cells has been observed.18,19 In contrast to autologous or allogeneic A-NK cells, which are difficult and laborious to generate in high purity in vitro, NK-92 cells can be easily expanded to large numbers and are readily available on demand in standardized quality for adoptive therapy, a prerequisite for the ongoing clinical development of NK-92.23
Previously, it has been shown that chimeric antigen receptor constructs can successfully be expressed in NK cells, resulting in efficient retargeting.25-27 In an attempt to further enhance the antitumoral activity of NK-92 cells and expand the range of tumor entities suitable for NK-92–based therapies, we have generated genetically modified NK-92 cells stably expressing a chimeric antigen receptor specific for the tumor-associated ErbB2 (HER2/neu) antigen. ErbB2 is a member of the EGF-receptor–related family of receptor tyrosine kinases. c-erbB2 gene amplification and ErbB2 protein overexpression have been observed in many human tumors of epithelial origin and have been linked with cancer development and progression.28,29 Various antibody-based strategies against ErbB2-expressing cancers have been developed and have confirmed the relevance of ErbB2 as a target for directed cancer therapy both in preclinical models and in clinical studies.30,31Recombinant, ErbB2-specific single-chain (sc) Fv antibody fragments have been produced and have been genetically fused to the signal transducing CD3 ζ chain of the T-cell receptor (TCR) complex or the γ chain of the immunoglobulin Fc receptor complex.32-34Such chimeric antigen receptors were originally developed as a means to bypass immunization and directly generate cytotoxic effector cells such as CD8+ T cells with predefined recognition specificity.35,36 Upon antigen binding, the chimeric receptors link to endogenous signaling pathways in the effector cell and generate activating signals similar to those initiated by the T-cell receptor complex.37,38 Expression of ErbB2-specific chimeric receptors in established and primary T cells facilitated specific, MHC-independent recognition and efficient lysis of ErbB2-expressing cancer cells in in vitro and in vivo model systems.33,34,39 40
Here we have transduced NK-92 cells with a retroviral vector encoding a chimeric antigen receptor that consists of the ErbB2-specific scFv(FRP5) antibody fragment, a flexible hinge region, and the CD3 ζ chain. In contrast to parental NK-92 cells, the genetically modified NK-92-scFv(FRP5)-ζ cells specifically and efficiently lysed ErbB2-expressing tumor cells of various origins, suggesting that this strategy might be suitable for the development of effective cell-based therapeutics for the treatment of ErbB2-expressing cancers.
Materials and methods
Cells and culture conditions
Human SKBR3, MDA-MB453, MDA-MB468, and T47D breast carcinoma cells, SKOV3 ovarian carcinoma cells, and A431 epidermoid cancer cells were maintained in Dulbecco modified Eagle medium (DMEM; Bio Whittaker, Verviers, Belgium) containing 10% heat-inactivated fetal bovine serum (FBS), 2 mM l-glutamine, 100 units/mL penicillin, and 100 μg/mL streptomycin. Human erythroleukemic K562 cells were grown in RPMI 1640 medium (Bio Whittaker) supplemented with 10% heat-inactivated FBS, 2 mM l-glutamine, 100 units/mL penicillin, and 100 μg/mL streptomycin. Mouse renal cell carcinoma cells stably transfected with the lacZ gene (Renca-lacZ), and Renca-lacZ/ErbB2 cells expressing human ErbB241 were maintained in RPMI 1640 medium containing 10% heat-inactivated FBS, 2 mM l-glutamine, 100 units/mL penicillin, 100 μg/mL streptomycin, 0.25 mg/mL Zeocin, and 0.48 mg/mL G418 (Renca-lacZ/ErbB2). Human NK-92 cells and transduced NK-92-scFv(FRP5)-ζ cells were propagated in X-VIVO 10 medium (Bio Whittaker) supplemented with 5% heat-inactivated human serum, 100 units/mL IL-2 (Proleukin, Chiron, Emeryville, CA), and 0.6 mg/mL G418 (NK-92-scFv(FRP5)-ζ).
Transduction of NK-92 cells
FLYA-JET-5-ζ cells producing pL-scFv(FRP5)-ζ-SN amphotropic virus42 were grown overnight in NK-92 medium. Culture supernatant was passed through a 0.2-μm filter and incubated with NK-92 cells in the presence of 8 μg/mL polybrene for 6 hours at 37°C. Then the cells were grown for 40 hours in fresh NK-92 medium before G418 was added to a final concentration of 0.6 mg/mL.
Analysis of expression of the chimeric scFv(FRP5)-ζ construct
Cell-surface expression of scFv(FRP5)-ζ was determined by fluorescence activated cell sorter (FACS) analysis. Single-cell suspensions (5 × 105) of NK-92-scFv(FRP5)-ζ or parental NK-92 cells were incubated for 30 minutes at 4°C with 1.5 μg of the Myc-tag specific monoclonal antibody (mAb) 9E10.43 Cells were washed twice with phosphate-buffered saline and then treated for another 30 minutes at 4°C with a fluorescein isothiocyanate (FITC)–labeled goat anti-mouse IgG (BD PharMingen, Heidelberg, Germany) secondary antibody. Fluorescence of cells was analyzed with a FACScan (Becton Dickinson, Heidelberg, Germany). For immunoblotting, 5 × 106NK-92-scFv(FRP5)-ζ or NK-92 cells were harvested, washed with phosphate-buffered saline, and incubated for 20 minutes at 4°C in 1 mL of lysis buffer containing 50 mM Tris-HCl, pH 7.5, 1% Triton X-100, 5 mM EGTA (ethylene glycol-bis(2-aminoethylether)-N,N,N′,N′-tetraacetic acid), 150 mM NaCl, and 1 mM phenylmethylsulfonyl fluoride. Cell lysates were cleared by centrifugation, separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) under reducing or nonreducing conditions, and immunoblotted with anti-CD3 ζ mAb (BD PharMingen).
Enrichment of scFv(FRP5)-ζ–expressing NK-92 cells
NK-92-scFv(FRP5)-ζ cells expressing high levels of chimeric antigen receptor were enriched by sorting with magnetic beads. G418-resistant cells were incubated with mAb 9E10 (1.5 μg/5 × 105 cells) and selected using goat anti-mouse IgG MicroBeads (Miltenyi Biotec, Bergisch Gladbach, Germany) and MACS LS+ separation columns (Miltenyi Biotec) according to the manufacturer's instructions.
