Mutations in the ZAP-70 protein tyrosine kinase gene result in a severe combined immunodeficiency (SCID) characterized by a selective inability to produce CD8+ T cells and a signal transduction defect in peripheral CD4+ cells. Transplantation of genetically modified hematopoietic progenitor cells that express the wild-type ZAP-70 gene may provide significant benefit to some of these infants. The feasibility of stem cell gene correction for human ZAP-70 deficiency was assessed using a ZAP-70 knock-out model. ZAP-70–deficient murine bone marrow progenitor cells were transduced with a retroviral vector expressing the human ZAP-70 gene. Engraftment of these cells in irradiated ZAP-70–deficient animals resulted in the development of mature CD4+ and CD8+ T cells. In marked contrast, both populations were absent in ZAP-70−/− mice undergoing transplantation with bone marrow progenitor cells transduced with a control vector. Importantly, ZAP-70–reconstituted T cells proliferated in response to T-cell receptor stimulation. Moreover, these ZAP-70–expressing T cells demonstrated a diverse T-cell receptor repertoire as monitored by the relative usage of each T-cell receptor β chain hypervariable region subfamily. The presence of ZAP-70 in B cells did not affect either lipopolysaccharide- or lipopolysaccharide/interleukin-4–mediated immunoglobulin isotype switching. Altogether, these data indicate that retroviral-mediated gene transfer of the ZAP-70 gene may prove to have a therapeutic benefit for patients with ZAP-70–SCID.
Introduction
Severe combined immunodeficiency (SCID) is caused by a variety of mutations that interfere with the differentiation or function of T and/or B lymphocytes. Patients with ZAP-70 deficiency have an unusual SCID phenotype characterized by the presence of normal numbers of nonfunctional CD4+ T cells and a selective absence of CD8 single-positive T cells in the periphery as well as in the thymus.1-4 ZAP-70 is a 70-kd protein tyrosine kinase that is recruited to the T-cell receptor (TCR) following its stimulation.5 It is expressed at approximately equivalent levels in thymocytes, mature T cells, and natural killer (NK) cells.6 Extensive studies in murine models as well as in human T-cell lines have demonstrated a critical role for this protein in T-cell ontogeny and activation.7
Like patients with other forms of SCID, ZAP-70–deficient patients present with opportunistic infections and failure to thrive. The disease is almost universally fatal in infancy unless treated by allogeneic hematopoietic stem cell (HSC) transplantation. However, for most patients, histocompatible donors are not available, and they therefore undergo transplantation with haploidentical T-cell–depleted or HLA-matched unrelated HSC grafts. Unfortunately, transplantation with nonhistocompatible HSCs is associated with a high rate of serious complications, such as graft-versus-host disease, delayed immune reconstitution, and abnormal B-cell differentiation.8-10Thus, the development of gene-based therapies could be beneficial for patients who do not have histocompatible HSC graft donors. Indeed, the elegant clinical trial performed by Fischer's group showed the success of this type of approach for infants with X-linked SCID (XSCID).11 Retroviral-mediated introduction of γc, the gene encoding the common γ chain of multiple cytokine receptors, into XSCID progenitor cells resulted in the development of functional T lymphocytes.
Our previous data, demonstrating that retroviral-mediated transfer of ZAP-70 into primary ZAP-70–deficient CD4+ T cells restores T-cell function,12 support the feasibility of gene therapy for ZAP-70 deficiency. Nevertheless, several important issues remain to be addressed: (1) Does introduction of the ZAP-70 gene into HSCs lead to CD4+ and CD8+ development in vivo? (2) Do the developing lymphoid cells exhibit a polyclonal TCR repertoire and normal immune responsiveness? (3) Does ectopic ZAP-70 expression in progenitor cells negatively affect the differentiation and function of non-T hematopoietic lineages? Importantly, a gene therapy approach for ZAP-70 deficiency provides challenges not encountered in XSCID patients: the ZAP-70 protein is normally expressed only in T-cell lineages, whereas γc is expressed in all hematopoietic cells.
ZAP-70 knock-out mice provide a tool used to address these issues. However, the phenotype of ZAP-70–deficient mice and humans are not identical, with ZAP-70–mutant mice exhibiting an earlier block in T-cell development at the CD4+CD8+ thymocyte stage.13 14 Although the bases for the differential role of ZAP-70 in human and murine T-cell development remain unclear, it is likely that compensatory mechanisms exist in ZAP-70–deficient patients that allow nonfunctional CD4+ T cells to mature and emigrate to the periphery. Nevertheless, ZAP-70 knock-out mice recapitulate many features of ZAP-70–deficient patients and offer an important model in which the validity of stem cell gene correction for this disease can be assessed.
Materials and methods
Retroviral constructs and producer cell lines
The murine leukemia virus (MLV)–based retroviral vector harboring the human ZAP-70 complementary DNA (cDNA) has been previously described.12 Briefly, ZAP-70 expression is under the control of the 5′ MLV long terminal repeat (LTR), and enhanced green fluorescent protein (EGFP) is situated 3′ of an internal ribosome entry site (IRES). Plasmid DNA was transfected into the GP + E-86 ecotropic packaging cell line,15 and cell populations expressing high levels of EGFP were selected by 2 sequential sorts on a FACS Vantage cell sorter (Becton Dickinson, San Jose, CA). A control MLV-based vector expressing EGFP alone, MND-EGFP, was kindly provided by D. Kohn (Los Angeles, CA) and modified as described.16All packaging cells were negative for the presence of replication-competent retrovirus. Cell lines were cultured in Dulbecco modified Eagle medium supplemented with 10% fetal bovine serum and maintained at 37°C in a 5% CO2 atmosphere. All vector-containing retroviral supernatants were harvested after a 24-hour incubation of near confluent cells in a humidified incubator at 32°C. Titers for the ZAP-70 and control EGFP retroviruses, as assessed on NIH-3T3 cells, were 2 × 105 and 1 × 106 transducing units per milliliter, respectively. The collected culture medium was filtered through 0.45-μm filters and stored at −80°C for further use.