Cytotoxicity assays
Cytotoxicity of parental NK-92 and transduced NK-92-scFv(FRP5)-ζ cells was analyzed in europium (Eu3+)-release assays. Target cells (5 × 106) were incubated for 10 minutes at 4°C in 800 μL of europium solution [50 mM HEPES, pH 7.4, 93 mM NaCl, 5 mM KCl, 2 mM MgCl2, 10 mM diethylenetriamine-pentaacetic acid (Sigma, Taufkirchen, Germany), 2 mM europium(III)-acetate (Sigma)], electroporated at 200 Ω, 960 μFa, 250 V using a GenePulser (Bio-Rad, Munich, Germany), and incubated for another 10 minutes on ice. Then the cells were washed once in 50 mL and another 4 times in 15 mL of RPMI 1640 medium supplemented with 5% heat-inactivated FBS (RPMI-FBS). Target cells (1 × 104 in 100 μL RPMI-FBS/well) were seeded in triplicates in 96-well tissue culture plates before the addition of 100 μL/well RPMI/FBS (to determine spontaneous lysis), or NK-92, or NK-92-scFv(FRP5)-ζ cells at various effector-to-target ratios (E/T). The plates were centrifuged for 2 minutes at × 50 g and incubated for 2 hours at 37°C. To determine maximal lysis, 100 μL target-cell suspension was incubated with 100 μL lysis buffer (PerkinElmer Wallac, Freiburg, Germany), followed by repeated freezing/thawing. Cells were centrifuged for 5 minutes at × 500 g, and 20 μL culture supernatant was collected and added to 200 μL/well of enhancement solution (PerkinElmer Wallac) in FluoroNunc 96-well plates (Nunc, Wiesbaden, Germany). After incubation on a shaker at 50 revolutions per minute for 15 minutes at room temperature, fluorescence was determined using an LKB-Wallac 1230 Arcus fluorometer (PerkinElmer Wallac). Specific cytotoxicity was calculated as % cytotoxicity = (experimental lysis − spontaneous lysis) × 100/(maximal lysis − spontaneous lysis).
TUNEL assays
For the detection of apoptotic target cells, TUNEL (TdT-mediated dUTP nick-end labeling) assays were performed. 104 SKBR3 cells/well were seeded on 8-well glass slides (Roth, Karlsruhe, Germany) and grown overnight at 37°C in a humidified chamber. The growth medium was exchanged, 3 × 104 NK-92 or NK-92-scFv(FRP5)-ζ cells were added (E/T ratio 3:1), and the cells were incubated for one hour at 37°C. After removal of medium and nonadherent effector cells, the samples were fixed with 4% paraformaldehyde in PBS, pH 7.4 for one hour at room temperature. For cell permeabilization, the slides were rinsed with PBS and incubated in permeabilization solution (0.1% Triton X-100, 0.1% sodium citrate) for 2 minutes on ice. For labeling of apoptotic cells, the in situ cell death detection kit, fluorescein (Roche Diagnostics, Mannheim, Germany), was used according to the manufacturer's instructions. The samples were embedded in Vectashield mounting medium with propidium iodide (Vector Laboratories, Burlingame, CA), covered with a coverslip, and analyzed under a Zeiss Axiophot fluorescence microscope (Carl Zeiss, Jena, Germany).
In vivo antitumor activity
NIH 3T3#3.7 tumor cells (5 × 105)34were mixed with 5 × 106 NK-92-scFv(FRP5)-ζ or parental NK-92 cells (E/T ratios of 10:1) in 0.1 mL phosphate-buffered saline and immediately injected into the flanks of CD-1 nu/nu mice (Charles River, Sulzfeld, Germany) (2 injections per animal). Tumor growth was followed by caliper measurements, and tumor volumes were calculated using the formula length × (width)2 × 0.4, and data were statistically analyzed. Significance of differences between treatment groups was calculated by double-sided Studentt test.
Results
Generation of NK-92 cells carrying a chimeric antigen receptor
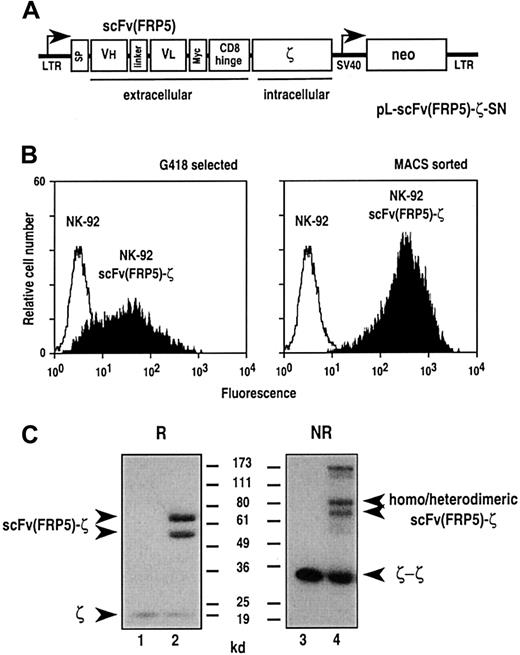
To provide established human NK-92 cells with chimeric antigen receptor, the cells were transduced with amphotropic retroviral vector pL-scFv(FRP5)-ζ-SN produced by the packaging cell line FLYA-JET-5-ζ.42 A schematic representation of the pLXSN-based44 pL-scFv(FRP5)-ζ-SN construct is shown in Figure 1A. The vector encodes under the control of the retroviral 5′ long terminal repeat (LTR), a fusion protein consisting of an immunoglobulin heavy-chain leader peptide (SP), the ErbB2-specific single-chain antibody fragment scFv(FRP5), a Myc-tag, the hinge region of murine CD8α (amino acids 105-165), and the murine CD3 ζ chain.39 Transduced cells were selected with G418, and surface expression of the chimeric scFv(FRP5)-ζ construct was verified by FACS analysis using the Myc-tag–specific mAb 9E10. As shown in Figure 1B, left panel, G418-resistant NK-92-scFv(FRP5)-ζ cells expressed the chimeric receptor on the cell surface, albeit at heterogeneous levels. To enrich cells expressing large amounts of the scFv(FRP5)-ζ construct, NK-92-scFv(FRP5)-ζ cells were separated using mAb 9E10 and immunomagnetic beads. FACS analysis of the selected cells revealed a more homogeneous surface expression of scFv(FRP5)-ζ at very high levels (Figure 1B, right panel). This NK-92-scFv(FRP5)-ζ cell population was used in subsequent experiments. Thereby, persistence of high-level transgene expression was routinely confirmed by FACS analysis (data not shown).