Retroviral transduction of BM cells and transplantation
Bone marrow (BM) precursor cells were isolated from donor mice following an intraperitoneal injection of 5-fluorouracil (5-FU) (150 mg/kg) 48 hours prior to harvest. BM cells flushed from femora and tibias from ZAP-70−/− mice, kindly provided by A. Singer (National Institutes of Health, Bethesda, MD), or wild-type (WT) (C57Bl/6J) donor mice were washed and incubated with a cocktail of biotinylated lineage (lin)–specific antibodies (αCD2, αCD4, αCD8, αB220, αNK1.1, αTER.119, αMac-1, αGr-1, αTCRαβ, αTCRγδ) followed by strepavidin Dynabeads (Dynal, Compiégne, France) to remove differentiated hematopoietic cells. After lin depletion, progenitor cells were washed and stimulated for 48 hours with 10 ng/mL murine interleukin-3 (IL-3) (Peprotech, Rocky Hill, NJ), 100 ng/mL human IL-6 (Amgen, Thousand Oaks, CA), and 100 ng/mL stem cell factor (rSCF) (Amgen) in Dulbecco modified Eagle medium supplemented with 15% fetal bovine serum (Hyclone, Logan, UT). Stimulated progenitor cells from ZAP-70−/− donor mice were transduced on fibronectin-coated plates (Takara Shuzo, Otsu Shiga, Japan)17 preloaded with retroviral vector particles. Preloading was performed by 2 sequential additions of 1.0 mL cell-free retroviral supernatants to wells (24-well plates) for 15 minutes at room temperature. After 48 hours of continued culture in the presence of the indicated cytokines, BM cells were washed and injected into the tail vein of lethally irradiated (9 Gy [900 rad]) ZAP-70−/− recipient mice. The analyses shown here were performed on 3 ZAP-70−/− recipient mice undergoing transplantation with EGFP-transduced ZAP-70−/− BM, 4 ZAP-70−/− recipient mice undergoing transplantation with ZAP-70–transduced BM, 4 ZAP-70−/− recipient mice undergoing transplantation with WT BM, and 3 WT mice not undergoing transplantation. All animal experiments were approved by the National Human Genome Research Institute (NHGRI) and Institut de Génétique Moléculaire de Montpellier (IGMM) animal care and use committees.
Western blot analysis
Cells (1 × 106) were lysed in a 1% Nonidet P-40 lysis buffer, resolved on sodium dodecyl sulfate–polyacrylamide gel electrophoresis, and transferred electrophoretically to nitrocellulose membranes as previously described.18 Membranes were blotted with an αZAP-70 monoclonal antibody (mAb) specific for human ZAP-70 (Upstate Biotechnology, Lake Placid, NY) or an αZAP-70 mAb that cross-reacts with both human and mouse ZAP-70 proteins (Transduction Laboratories, Lexington, KY). Protein loading was verified with either an α-actin polyclonal antibody (Santa Cruz, Santa Cruz, CA) or an αErk2 mAb (Transduction Laboratories). Immunoreactive proteins were visualized using enhanced chemiluminescence.
Flow cytometry analysis
Phycoerythrin (PE)- and CyChrome (Cy)-conjugated αCD3ε, αCD4, αCD8α, αB220, αCD62L, αCD25, αCD69, αIgG1, αIgG3, αIgM, αCD5, and αTCRβ mouse mAbs were purchased from Pharmingen (San Diego, CA). Cells were incubated with antibodies for 20 minutes and then washed in phosphate-buffered saline before analysis on a FACSCalibur (Becton Dickinson, San Jose, CA). Cells transduced with retroviral vectors harboring EGFP were identified by their FL-1 autofluorescence.
Splenocyte proliferation
Splenocytes obtained 18 weeks following bone marrow transplantation (BMT) were seeded in triplicate in 96-well plates (1 × 105 cells per well). Cells were cultured in 100 μL RPMI media supplemented with 10% fetal calf serum and 50 μM 2-mercaptoethanol in the absence or presence of soluble αCD3 and αCD28 mAbs (1 and 2 μg/mL, respectively; Pharmingen) or lipopolysaccharide (LPS) (20 μg/mL; Sigma, St Louis, MO). After 48 hours of culture, cells were pulsed with [3H]thymidine (0.5 μCi [18.5 KBq] per well) (NEN, Dupont, Boston, MA) for 16 hours, harvested, and incorporated radioactivity was determined using a scintillation counter.
In vitro immunoglobulin isotype switching assay
Splenocytes obtained 18 weeks after BMT were cultured in RPMI medium supplemented with 10% fetal calf serum at a concentration of 1 × 106 cells per milliliter. For the induction of isotype switching, LPS (20 μg/mL) or LPS plus IL-4 (25 ng/mL; Peprotech) was added. After 6 days of culture, cells were collected and stained with a Cy-conjugated αB220 mAb together with either αIgG1 or αIgG3 mAbs and analyzed by fluorescence-activated cell sorting (FACS).
T-cell repertoire analysis
Total RNA was prepared from splenocytes obtained 18 weeks after BMT using RNAlater (Ambion, Austin, TX). A total of 2 μg RNA was reverse transcribed with random hexanucleotides (Pharmacia Biotech, Orsay, France) using Moloney MLV reverse transcriptase (RT; Gibco, Cergy, France). The cDNAs were amplified (40 cycles) in a 25-μL reaction mixture with 1 of the 24 TCRBV (TCRβ chain hypervariable region) subfamily–specific primers and a Cβ primer recognizing the 2 constant regions Cβ1 and Cβ2 of the β chain of the TCR, as previously described.19,20 A total of 2 μL of the 24 TCRBV/Cβ–first-run polymerase chain reaction (PCR) products was subjected to 2 cycles of elongation using a Cβ dye-labeled (6-Fam) primer allowing PCR products to be detected on a 337 automated DNA sequencer (Applied Biosystems); 1 μL of the PCR product was loaded on a 6% acrylamide sequencing gel and analyzed for size and fluorescence intensity using Immunoscope software. The TCRBV nomenclature proposed by Arden et al was used in this study.21
Statistical analyses
Statistical significance was determined using a Studentt test with a 1-tailed distribution and 2-sample equal variance. Data were considered to be statistically different forP < .05. All data are presented as mean ± SD.
Results
Introduction of ZAP-70 into hematopoietic progenitor cells
To assess the feasibility of a stem cell–based gene therapy strategy for ZAP-70–deficient patients, the ability of a human ZAP-70–expressing retroviral vector to support T-cell hematopoiesis in a ZAP-70–deficient mouse model was studied. BM from ZAP-70–deficient mice was harvested following treatment with 5-FU, and differentiated hematopoietic cells were removed using a cocktail of lin-specific antibodies. The remaining lin− BM progenitor cells were prestimulated for 48 hours with IL-3, IL-6, and SCF and then transduced with either a control EGFP or a ZAP-70/EGFP MLV–based vector packaged in the GP + E86 ecotropic cell line (Figure1A).
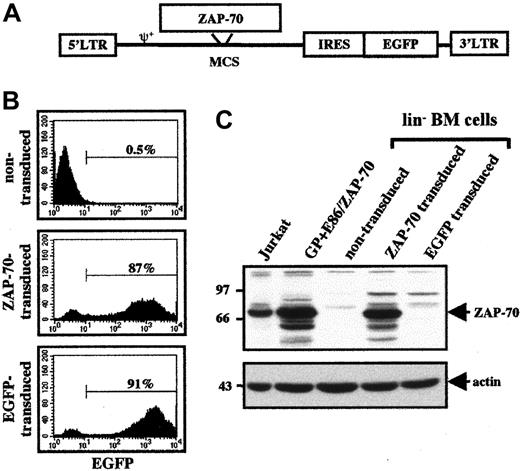
Transduction of ZAP-70−/− BM cells with a ZAP-70–expressing MLV-based vector.
(A) Schematic representation of the MLV-based ZAP-70/EGFP vector harboring the WT ZAP-70 cDNA. The relative positions of the LTRs, packaging signal (ψ), ZAP-70 cDNA, IRES, and EGFP cDNA are indicated. (B) Stimulated lin− BM progenitor cells were incubated with the appropriate retroviral supernatants. Cells were then expanded for 48 hours and assessed for EGFP expression by flow cytometry. Representative histograms of the nontransduced, ZAP-70/EGFP–transduced, and EGFP-transduced lin− BM cells are shown, and the percentages of EGFP+ cells are indicated. (C) Expression of ZAP-70 in transduced ZAP-70−/− lin− BM cells as well as in the GP + E86 packaging cell line harboring the ZAP-70–expressing retroviral vector and a human T-cell line, Jurkat, was assessed in Western blots using an αZAP-70 mAb. Protein loading was monitored by immunoblotting with an α-actin mAb.
Transduction of ZAP-70−/− BM cells with a ZAP-70–expressing MLV-based vector.
(A) Schematic representation of the MLV-based ZAP-70/EGFP vector harboring the WT ZAP-70 cDNA. The relative positions of the LTRs, packaging signal (ψ), ZAP-70 cDNA, IRES, and EGFP cDNA are indicated. (B) Stimulated lin− BM progenitor cells were incubated with the appropriate retroviral supernatants. Cells were then expanded for 48 hours and assessed for EGFP expression by flow cytometry. Representative histograms of the nontransduced, ZAP-70/EGFP–transduced, and EGFP-transduced lin− BM cells are shown, and the percentages of EGFP+ cells are indicated. (C) Expression of ZAP-70 in transduced ZAP-70−/− lin− BM cells as well as in the GP + E86 packaging cell line harboring the ZAP-70–expressing retroviral vector and a human T-cell line, Jurkat, was assessed in Western blots using an αZAP-70 mAb. Protein loading was monitored by immunoblotting with an α-actin mAb.
Retroviral transduction using either control EGFP or ZAP-70/EGFP virions resulted in gene transfer in more than 85% of progenitor cells, as assessed by expression of the EGFP marker (Figure 1B). Moreover, introduction of the ZAP-70/EGFP vector resulted in a high level of ZAP-70 expression in the lin− BM progenitor cells. This level of expression was approximately 2-fold to 3-fold higher than that observed in the control Jurkat T-cell line, demonstrating the efficacy of the LTR promoter in the retroviral vector (Figure 1C). As expected, ZAP-70 was not detected in nontransduced BM cells or in BM cells transduced with the control EGFP vector.
Thymic differentiation in ZAP-70−/− mice following introduction of the ZAP-70 gene in lin−BM cells
Thymic differentiation was assessed at 18 weeks following transplantation of ZAP-70−/− mice with transduced BM progenitor cells to assess the effects of transgene expression on long-term differentiation. The block in ZAP-70 deficiency occurs at a relatively late state of T-cell development as compared with many other types of genetic immunodeficiencies: at the double-positive (DP) CD4+CD8+ stage.13 Because DP cells constitute the majority of thymocytes, it was not surprising that neither transduction with the control EGFP vector nor with the ZAP-70/EGFP vector significantly affected thymic cellularity: 2.4 × 107 ± 1.5 × 107 and 3.8 × 107 ± 2.1 × 107, respectively, vs 5.6 × 107 ± 3.8 × 107 in ZAP-70–deficient mice undergoing transplantation with WT BM (P > .05). Within the nontransduced ZAP-70–deficient thymocyte populations, there were only very few single-positive (SP) thymocytes, and the relative partition of the DP and SP populations was unchanged in those thymocytes harboring the control EGFP vector (Figure2A). In marked contrast, within the population of thymocytes expressing the ZAP-70/EGFP vector, the percentages of SP thymocytes were significantly elevated (20.8% ± 9.7% and 8.6% ± 4.0% for CD4+ and CD8+ thymocytes; P < .05 for both conditions). Notably, these levels were not statistically different from those detected in ZAP-70–deficient mice undergoing transplantation with WT BM (12.9% ± 5.1% and 4.1% ± 1.3% for CD4+ and CD8+ thymocytes; P > .05 for both conditions) (Figure 2A).
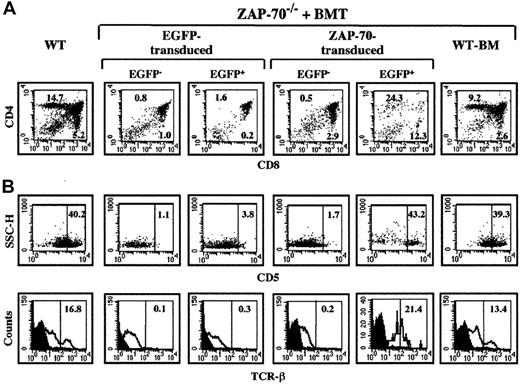
Thymocyte differentiation in ZAP-70−/− mice undergoing transplantation with ZAP-70 genecorrected BM cells.
Thymocytes were harvested from euthanized animals at 18 weeks after BMT and analyzed by flow cytometry. (A) Thymocytes were stained with PE-conjugated αCD8 and Cy-conjugated αCD4 mAbs. The percentages of CD4+ and CD8+ SP thymocytes are indicated in each dot plot. (B) As a measure of thymocyte differentiation, expression of CD5 and TCRβ was assessed in the EGFP− and EGP+ thymocyte populations of ZAP-70−/− mice undergoing transplantation with control EGFP or ZAP-70/EGFP retroviral vectors. Expression of these 2 markers was also monitored in the total thymocyte population of WT mice and ZAP-70−/− mice undergoing transplantation with WT BM. The percentages of CD5hi and TCRβhi cells are indicated. Histograms are representative of data obtained from 3 to 4 mice in each group.
Thymocyte differentiation in ZAP-70−/− mice undergoing transplantation with ZAP-70 genecorrected BM cells.
Thymocytes were harvested from euthanized animals at 18 weeks after BMT and analyzed by flow cytometry. (A) Thymocytes were stained with PE-conjugated αCD8 and Cy-conjugated αCD4 mAbs. The percentages of CD4+ and CD8+ SP thymocytes are indicated in each dot plot. (B) As a measure of thymocyte differentiation, expression of CD5 and TCRβ was assessed in the EGFP− and EGP+ thymocyte populations of ZAP-70−/− mice undergoing transplantation with control EGFP or ZAP-70/EGFP retroviral vectors. Expression of these 2 markers was also monitored in the total thymocyte population of WT mice and ZAP-70−/− mice undergoing transplantation with WT BM. The percentages of CD5hi and TCRβhi cells are indicated. Histograms are representative of data obtained from 3 to 4 mice in each group.