Transduction of NK-92 cells with retroviral vector pL-scFv(FRP5)-ζ-SN.
(A) Schematic representation of the pL-scFv(FRP5)-ζ-SN construct. The Moloney murine leukemia virus 5′ long terminal repeat (LTR) controls the expression of the chimeric antigen receptor scFv(FRP5)-ζ, which consists of an N-terminal immunoglobulin heavy-chain leader peptide (SP), the ErbB2-specific single-chain antibody scFv(FRP5), a Myc-tag, the hinge region of murine CD8α, and the murine CD3 ζ chain. The neomycin-resistance gene for G418 selection of transduced cells is driven by the SV40 early promoter. (B) Surface expression of chimeric scFv(FRP5)-ζ antigen receptor. NK-92 cells successfully transduced with the retroviral vector (NK-92-scFv(FRP5)-ζ cells) were selected with G418 (left panel). Cells expressing high levels of the chimeric antigen receptor were enriched by sorting with Myc-tag specific mAb 9E10 and goat anti-mouse IgG–coated magnetic beads (right panel). After each selection step surface expression of scFv(FRP5)-ζ was determined by FACS analysis using mAb 9E10 and FITC-labeled goat anti-mouse secondary antibody . Parental NK-92 cells served as a control. (C) Immunoblot analysis of scFv(FRP5)-ζ expression. Lysates of NK-92 (lanes 1 and 3) and transduced NK-92-scFv(FRP5)-ζ cells (lanes 2 and 4) were separated by SDS-PAGE under nonreducing (NR) or reducing (R) conditions. Immunoblot analysis was performed with a CD3 ζ chain–specific mAb followed by horseradish peroxidase (HRP)–conjugated anti-mouse antibody and chemiluminescent detection. The positions of endogenous and chimeric CD3 ζ proteins and of molecular weight standards (kd) are indicated.
Transduction of NK-92 cells with retroviral vector pL-scFv(FRP5)-ζ-SN.
(A) Schematic representation of the pL-scFv(FRP5)-ζ-SN construct. The Moloney murine leukemia virus 5′ long terminal repeat (LTR) controls the expression of the chimeric antigen receptor scFv(FRP5)-ζ, which consists of an N-terminal immunoglobulin heavy-chain leader peptide (SP), the ErbB2-specific single-chain antibody scFv(FRP5), a Myc-tag, the hinge region of murine CD8α, and the murine CD3 ζ chain. The neomycin-resistance gene for G418 selection of transduced cells is driven by the SV40 early promoter. (B) Surface expression of chimeric scFv(FRP5)-ζ antigen receptor. NK-92 cells successfully transduced with the retroviral vector (NK-92-scFv(FRP5)-ζ cells) were selected with G418 (left panel). Cells expressing high levels of the chimeric antigen receptor were enriched by sorting with Myc-tag specific mAb 9E10 and goat anti-mouse IgG–coated magnetic beads (right panel). After each selection step surface expression of scFv(FRP5)-ζ was determined by FACS analysis using mAb 9E10 and FITC-labeled goat anti-mouse secondary antibody . Parental NK-92 cells served as a control. (C) Immunoblot analysis of scFv(FRP5)-ζ expression. Lysates of NK-92 (lanes 1 and 3) and transduced NK-92-scFv(FRP5)-ζ cells (lanes 2 and 4) were separated by SDS-PAGE under nonreducing (NR) or reducing (R) conditions. Immunoblot analysis was performed with a CD3 ζ chain–specific mAb followed by horseradish peroxidase (HRP)–conjugated anti-mouse antibody and chemiluminescent detection. The positions of endogenous and chimeric CD3 ζ proteins and of molecular weight standards (kd) are indicated.
Expression of scFv(FRP5)-ζ was also analyzed by immunoblotting. Cell lysates of NK-92 and NK-92-scFv(FRP5)-ζ cells were separated by SDS-PAGE under reducing or nonreducing conditions and immunoblotted with a ζ-chain–specific mAb. The results are shown in Figure 1C. Under reducing conditions, endogenous ζ chain was detected as an 18 kd band in lysates of both NK-92 (lane 1) and NK-92-scFv(FRP5)-ζ cells (lane 2). Two additional bands of approximately 54 kd and 68 kd were observed in NK-92-scFv(FRP5)-ζ cells but were absent in parental NK-92 cells. While the lower band corresponds well with the calculated size of chimeric scFv(FRP5)-ζ protein (52 kd), the larger protein species is most likely the result of complex glycosylation of the chimeric receptor within the scFv antibody fragment and hinge region.34 Under nonreducing conditions, a series of additional bands were observed in lysates of NK-92-scFv(FRP5)-ζ cells (lane 4) representing scFv(FRP5)-ζ homodimers and heterodimers of scFv(FRP5)-ζ and endogenous ζ chain. Homodimeric CD3 ζ could be detected in lysates of both cell populations.
Cytotoxic activity of NK-92-scFv(FRP5)-ζ cells
NK-92 cells are highly cytotoxic against malignant cells of various origins such as leukemia, lymphoma, and myeloma.18To investigate whether genetic manipulation resulted in an alteration of the intrinsic cytotoxic properties, the cell-killing activity of NK-92-scFv(FRP5)-ζ and parental NK92 cells toward different tumor cell lines was compared in europium (Eu3+) release assays. Target cells were electroporated with an Eu3+-containing solution before incubation for 2 hours with NK-92-scFv(FRP5)-ζ or parental NK-92 cells at different E/T ratios. First, human K562 erythroleukemic cells that were previously shown to be highly sensitive for NK-92–mediated killing were tested.18 21 As shown in Figure 2A, K562 cells were lysed equally well by unmodified NK-92 and transduced NK-92-scFv(FRP5)-ζ cells. Even at low E/T ratios, no significant differences between the 2 effector cell populations were detected, demonstrating that expression of the chimeric scFv(FRP5)-ζ protein did not alter the intrinsic cytotoxic activity toward NK-92–sensitive targets.