To more closely examine the differentiation of ZAP-70–transduced thymocytes in these animals, surface expression of the CD5 and TCRβ differentiation markers was monitored in the nontransduced (EGFP−) and transduced (EGFP+) populations. Expression of the CD5 activation marker22 was significantly augmented in thymocytes transduced with the ZAP-70 vector as compared with control EGFP-transduced thymocytes (35.2% ± 11.8% vs 2.7% ± 1.6%; P < .05, Figure 2B). The percentage of ZAP-70–transduced thymocytes expressing CD5hi was equivalent to that observed in WT mice and ZAP-70–deficient mice undergoing transplantation with WT BM. A similar profile was observed for TCRβ expression with elevated levels of this protein in ZAP-70–transduced thymocytes, equivalent to that observed in the WT thymocyte population (Figure 2B). Collectively, these results show that transfer of the ZAP-70 gene eliminates the block in thymocyte differentiation and provides the transduced thymocytes with a selective advantage.
Phenotype and function of peripheral T cells in ZAP-70–transduced mice
We next assessed the presence of peripheral splenic T cells in ZAP-70–treated mice. As expected, peripheral T cells were not observed in ZAP-70–deficient mice transduced with the control vector. However, introduction of ZAP-70 allowed the development of CD3+ T lymphocytes. Both mature CD4+ and CD8+ T cells were detected in the spleen as well as in the peripheral circulation of all 4 ZAP-70–transduced mice (Figure 3A and data not shown). The activation status of these lymphocytes was determined by assessing the expression of the CD25 and CD69 activation markers. CD4+ T cells from ZAP-70−/− mice undergoing transplantation with ZAP-70–transduced progenitor cells expressed the CD25 activation marker at levels equivalent to that observed in WT mice and ZAP-70–deficient mice undergoing transplantation with WT BM (Figure 3B). Expression of the CD69 activation marker was also detected in ZAP-70–transduced CD4+ T cells, albeit at higher levels than in WT mice (52.8% ± 5.9% vs 35.7% ± 3.2%; P < .05). Although both naive and memory T-cell populations were observed in reconstituted mice 18 weeks after BMT, the percentage of naive CD4+ T cells (CD62L+) was reduced in the mice grafted with ZAP-70–transduced progenitors as compared with mice undergoing transplantation with WT BM (12.7% ± 4.8% vs 49.6% ± 6.6%; P < .005, Figure 3B). Intriguingly, increases in the percentages of activated (CD69+) and memory (CD62L−) CD4+ T cells were at least related in part to the pretransplantation irradiation and/or post-BMT immunoreconstitution because these populations were also augmented in ZAP-70–deficient mice undergoing transplantation with WT BM as compared with WT mice not undergoing transplantation (Figure3B).
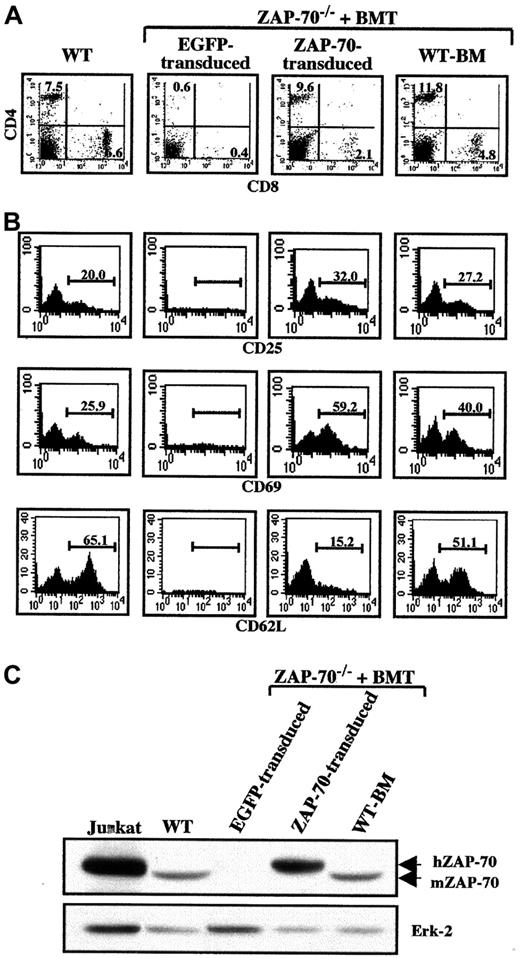
T-lymphocyte reconstitution in ZAP-70–transduced mice.
Splenocytes were collected from euthanized animals at 18 weeks following BMT and analyzed by flow cytometry. (A) Cells were stained with PE-conjugated αCD8 and Cy-conjugated αCD4 mAbs. The percentages of CD4+ and CD8+ T cells in each dot blot are indicated. (B) The activation status of the CD4+ T-cell population was determined using PE-conjugated αCD25 and αCD69 mAbs, and the relative percentages of naive and memory CD4+ T cells were monitored with a mAb recognizing CD62L (CD62L+ and CD62L−, respectively). The percentages of stained cells are indicated in each histogram. These parameters could not be analyzed in ZAP-70−/− mice undergoing transplantation with ZAP-70−/− progenitor cells harboring the EGFP vector. Data are representative of results obtained from 3 to 4 mice in each group. (C) Expression of ZAP-70 in EGFP-transduced splenocytes, ZAP-70–transduced splenocytes, and ZAP-70−/− mice undergoing transplantation with WT BM as well as WT mice and the human T-cell line, Jurkat, was assessed in Western blots using an αZAP-70 mAb that reacts against both human (hZAP-70) and murine ZAP-70 (mZAP-70). Protein loading was monitored by immunoblotting with an αErk2 mAb. Data are representative of results obtained using splenocytes isolated from 2 mice in each group.
T-lymphocyte reconstitution in ZAP-70–transduced mice.
Splenocytes were collected from euthanized animals at 18 weeks following BMT and analyzed by flow cytometry. (A) Cells were stained with PE-conjugated αCD8 and Cy-conjugated αCD4 mAbs. The percentages of CD4+ and CD8+ T cells in each dot blot are indicated. (B) The activation status of the CD4+ T-cell population was determined using PE-conjugated αCD25 and αCD69 mAbs, and the relative percentages of naive and memory CD4+ T cells were monitored with a mAb recognizing CD62L (CD62L+ and CD62L−, respectively). The percentages of stained cells are indicated in each histogram. These parameters could not be analyzed in ZAP-70−/− mice undergoing transplantation with ZAP-70−/− progenitor cells harboring the EGFP vector. Data are representative of results obtained from 3 to 4 mice in each group. (C) Expression of ZAP-70 in EGFP-transduced splenocytes, ZAP-70–transduced splenocytes, and ZAP-70−/− mice undergoing transplantation with WT BM as well as WT mice and the human T-cell line, Jurkat, was assessed in Western blots using an αZAP-70 mAb that reacts against both human (hZAP-70) and murine ZAP-70 (mZAP-70). Protein loading was monitored by immunoblotting with an αErk2 mAb. Data are representative of results obtained using splenocytes isolated from 2 mice in each group.
ZAP-70 expression in these transduced T cells was assessed using a ZAP-70–specific mAb that cross-reacts with human and murine ZAP-70. Importantly, ZAP-70 was expressed in splenocytes from ZAP-70–transduced animals at levels that appear to be approximately 3-fold higher than endogenous ZAP-70 levels (Figure 3C). However, in these experiments it is not possible to directly compare expression of endogenous and ectopic ZAP-70 because it is not known whether the αZAP-70 mAb recognizes human and murine ZAP-70 with the same affinity. Nevertheless, the data suggest that retroviral-mediated introduction of ZAP-70 into murine hematopoietic progenitor cells results in a high level of ZAP-70 expression in the differentiated splenic T and B cells.
Splenocytes were then analyzed for their ability to respond to TCR stimulation. Incorporation of [3H]thymidine was measured 3 days following activation with αCD3/αCD28 mAbs. As expected, splenocytes from EGFP-transduced ZAP-70–deficient mice did not respond to CD3/CD28 engagement (Table 1). Splenocytes from ZAP-70–transduced mice exhibited a considerable CD3/CD28-induced proliferative response (P < .05 vs EGFP-transduced splenocytes), albeit at levels lower than that observed in WT mice or ZAP-70–deficient mice undergoing transplantation with WT BM cells (Table 1). However, the percentages of T cells in the spleens of ZAP-70–transduced mice were significantly lower than that observed in mice undergoing transplantation with WT BM (13.9% ± 2.0% vs 24.4% ± 3.6% for 4 animals in each group; P < .05). Thus, at the onset of the culture, the absolute number of T cells present in wells seeded with splenocytes from ZAP-70–transduced mice was approximately 2-fold lower than in wells seeded with splenocytes from ZAP-70–deficient animals undergoing transplantation with WT BM.
Splenocyte proliferation in ZAP-70–deficient mice following gene transfer
| . | Medium . | αCD3/αCD28 . | LPS . |
|---|---|---|---|
| WT mice | 702 ± 97 | 74 146 ± 1 821 | 61 841 ± 871 |
| ZAP-70−/− mice | |||
| EGFP transduced | 497 ± 84 | 1 424 ± 262 | 61 050 ± 2 660 |
| ZAP-70 transduced | 690 ± 163 | 11 553 ± 1 203 | 48 732 ± 3 944 |
| WT BM | 424 ± 51 | 58 456 ± 2 231 | 47 541 ± 2 392 |
| . | Medium . | αCD3/αCD28 . | LPS . |
|---|---|---|---|
| WT mice | 702 ± 97 | 74 146 ± 1 821 | 61 841 ± 871 |
| ZAP-70−/− mice | |||
| EGFP transduced | 497 ± 84 | 1 424 ± 262 | 61 050 ± 2 660 |
| ZAP-70 transduced | 690 ± 163 | 11 553 ± 1 203 | 48 732 ± 3 944 |
| WT BM | 424 ± 51 | 58 456 ± 2 231 | 47 541 ± 2 392 |
Splenocytes obtained from euthanized animals at 18 weeks after BMT were seeded in triplicate in 96-well flat-bottom plates. T- and B-cell proliferation were monitored in the presence of αCD3/αCD28 mAbs and LPS, respectively. Cells were cultured for 48 hours and pulsed with [3H]thymidine (0.5 μCi [18.5 KBq] per well) for the final 16 hours of culture. Cells were then harvested, incorporated radioactivity was determined on a scintillation counter (counts per minute), and mean values ± SD of triplicate cultures are shown. Data are representative of results obtained from 3 mice in each group.
Repertoire of ZAP-70–corrected T cells
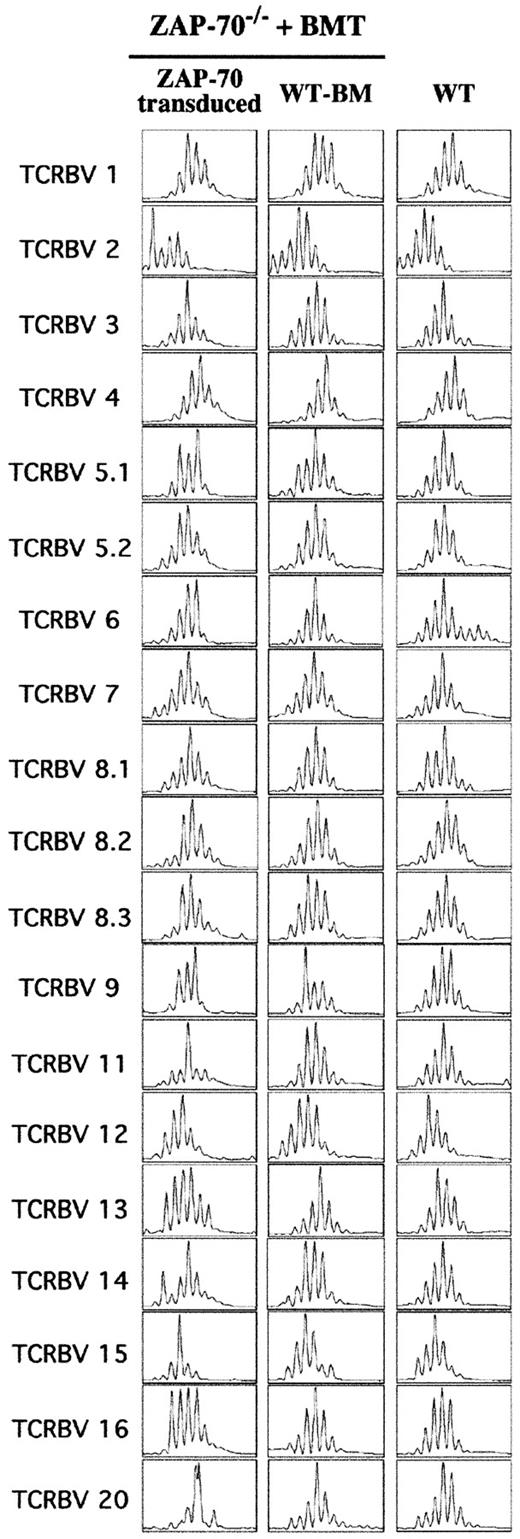
Because the absolute number of T cells in ZAP-70–transduced mice was lower than that observed in WT mice, it was important to further characterize the nature of these lymphocytes, especially with regard to their polyclonality. Thus, the relative usage of each TCRBV within the global T-cell population was examined using the Immunoscope method.19 20 This method is based on an RT-PCR of the hypervariable complementarity determining region 3 (CDR3), allowing the lengths of the RNAs encoding the β chain of the TCR to be analyzed. A Gaussian distribution of the CDR3 lengths is indicative of a diverse and nonbiased T-cell population. A total of 24 PCRs, corresponding to the 24 different TCRBV subfamilies, were performed and, notably, RT-PCR signals were detected for each of the 24 TCRBVs in mice undergoing transplantation with ZAP-70–transduced precursor cells. However, 5 sets of primers, corresponding to TCRBV families 5.3, 10, 17, 18, and 19, did not allow optimal amplification both in WT mice and ZAP-70–transduced mice and, as such, data corresponding to the remaining 19 TCRBV families were compared. Analyses of 3 mice in each group demonstrated that most TCRBV profiles in the ZAP-70–transduced mice were Gaussian, similar to that observed in both WT mice and ZAP-70–deficient mice undergoing transplantation with WT BM cells (Figure 4 and data not shown). Thus, introduction of ZAP-70 into progenitor cells allows for a polyclonal T-cell selection in the thymus and, more importantly, the maintenance of a diverse lymphocyte population in the periphery. Of note, the profiles from 3 TCRBV subfamilies (9, 11, and 20) were not strictly Gaussian in the ZAP-70–corrected mice (Figure 4 and data not shown), but the physiologic significance of this observation remains to be determined.