Cytotoxic activity of NK-92-scFv(FRP5)-ζ cells.
Lysis of K562 human erythroleukemic cells (A), ErbB2-negative MDA-MB468 (B), and ErbB2-overexpressing MDA-MB453 human breast carcinoma cells (C) by NK-92 and transduced NK-92-scFv(FRP5)-ζ cells was analyzed in europium (Eu3+) release assays. Target cells were labeled with Eu3+ solution by electroporation and then incubated with NK-92-scFv(FRP5)-ζ (○) or parental NK-92 cells (●) at different E/T ratios for 2 hours. Release of Eu3+ complexes into the medium was determined by fluorimetric analysis and served as a measure for target-cell lysis. Specific lysis was calculated as described in “Materials and methods.”
Cytotoxic activity of NK-92-scFv(FRP5)-ζ cells.
Lysis of K562 human erythroleukemic cells (A), ErbB2-negative MDA-MB468 (B), and ErbB2-overexpressing MDA-MB453 human breast carcinoma cells (C) by NK-92 and transduced NK-92-scFv(FRP5)-ζ cells was analyzed in europium (Eu3+) release assays. Target cells were labeled with Eu3+ solution by electroporation and then incubated with NK-92-scFv(FRP5)-ζ (○) or parental NK-92 cells (●) at different E/T ratios for 2 hours. Release of Eu3+ complexes into the medium was determined by fluorimetric analysis and served as a measure for target-cell lysis. Specific lysis was calculated as described in “Materials and methods.”
Next, the cell-killing activity of NK-92 and NK-92-scFv(FRP5)-ζ cells against human MDA-MB468 and MDA-MB453 breast carcinoma cells was investigated. MDA-MB468 cells do not express detectable levels of ErbB2, whereas MDA-MB453 cells highly overexpress the scFv(FRP5) target antigen (Table 1). ErbB2-negative MDA-MB468 cells were completely resistant to NK-92–mediated killing, even at high E/T ratios (Figure 2B). As expected, this was not changed by expression of the ErbB2-specific scFv(FRP5)-ζ protein in NK-92-scFv(FRP5)-ζ cells. Similarly, MDA-MB453 cells were completely resistant to parental NK-92 cells. However, these ErbB2-overexpressing cells were efficiently lysed by NK-92-scFv(FRP5)-ζ cells, even at low E/T ratios (more than 70% specific lysis at an E/T of 1:1) (Figure2C). These results demonstrate that expression of the chimeric scFv(FRP5)-ζ antigen receptor enables NK-92-scFv(FRP5)-ζ cells to eliminate target cells expressing the relevant surface antigen and to overcome these cells' intrinsic resistance to NK cell–mediated lysis.
ErbB2 expression levels in human tumor cell lines and sensitivity toward NK-92-scFv(FRP5)-ζ cells
| Cell line . | Origin . | No. of ErbB2 receptors* . | Lysis by NK-92 (%)† E/T 10:1 . | Lysis by NK-92-scFv(FRP5)-ζ (%) E/T 10:1 . |
|---|---|---|---|---|
| K562 | Erythroid leukemia | — | 60 | 59 |
| MDA-MB468 | Breast carcinoma | < 5 × 103 | < 10 | < 10 |
| MDA-MB453 | Breast carcinoma | 1 × 106 | < 10 | 91 |
| SKBR3 | Breast carcinoma | 1 × 106 | < 10 | 93 |
| SKOV3 | Ovarian carcinoma | 1 × 105 | < 10 | 68 |
| A431 | Squamous cell carcinoma | 5 × 104 | < 10 | 62 |
| T47D | Breast carcinoma | 3 × 104 | 16 | 74 |
| Cell line . | Origin . | No. of ErbB2 receptors* . | Lysis by NK-92 (%)† E/T 10:1 . | Lysis by NK-92-scFv(FRP5)-ζ (%) E/T 10:1 . |
|---|---|---|---|---|
| K562 | Erythroid leukemia | — | 60 | 59 |
| MDA-MB468 | Breast carcinoma | < 5 × 103 | < 10 | < 10 |
| MDA-MB453 | Breast carcinoma | 1 × 106 | < 10 | 91 |
| SKBR3 | Breast carcinoma | 1 × 106 | < 10 | 93 |
| SKOV3 | Ovarian carcinoma | 1 × 105 | < 10 | 68 |
| A431 | Squamous cell carcinoma | 5 × 104 | < 10 | 62 |
| T47D | Breast carcinoma | 3 × 104 | 16 | 74 |
NK-92-scFv(FRP5)-ζ cytotoxicity against NK-92–resistant cells is strictly dependent on binding of the chimeric antigen receptor to ErbB2
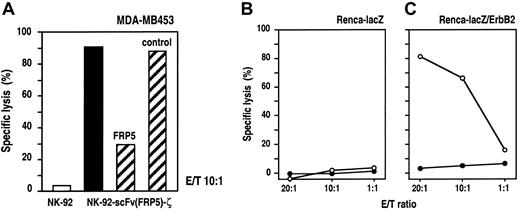
To further confirm the specificity of target-cell recognition and to analyze the dependence on ErbB2 binding for lytic activity, we performed Eu3+-release assays in the presence of the parental mAb FRP550 as a specific competitor for scFv(FRP5) binding. NK-92-scFv(FRP5)-ζ cells and ErbB2-expressing MDA-MB453 cells were incubated at an E/T ratio of 10:1 for 2 hours in the presence of 30 μg/mL mAb FRP5 or an isotype-matched control antibody. As shown in Figure 3A, NK-92-scFv(FRP5)-ζ–mediated killing of MDA-MB453 cells was markedly reduced in the presence of mAb FRP5 (30% vs 91% specific lysis) but remained unaffected by an irrelevant control mAb (88% specific lysis). As shown before, parental NK-92 cells displayed no significant lytic activity toward MDA-MB453 cells (3% lysis). These results confirm that cytotoxicity of NK-92-scFv(FRP5)-ζ cells toward NK-92–resistant targets is strictly dependent on binding of the chimeric antigen receptor to the ErbB2 target antigen.