Diverse T-cell repertoire following gene therapy of ZAP-70−/− mice.
The TCRBV repertoire was assessed by a comparison of TCR CDR3 size distribution (Immunoscope profiles) of splenocytes obtained from WT mice and ZAP-70−/− mice undergoing transplantation with either ZAP-70–transduced progenitor cells or WT BM. Twenty-four PCR products were generated by reverse transcription with 24 different TCRBV subfamily-specific primers and 1 constant β consensus primer (Cβ), followed by a runoff reaction with a fluorescent Cβ primer. The graphs represent fluorescence intensity in arbitrary units (y-axis) plotted against CDR3 size (x-axis). The size distributions within 19 TCRBV families from 1 of 4 analyzed mice in each of the 3 groups are shown.
Diverse T-cell repertoire following gene therapy of ZAP-70−/− mice.
The TCRBV repertoire was assessed by a comparison of TCR CDR3 size distribution (Immunoscope profiles) of splenocytes obtained from WT mice and ZAP-70−/− mice undergoing transplantation with either ZAP-70–transduced progenitor cells or WT BM. Twenty-four PCR products were generated by reverse transcription with 24 different TCRBV subfamily-specific primers and 1 constant β consensus primer (Cβ), followed by a runoff reaction with a fluorescent Cβ primer. The graphs represent fluorescence intensity in arbitrary units (y-axis) plotted against CDR3 size (x-axis). The size distributions within 19 TCRBV families from 1 of 4 analyzed mice in each of the 3 groups are shown.
Differentiation and function of non–T-cell lineages following ZAP-70 gene transfer in hematopoietic progenitor cells
Because ZAP-70 is not expressed in non–T-cell lineages in WT mice, it was important to determine whether there were any transgene-related adverse effects in other hematopoietic cell lineages. Transduction of hematopoietic progenitor cells with the ZAP-70/EGFP vector did not alter the absolute numbers of white blood cells, red blood cells, splenic B cells, or splenic myeloid cells as compared with normal mice or ZAP-70–deficient mice transduced with WT BM (Table2). Interestingly, ZAP-70–deficient mice transduced with the control EGFP retroviral vector had an increased number of splenic myeloid cells as compared with all other groups of mice, which was associated with a splenomegaly in 2 of 3 animals (Table2). This phenotype was likely due to the immunodepressed status of the mice, especially in the context of lethal irradiation and BMT. Indeed, this phenotype has also been observed in another SCID mice model: lethally irradiated XSCID mice undergoing transplantation with XSCID progenitor cells (M.O. and F.C., unpublished observations, June 2000).
Differentiation of hematopoietic cell lineages following ZAP-70 gene transfer
| . | No. . | WBCs per milliliter . | RBCs per microliter . | Splenic B cells (B220+/IgM+) . | Splenic myeloid cells (Mac-1+/Gr-1+) . |
|---|---|---|---|---|---|
| WT mice | 3 | ND | ND | 112.7 ± 10.6 | 3.2 ± 1.1 |
| Zap-70−/− mice | |||||
| EGFP transduced | 3 | 16.8 ± 2.2 | 8.5 ± 0.8 | 46.8 ± 40.7 | 32.6 ± 29.9 |
| Zap-70 transduced | 4 | 20.9 ± 2.8 | 7.6 ± 0.5 | 58.0 ± 24.6 | 3.0 ± 1.3 |
| WT-BM | 4 | 17.9 ± 5.1 | 8.3 ± 0.6 | 55.6 ± 15.8 | 4.9 ± 1.3 |
| . | No. . | WBCs per milliliter . | RBCs per microliter . | Splenic B cells (B220+/IgM+) . | Splenic myeloid cells (Mac-1+/Gr-1+) . |
|---|---|---|---|---|---|
| WT mice | 3 | ND | ND | 112.7 ± 10.6 | 3.2 ± 1.1 |
| Zap-70−/− mice | |||||
| EGFP transduced | 3 | 16.8 ± 2.2 | 8.5 ± 0.8 | 46.8 ± 40.7 | 32.6 ± 29.9 |
| Zap-70 transduced | 4 | 20.9 ± 2.8 | 7.6 ± 0.5 | 58.0 ± 24.6 | 3.0 ± 1.3 |
| WT-BM | 4 | 17.9 ± 5.1 | 8.3 ± 0.6 | 55.6 ± 15.8 | 4.9 ± 1.3 |
Peripheral blood samples were collected from the retro-orbital sinus 8 weeks post-BMT, and white blood cells (WBCs) and red blood cells (RBCs) were enumerated (× 106 ± SD). The numbers of B and myeloid cells in the spleens (× 106± SD) were determined at 18 weeks after transplantation following splenocyte staining with the indicated antibodies. Mean ± SD were obtained from 3 mice in each group.
ND indicates not done.
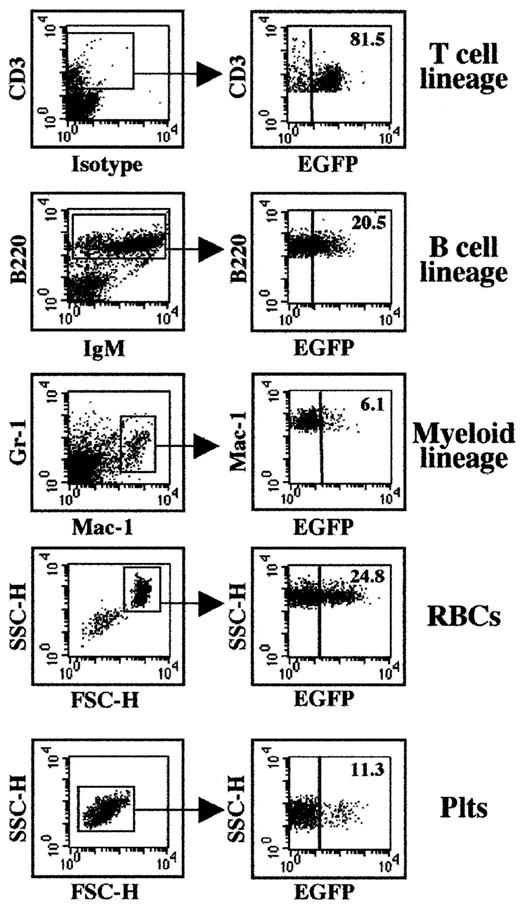
Following transplantation of progenitor cells transduced with the ZAP-70/EGFP vector, the presence of the retroviral vector in differentiated cell lineages was determined by concurrent analyses of lin marker and EGFP expression. Because ZAP-70 is required for T-cell differentiation, the finding that most peripheral T cells were transduced was expected (Figure 5). Notably, transgene expression was observed in all other assessed lineages, including short-lived granulocytes. Thus, these data suggest that at least a subset of the ZAP-70/EGFP–transduced lin− BM cells were primitive progenitors. The level of transgene expression varied both between individual mice as well as between the various non–T-cell lineages (6%-25%, Figure 5 and data not shown). Further work will be necessary to determine the etiology of this variation.
Expression of the EGFP marker gene in differentiated hematopoietic cells following transplantation of ZAP-70/EGFP–transduced BM cells.
The percentages of transduced splenic T cells, B cells, and myeloid cells, as well as red blood cells (RBCs) and platelets (Plts), were assessed in ZAP-70–deficient mice undergoing transplantation with ZAP-70/EGFP–transduced BM progenitor cells. Splenic T, B, and myeloid cells were gated based on CD3, B220, and Gr-1/Mac-1 expression, respectively (left panel, gated populations are indicated). RBCs and Plts were identified based on specific FSC/SSC profiles as previously described.33 34Expression of EGFP in each lineage was determined in the designated gate, and the percentages of positive cells are indicated (right panel). Representative data from 1 of 3 ZAP-70–transduced mice are shown.
Expression of the EGFP marker gene in differentiated hematopoietic cells following transplantation of ZAP-70/EGFP–transduced BM cells.
The percentages of transduced splenic T cells, B cells, and myeloid cells, as well as red blood cells (RBCs) and platelets (Plts), were assessed in ZAP-70–deficient mice undergoing transplantation with ZAP-70/EGFP–transduced BM progenitor cells. Splenic T, B, and myeloid cells were gated based on CD3, B220, and Gr-1/Mac-1 expression, respectively (left panel, gated populations are indicated). RBCs and Plts were identified based on specific FSC/SSC profiles as previously described.33 34Expression of EGFP in each lineage was determined in the designated gate, and the percentages of positive cells are indicated (right panel). Representative data from 1 of 3 ZAP-70–transduced mice are shown.
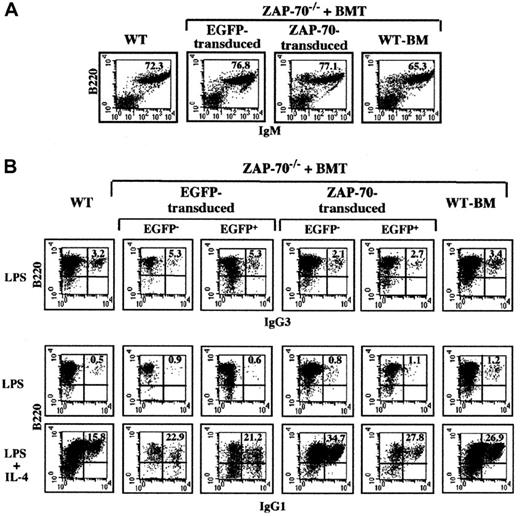
The percentage of splenic B lymphocytes (B220+/IgM+) was equivalent in WT mice and ZAP-70–deficient mice transduced with the control EGFP vector and the ZAP-70/EGFP vector, ranging from 65% to 77% (Figure6A). Importantly, introduction of ZAP-70 had no adverse effects on LPS-induced B-cell proliferation because splenocytes from all groups of treated and control mice proliferated at comparable levels (Table 1). Additionally, the function of splenic B lymphocytes was studied by performing in vitro immunoglobulin (Ig) isotype switching assays. It has previously been shown that LPS stimulation results in IgG3 production and B cells secrete IgG1 in response to LPS plus IL-4.23 24 Upon treatment with LPS, approximately 2.5% to 4.0% of all splenocytes expressed IgG3 regardless of the type of BMT. IgG1 expression was very low in all the B-cell populations treated with LPS alone (< 1%), whereas the combination of LPS and IL-4 resulted in the production of IgG1 in approximately 25% of B cells (Figure 6B). The presence of the ZAP-70 transgene did not alter this response; equivalent responses were observed in the untransduced (EGFP−) and ZAP-70–transduced (EGFP+) populations. Thus, neither the differentiation nor function of B cells appears to be adversely affected by the introduction of ZAP-70 in hematopoietic progenitor cells.
B-cell development and Ig isotype switching following ZAP-70 gene therapy.
(A) The percentages of splenic B cells present in WT mice and ZAP-70−/− mice undergoing transplantation with WT BM or ZAP-70−/− progenitor cells transduced with either EGFP or ZAP-70 retroviral vectors were assessed by staining with a PE-conjugated αB220 mAb and a Cy-conjugated αIgM mAb. The percentages of B220+/IgM+ cells are noted. (B) To assess IgG3 and IgG1 isotype switching, splenocytes were cultured in the presence of LPS and LPS plus IL-4, respectively. After 6 days of culture, cells were collected and stained with αIgG1 and αIgG3 mAbs. The percentages of B220+/IgG3+ and B220+/IgG1+ cells within the EGFP−and EGFP+ splenocyte populations of ZAP-70−/−mice undergoing transplantation with control EGFP or ZAP-70/EGFP retroviral vectors are indicated. Data are representative of results obtained using B cells isolated from 3 individual mice in each group.
B-cell development and Ig isotype switching following ZAP-70 gene therapy.
(A) The percentages of splenic B cells present in WT mice and ZAP-70−/− mice undergoing transplantation with WT BM or ZAP-70−/− progenitor cells transduced with either EGFP or ZAP-70 retroviral vectors were assessed by staining with a PE-conjugated αB220 mAb and a Cy-conjugated αIgM mAb. The percentages of B220+/IgM+ cells are noted. (B) To assess IgG3 and IgG1 isotype switching, splenocytes were cultured in the presence of LPS and LPS plus IL-4, respectively. After 6 days of culture, cells were collected and stained with αIgG1 and αIgG3 mAbs. The percentages of B220+/IgG3+ and B220+/IgG1+ cells within the EGFP−and EGFP+ splenocyte populations of ZAP-70−/−mice undergoing transplantation with control EGFP or ZAP-70/EGFP retroviral vectors are indicated. Data are representative of results obtained using B cells isolated from 3 individual mice in each group.
Discussion
The successful retroviral gene transfer trial for XSCID patients11 has provided an enormous boost to gene therapy–based strategies for many other pathologies and especially for other types of SCID. We previously demonstrated that retroviral-mediated introduction of ZAP-70 into CD4+ T cells from ZAP-70–deficient patients results in the reconstitution of their function.12 Nevertheless, because CD8+ T cells are not present in these patients, this type of approach would not reconstitute the cytotoxic arm of the immune system. We therefore used a murine model to determine whether a hematopoietic progenitor cell–based protocol could be beneficial for ZAP-70–deficient patients. Here, we report that T-lymphocyte differentiation and function is reconstituted following gene transfer of ZAP-70 into deficient progenitor cells.
ZAP-70 deficiency provides several challenges not encountered in gene therapy strategies for either XSCID or adenosine deaminase deficiency. Specifically, the gene products in both of these latter pathologies are normally expressed in all hematopoietic lineages, whereas ZAP-70 is expressed only in T lin cells.1-3 As expected, ectopic ZAP-70/EGFP was expressed in most T cells in ZAP-70–deficient mice undergoing transplantation with gene-corrected progenitor cells. Importantly, though, all other hematopoietic lineages were transduced, albeit at lower levels than that observed in T cells. Moreover, Ig isotype switching and B-cell proliferation was not modulated by the presence of the ZAP-70 transgene. Altogether, these data suggest that ectopic ZAP-70 expression was associated with neither a selective advantage nor disadvantage in non–T-cell lineages.
Although the mice used in these studies were irradiated prior to transplantation, ZAP-70–deficient patients participating in a clinical gene transfer protocol would not be conditioned. Thus, upon introduction of ZAP-70–corrected progenitor cells in an environment of high numbers of ZAP-70–deficient stem cells, it is likely that the percentages of transduced non-T lin cells would be very low. This hypothesis is based on the finding that, following stem cell gene therapy for adenosine deaminase deficiency, the percentage of transduced T cells present in the periphery was 2 to 3 logs higher than the percentage of transduced granulocytes.25 Furthermore, in gene-corrected XSCID patients, the percentage of transduced T cells was essentially 100% but the percentage of transduced granulocytes was only about 0.1%.11 The huge discrepancies are explained by the observation that adenosine deaminase and γc are required for T-cell survival and differentiation, respectively, but neither is necessary for granulocyte survival. A similar phenomenon would likely be observed following stem cell gene therapy for ZAP-70 deficiency because (1) we have demonstrated that ZAP-70–reconstituted CD4+ lymphocytes have a selective advantage over their nonreconstituted counterparts12 and (2) following a nonconditioned haploidentical BMT for ZAP-70 deficiency, Knobloch and coworkers found that donor cells were found as a portion of the T-cell fraction but no B cells of donor origin were detectable.26In fact, engraftment of donor cells was restricted to the T-cell lineage. Importantly, in this patient who underwent transplantation, engraftment of small numbers of donor T cells (6% ± 2%) was sufficient to mediate specific immune functions.26 This is notable because it appears that following transfer of the ZAP-70 gene into murine progenitor cells, the absolute numbers of peripheral T cells were approximately 2-fold lower than that observed in WT mice (1.1 × 107 ± 0.4 × 107 vs 2.3 × 107 ± 0.9 × 107 T splenocytes, respectively).
One of the primordial questions in hematopoietic gene therapy protocols concerns the identity of HSCs. Human hematopoietic cells expressing the CD34 marker are capable of differentiating into T and NK cells in vivo as well as in multiple experimental systems.11,27Nevertheless, it is clear that CD34+ cells represent a very heterogeneous group of cells and, moreover, several researchers have suggested that CD34− cells that do not express lin-specific markers may represent an even more primitive cell type than CD34+ cells.28 This question is even more confounding in the murine model because CD34 expression is not a marker of an HSC. In previous studies assessing γc gene transfer, HSCs were enriched by pretreating mice with 5-FU.16,23,29-31 Here, we first treated mice with 5-FU and then further purified HSCs by eliminating lin-expressing cells. Because these mice were lethally irradiated and their hematopoietic system was reconstituted following transplantation of these lin− cells, a portion of the 5 × 105 injected cells were primitive progenitors. Furthermore, at least some of these primitive progenitor cells were transduced because at 18 weeks after transplantation transgene-expressing cells were found in all hematopoietic lineages. Nevertheless, many of the transduced cells were probably not HSCs because although the initial percentages of transduced lin− BM cells exceeded 80%, the percentages of differentiated non-T hematopoietic cells harboring the ZAP-70 vector were lower (6%-25%). This is in agreement with data reported by Larochelle and coworkers demonstrating that only a small subpopulation of human CD34+ cells is capable of multilineage hematopoietic differentiation (SCID repopulating cells), and this subpopulation is relatively resistant to MLV-based retroviral transduction.32 Thus, studies elucidating the identity of HSCs and the conditions necessary for their transduction will improve the success of future clinical trials.
The level of ZAP-70 expressed from this vector was sufficient to restore T lymphopoiesis in ZAP-70–deficient mice but, as noted earlier, the phenotype of ZAP-70–deficient mice and humans are distinct. ZAP-70–mutant mice exhibit an earlier block in T-cell development, at the CD4+CD8+ thymocyte stage,13,14 whereas ZAP-70–deficient patients develop mature, albeit nonfunctional, CD4+ T cells.1-3This difference in phenotype raises the possibility that ectopic ZAP-70 expression has different consequences in mice and humans. We therefore assessed whether expression of ectopic ZAP-70 modulates human T-cell differentiation. To this end, the ZAP-70/EGFP retroviral vector was introduced into human fetal liver CD34+ cells and injected into a human fetal thymic organ that had already undergone transplantation in a SCID mouse (SCID-hu model). Importantly, the partition of the various thymocyte subsets was not altered by transduction with the ZAP-70 vector (K. Weijer, N.N., N.T., and H. Spits, unpublished observations, September 1999).
Further studies will be necessary to determine the etiology of the decreased numbers of naive T cells relative to memory T cells in the ZAP-70–reconstituted mice. Intriguingly, the percentages of naive CD4+ T cells were also relatively reduced in 2 γc-reconstituted murine models.16 29 Thus, it is possible that an increased homeostatic proliferation, in the context of lymphopenia, results in the higher number of memory T cells observed in both reconstituted XSCID and ZAP-70 knock-out mice. Alternatively, it is possible that the relative decrease in naive T cells observed in ZAP-70–reconstituted mice can be attenuated by improved transgene expression.
The level of ectopic ZAP-70 in peripheral splenocytes of animals undergoing transplantation appeared significant (Figure 3), but it may be important to optimize this vector prior to use in a clinical ZAP-70 gene therapy trial. In this regard, it is notable that the outcome of gene therapy for murine XSCID has been reported to be dependent on the MLV-based retroviral vector used to express the γc transgene.31 The ZAP-70–expressing retroviral vector used in this study is “standard” in that (1) the LTR is derived from the Moloney MLV, (2) there are no modifications in the LTR control regions or primer binding site, and (3) the 5′ splice acceptor site is a “cryptic site.” Thus, modifications in the vector may improve transgenic ZAP-70 expression. Finally, although high levels of tissue-specific expression have been difficult to achieve in the context of MLV-based vectors, new data suggest that use of T cell–specific promoters in HIV-based vectors is feasible (D. Klatzmann, personal communication, December 2001). Thus, such an approach may be beneficial