Specificity of NK-92-scFv(FRP5)-ζ cells.
(A) Competition of ErbB2 binding. ErbB2-expressing MDA-MB453 cells were incubated with NK-92-scFv(FRP5)-ζ cells at an E/T ratio of 10:1 for 2 hours in the absence of competitor (filled bar) or in the presence of 30 μg/mL ErbB2-specific mAb FRP5 or an isotype-matched control antibody as indicated (hatched bars). Parental NK-92 cells were included as a control (open bar). Specific lysis was determined in europium-release assays as described in the legend of Figure 2. (B-C) Cytotoxic activity of NK-92-scFv(FRP5)-ζ against transfected murine cell lines. Renca-lacZ murine renal cell carcinoma cells expressing theEscherichia coli β-galactosidase gene (B) and Renca-lacZ/ErbB2 cells further transfected with a human c-erbB2 cDNA construct (C) were incubated with NK-92-scFv(FRP5)-ζ (○) or parental NK-92 cells (●) at different E/T ratios for 2 hours. Specific lysis was determined in europium-release assays as described in the legend of Figure 2.
Specificity of NK-92-scFv(FRP5)-ζ cells.
(A) Competition of ErbB2 binding. ErbB2-expressing MDA-MB453 cells were incubated with NK-92-scFv(FRP5)-ζ cells at an E/T ratio of 10:1 for 2 hours in the absence of competitor (filled bar) or in the presence of 30 μg/mL ErbB2-specific mAb FRP5 or an isotype-matched control antibody as indicated (hatched bars). Parental NK-92 cells were included as a control (open bar). Specific lysis was determined in europium-release assays as described in the legend of Figure 2. (B-C) Cytotoxic activity of NK-92-scFv(FRP5)-ζ against transfected murine cell lines. Renca-lacZ murine renal cell carcinoma cells expressing theEscherichia coli β-galactosidase gene (B) and Renca-lacZ/ErbB2 cells further transfected with a human c-erbB2 cDNA construct (C) were incubated with NK-92-scFv(FRP5)-ζ (○) or parental NK-92 cells (●) at different E/T ratios for 2 hours. Specific lysis was determined in europium-release assays as described in the legend of Figure 2.
NK-92 cells display no xenogeneic cytotoxic effects against murine cells in SCID mouse models.19,20 To analyze whether the expression of human ErbB2 on the surface of murine cells is sufficient to allow recognition and cytolysis by NK-92-scFv(FRP5)-ζ cells, we conducted Eu3+-release cytotoxicity assays using murine renal cell carcinoma (Renca) cells transfected with the β-galactosidase gene (Renca-lacZ) and Renca-lacZ cells stably transfected with human c-erbB2-cDNA (Renca-lacZ/ErbB241) as target cells. The results are shown in Figure 3B and C. As expected, neither parental NK-92 nor transduced NK-92-scFv(FRP5)-ζ cells showed significant cytotoxic effects toward Renca-lacZ cells at all E/T ratios analyzed (panel B). In contrast, Renca-lacZ/ErbB2 cells, although completely resistant to NK-92, were efficiently lysed by NK-92-scFv(FRP5)-ζ cells (panel C). These results demonstrate that expression of the ErbB2 receptor on the surface of target cells alone is sufficient to mediate recognition and lysis by NK-92-scFv(FRP5)-ζ cells.
NK-92-scFv(FRP5)-ζ cytotoxicity against tumor cells of epithelial origin correlates with ErbB2 expression levels
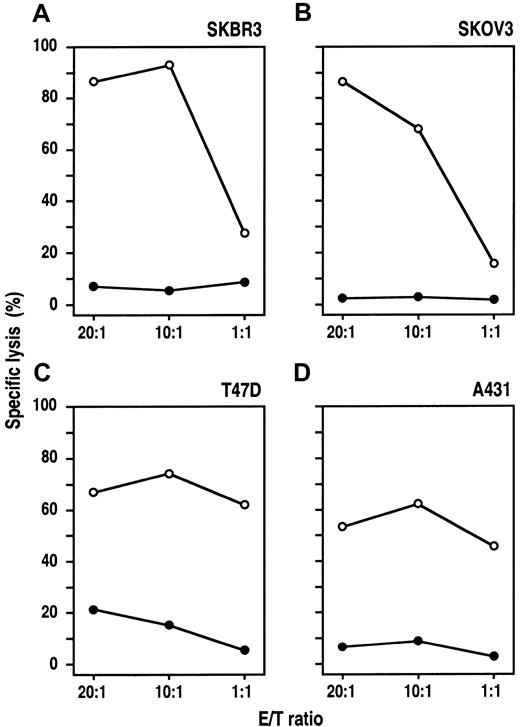
MDA-MB453 and Renca-lacZ/ErbB2 cells proved to be highly sensitive to NK-92-scFv(FRP5)-ζ–mediated lysis. These tumor cells express very high levels of the target antigen carrying approximately 1 million (MDA-MB453, Table 1) or even more (Renca-lacZ/ErbB241) ErbB2 molecules per cell. To investigate the activity of NK-92-scFv(FRP5)-ζ against target cells expressing varying ErbB2 levels, we analyzed a panel of human tumor cell lines derived from different cancers of epithelial origin. SKBR3 breast carcinoma and SKOV3 ovarian carcinoma cells carry large numbers of ErbB2 receptors, whereas A431 squamous cell carcinoma and T47D breast carcinoma cells express moderate ErbB2 levels (Table 1). The cells were labeled with europium and incubated for 2 hours with NK-92-scFv(FRP5)-ζ or parental NK-92 cells at different E/T ratios. As shown in Figure4, SKBR3, SKOV3, and A431 cells were completely resistant to NK-92. T47D cells at high E/T ratios were lysed by NK-92, but only to a low degree (< 25% specific lysis at an E/T ratio of 20:1). In contrast, all 4 cell lines were efficiently killed by NK-92-scFv(FRP5)-ζ. Thereby, A431 and T47D cells expressing moderate ErbB2 levels displayed NK-92-scFv(FRP5)-ζ sensitivity comparable to that of the K562 NK cell target (Table 1). With SKBR3 and SKOV3 cells expressing high ErbB2 levels, even more pronounced cytotoxic activity was observed (specific lysis > 90%), suggesting that NK-92-scFv(FRP5)-ζ cytotoxicity toward cells resistant to parental NK-92 correlates with the amount of ErbB2 on the target-cell surface.
Cytotoxic activity of NK-92-scFv(FRP5)-ζ against ErbB2-expressing cancer cell lines.
Human SKBR3 (A) and T47D (C) breast carcinoma cells, SKOV3 ovarian carcinoma cells (B), and A431 squamous cell carcinoma cells (D) were incubated with NK-92-scFv(FRP5)-ζ (○) or parental NK-92 cells (●) at different E/T ratios for 2 hours. Specific lysis was determined in europium-release assays as described in the legend of Figure2.
Cytotoxic activity of NK-92-scFv(FRP5)-ζ against ErbB2-expressing cancer cell lines.
Human SKBR3 (A) and T47D (C) breast carcinoma cells, SKOV3 ovarian carcinoma cells (B), and A431 squamous cell carcinoma cells (D) were incubated with NK-92-scFv(FRP5)-ζ (○) or parental NK-92 cells (●) at different E/T ratios for 2 hours. Specific lysis was determined in europium-release assays as described in the legend of Figure2.
NK-92-scFv(FRP5)-ζ cells rapidly induce apoptosis and completely eliminate target cells at low E/T ratios
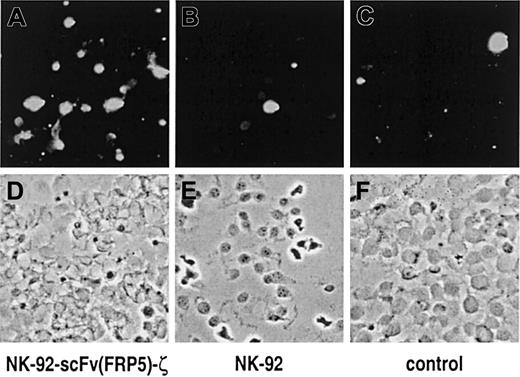
The consequences of NK-92-scFv(FRP5)-ζ activity on cells expressing high levels of ErbB2 were investigated further. SKBR3 breast cancer cells were grown on glass slides and then incubated for one hour with NK-92-scFv(FRP5)-ζ or parental NK-92 cells at an E/T ratio of 3:1. NK cell–mediated induction of apoptosis was analyzed by TUNEL assay and fluorescence microscopy. The results are shown in Figure5. A marked increase in the number of apoptotic SKBR3 cells was detected upon incubation with retargeted NK-92-scFv(FRP5)-ζ cells (panel A) when compared to cells grown in the presence of parental NK-92 (panel B) or without the addition of NK cells (panel C).
NK cell–mediated induction of apoptosis in breast carcinoma cells.
SKBR3 cells were incubated for one hour with NK-92-scFv(FRP5)-ζ (A, D) or parental NK-92 cells (B, E) at an E/T ratio of 3:1. Control cells were cultured in the absence of NK cells (C, F). Apoptotic cells were labeled with fluorescein using the TUNEL method and visualized under a fluorescence microscope with blue light excitation. Fluorescent images of representative fields are shown in (A) to (C); phase contrast images of the same sections are shown in (D) to (F).
NK cell–mediated induction of apoptosis in breast carcinoma cells.
SKBR3 cells were incubated for one hour with NK-92-scFv(FRP5)-ζ (A, D) or parental NK-92 cells (B, E) at an E/T ratio of 3:1. Control cells were cultured in the absence of NK cells (C, F). Apoptotic cells were labeled with fluorescein using the TUNEL method and visualized under a fluorescence microscope with blue light excitation. Fluorescent images of representative fields are shown in (A) to (C); phase contrast images of the same sections are shown in (D) to (F).
NK-92-scFv(FRP5)-ζ cells inhibit the in vivo growth of ErbB2-expressing tumor cells
Tumorigenic mouse NIH 3T3 fibroblasts (NIH 3T3#3.7 cells) expressing human ErbB2 oncogenically activated by a mutation in its transmembrane domain34 were employed as target cells to determine antitumoral activity of NK-92-scFv(FRP5)-ζ in vivo. Similar to murine Renca-lacZ/ErbB2 cells, in in vitro cytotoxicity assays, NIH 3T3#3.7 cells were completely resistant to cytolytic activity of parental NK-92 but were efficiently and specifically lysed by NK-92-scFv(FRP5)-ζ cells (82% and 98% lysis at E/T ratios of 1:1 and 10:1; data not shown). As shown in Figure6, subcutaneous injection of NIH 3T3#3.7 cells into CD-1 nude mice led to rapid tumor formation. Treatment with parental NK-92 cells had no effect on tumor growth in comparison to untreated controls. In contrast, simultaneous administration of tumor cells and NK-92-scFv(FRP5)-ζ cells resulted in marked suppression of tumor growth for several days. Differences between NK-92-scFv(FRP5)-ζ–treated and untreated groups were statistically significant at early time points (P < .05, day 7), and between NK-92-scFv(FRP5)-ζ and NK-92–treated groups at all time points (P < .05). Because of tumor growth at later time points in all animals, the experiment was terminated on day 14.
In vivo antitumor activity of NK-92-scFv(FRP5)-ζ cells.
5 × 105 NIH 3T3#3.7 tumor cells stably transfected with oncogenically activated human ErbB2 cDNA were mixed with NK-92-scFv(FRP5)-ζ cells (●), parental NK-92 cells (○) at an E/T ratio of 10:1, or saline (■) and immediately injected subcutaneously into the flanks of CD-1 nude mice. Tumor growth was followed by caliper measurements, and tumor volumes were calculated. The standard deviation is represented by error bars.
In vivo antitumor activity of NK-92-scFv(FRP5)-ζ cells.
5 × 105 NIH 3T3#3.7 tumor cells stably transfected with oncogenically activated human ErbB2 cDNA were mixed with NK-92-scFv(FRP5)-ζ cells (●), parental NK-92 cells (○) at an E/T ratio of 10:1, or saline (■) and immediately injected subcutaneously into the flanks of CD-1 nude mice. Tumor growth was followed by caliper measurements, and tumor volumes were calculated. The standard deviation is represented by error bars.
NK-92-scFv(FRP5)-ζ efficiently lyse primary human breast cancer cells
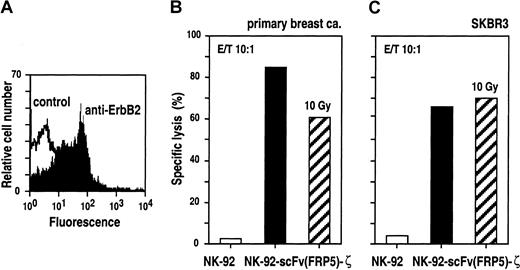
To analyze cytotoxic effects of NK-92-scFv(FRP5)-ζ against ErbB2-expressing primary cancer cells, tumor cells from a pleural effusion of a patient suffering from metastatic breast cancer were enriched by in vitro culture for 21 days.51 Expression of elevated levels of ErbB2 on the surface of these cells was confirmed by FACS analysis (Figure 7A) before labeling with europium and incubating for 2 hours with NK-92-scFv(FRP5)-ζ or parental NK-92 cells at E/T ratios of 10:1. The results are shown in Figure 7B. Similar to ErbB2-overexpressing established tumor cell lines, primary breast cancer cells were completely resistant to NK-92. In contrast, incubation with retargeted NK-92-scFv(FRP5)-ζ resulted in specific and efficient target-cell killing (85% specific lysis).
Cytotoxic activity of untreated and irradiated NK-92-scFv(FRP5)-ζ against primary and established human breast cancer cells.
Breast cancer cells were collected from a pleural effusion of a patient suffering from metastatic breast cancer. ErbB2 expression on the surface of primary cancer cells was confirmed by FACS analysis (A). Primary (B) and established SKBR3 breast cancer cells (C) were incubated with NK-92-scFv(FRP5)-ζ (filled bars), NK-92-scFv(FRP5)-ζ irradiated with a dose of 10 Gy (hatched bars), or parental NK-92 cells (open bars) at an E/T ratio of 10:1 for 2 hours. Specific lysis was determined in europium-release assays as described in the legend of Figure 2.
Cytotoxic activity of untreated and irradiated NK-92-scFv(FRP5)-ζ against primary and established human breast cancer cells.
Breast cancer cells were collected from a pleural effusion of a patient suffering from metastatic breast cancer. ErbB2 expression on the surface of primary cancer cells was confirmed by FACS analysis (A). Primary (B) and established SKBR3 breast cancer cells (C) were incubated with NK-92-scFv(FRP5)-ζ (filled bars), NK-92-scFv(FRP5)-ζ irradiated with a dose of 10 Gy (hatched bars), or parental NK-92 cells (open bars) at an E/T ratio of 10:1 for 2 hours. Specific lysis was determined in europium-release assays as described in the legend of Figure 2.
In ongoing clinical studies, adoptive transfer of NK-92 is carried out upon irradiation with 10 Gy to block possible in vivo proliferation of the cells.23 Because similar safety measures would be important for the use of retargeted NK-92 variants in cancer patients, we tested the effects of irradiation on NK-92-scFv(FRP5)-ζ cells. In [3H]thymidine incorporation and cell viability assays, it was found that upon irradiation with 10 Gy, NK-92-scFv(FRP5)-ζ cells, like parental NK-92 cells, completely ceased proliferation. However, more than 60% of the cells remained viable for at least another 3 days (data not shown). Cytolytic activity of irradiated NK-92-scFv(FRP5)-ζ cells was investigated using ErbB2-overexpressing primary and established breast cancer cells as targets at E/T ratios of 10:1. As shown in Figures 7B and C, NK-92-scFv(FRP5)-ζ cells irradiated with 10 Gy retained marked cell-killing activity toward primary breast cancer cells (61% specific lysis) and established SKBR3 cells (70% specific lysis).
Discussion
Chimeric receptors facilitate the generation of antigen-specific effector cells independent from the availability of T cells carrying a suitable natural T-cell receptor, and allow to bypass MHC-restricted recognition of peptide antigens as a requirement for the initiation of cytolytic effector functions.35,36 This might help to overcome some of the limitations inherent to adoptive transfer of tumor-infiltrating lymphocytes or lymphokine-activated killer cells such as heterogeneity of effector cell populations and poorly defined target specificity, whereas the principal advantages of a cell-based therapy, that is, the high intrinsic cytotoxic potential of the effector cells and their capacity to extravasate and home to tumor sites, could be retained. Despite these potential benefits, however, so far only a limited number of clinical trials with retargeted T cells have been initiated,52 53 at least in part because of the requirement for efficient transduction of patient-derived effector cells and expansion in quantities sufficient for therapy. Employing retargeted cytotoxic cell lines for adoptive transfer in an allogeneic setting might help to overcome some of the current limitations and could result in the development of more generally applicable cell therapeutics.
As a first step in this direction, here we have generated a genetically modified variant of the continuously growing cytotoxic NK cell line NK-92. NK-92 cells were previously shown to be highly cytotoxic against established leukemia, lymphoma, and melanoma cell lines.18-20 In addition, more than half of the newly diagnosed and relapsed primary AML, T-ALL, B-lineage-ALL, and CML cells tested proved to be sensitive to NK-92–mediated cell killing in vitro and in severe combined immunodeficiency disease (SCID) mouse models in vivo, whereas normal donor-derived human bone marrow hematopoietic cells were insensitive to cytolysis by NK-92.19 In contrast to malignant cells of hematologic origin, however, the proportion of NK-92–sensitive cancer cells derived from solid tumors appears to be significantly lower. In the present study, 6 of 7 established tumor cell lines and primary cancer cells originating from human breast, ovarian, and squamous cell carcinomas, and expressing elevated levels of receptor tyrosine kinases such as ErbB2 or the closely related EGF receptor (MDA-MB468 cells) were completely resistant to NK-92–mediated lysis, with the remaining cell line being only weakly sensitive.
To extend NK-92 cytotoxicity to these cancer cell types and to enhance tumor-specific targeting, we have transduced NK-92 cells with a retroviral vector encoding an ErbB2-specific chimeric antigen receptor. After antibiotic selection and immunomagnetic separation, we could establish an NK-92 cell population that displays homogeneous and high-level surface expression of the chimeric receptor, which has not changed substantially during continuous propagation for more than one year. Thereby, transduction with the retroviral vector does not appear to have altered the general features of the resulting NK-92-scFv(FRP5)-ζ cells. The growth kinetics of NK-92-scFv(FRP5)-ζ cells and parental NK-92 cells are very similar (data not shown). Also, the high cytotoxic potential against malignant cells of hematologic origin was retained, as indicated by the continued ability of NK-92-scFv(FRP5)-ζ cells to lyse erythroleukemic K562 cells. Likewise, expression of the chimeric antigen receptor did not change the activity of NK-92 against ErbB2-negative tumor cells of epithelial origin. Human MDA-MB468 breast carcinoma cells and murine Renca-lacZ cells, which both are not affected by parental NK-92 cells, remained resistant to NK-92-scFv(FRP5)-ζ cells. In contrast, all ErbB2-expressing tumor cell lines and primary cancer cells tested so far that were insensitive to NK-92–mediated killing were efficiently lysed by NK-92-scFv(FRP5)-ζ cells, demonstrating that resistance could be overcome alone by expression of the chimeric antigen receptor. Even at low E/T ratios, NK-92-scFv(FRP5)-ζ cells rapidly induced apoptosis in ErbB2-expressing target cells, and upon prolonged incubation, completely eliminated tumor cells from the culture. Thereby, cytotoxic activity of NK-92-scFv(FRP5)-ζ cells was inhibited if interaction of the chimeric receptor with the target antigen was blocked, further confirming the specificity of tumor-cell recognition. Specific antitumoral effects of NK-92-scFv(FRP5)-ζ cells were also observed when these cells were implanted simultaneously with ErbB2-transformed fibroblasts into nude mice. Compared with untreated animals or animals treated with parental NK-92 cells, tumor growth was delayed for several days. This indicates that NK-92-scFv(FRP5)-ζ cells retained specificity and cytolytic activity in vivo even though no exogenous IL-2 was included in the treatment schedule.
NK-92 cells were originally established from peripheral blood lymphocytes of a patient with large granular lymphoma.21Adoptive transfer of such cells in immunocompromised patients could carry the risk of engraftment and induction of secondary lymphoma, which would prohibit clinical use of NK-92. This was investigated independently by 2 groups using murine model systems. Thereby, repeated injection of NK-92 cells did not result in permanent engraftment in immunocompromised SCID or NK cell–deficient pfp-Rag-2 mice, despite the ongoing administration of recombinant IL-2.19,20 In addition, histopathological analysis of bone marrow, spleen, kidney, lung, and brain did not reveal any signs of toxicity.19Irradiation of NK-92 cells with 5 Gy was shown to prevent further cell division, whereas substantial cytotoxicity could be retained for up to 48 hours upon irradiation with 10 Gy.54 Preliminary results based on 8 patients from an ongoing phase I clinical trial suggest that treatment with 2 transfusions of up to 3 × 109 irradiated NK-92 cells/m2 body surface 48 hours apart is generally well tolerated.23Irradiation might also be suitable to block in vivo proliferation of genetically modified NK-92-scFv(FRP5)-ζ cells. Upon irradiation with 2 to 20 Gy, effects on proliferation and viability of NK-92-scFv(FRP5)-ζ and parental NK-92 cells were indistinguishable. Furthermore, although proliferation of NK-92-scFv(FRP5)-ζ cells was completely blocked after irradiation with 10 Gy, cytolytic activity of irradiated cells toward ErbB2-expressing targets was comparable to that of untreated NK-92-scFv(FRP5)-ζ cells.
Expression of ErbB2 on the tumor-cell surface appears to be sufficient to enable recognition and lysis by NK-92-scFv(FRP5)-ζ cells. NK-92–resistant murine Renca-lacZ cells became highly sensitive to the genetically modified variant after stable transfection with a human c-erbB2 cDNA construct. NK-92-scFv(FRP5)-ζ cell cytolytic activity is retained toward target cells expressing varying levels of ErbB2. Although human tumor cells displaying more moderate amounts of ErbB2 were slightly less sensitive to NK-92-scFv(FRP5)-ζ–mediated killing, specific lysis still exceeded 60% at an E/T ratio of 10:1. These findings correspond well with recent studies addressing the influence of target antigen and receptor densities on cytotoxic activity. Whereas T cells with large amounts of a chimeric receptor specific for the tumor-associated G250 antigen were able to lyse tumor cells expressing high or low G250 levels, T cells carrying fewer receptors were only triggered for cytolysis and lymphokine production if target cells expressed relatively high antigen levels.55 Similarly, the NK-92-scFv(FRP5)-ζ cells used in our study express high levels of the chimeric antigen receptor and require only moderately elevated ErbB2 levels for target-cell killing. This suggests that tumors consisting of cells with variable ErbB2 densities might still be effectively targeted by NK-92-scFv(FRP5)-ζ cells. In the adult, ErbB2 expression in normal tissues is limited to epithelial cells in the digestive tract, reproductive tract, breast, kidney, and skin, and is considerably lower than in tumors suitable for ErbB2-directed therapy.56 Although this makes adverse reactions due to ErbB2 retargeted effector cells less likely to occur, tightly controlled expression of the chimeric antigen receptor (using, for example, a tetracycline-regulated promoter57) or inclusion of suicide gene constructs58 might be suitable strategies to relieve remaining safety concerns.
We have generated a genetically modified variant of the well-characterized, continuously growing cytotoxic NK-92 cell line that, in contrast to the parental cells, specifically recognizes and efficiently lyses ErbB2-expressing tumor cells of various origins. Initial characterization of the characteristics of NK-92-scFv(FRP5)-ζ cells has led to promising results, suggesting that further development of retargeted NK-92 variants is justified and might result in the generation of potent cell-based therapeutics for the treatment of ErbB2-expressing malignancies.
The authors thank Dr Boris Brill for helpful suggestions on the animal studies and Drs Christine Solbach and Gunter von Minckwitz for providing primary human breast cancer cells.
Supported in part by a grant from the German Bundesministerium für Bildung, Wissenschaft, Forschung und Technologie (BMBF) and a fellowship from the Stiftung Hämotherapie-Forschung to T.T.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Winfried Wels, Chemotherapeutisches Forschungsinstitut, Georg-Speyer-Haus, Paul-Ehrlich-Straße 42-44, D-60596 Frankfurt am Main, Germany; e-mail: wels@em.uni-frankfurt.de.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal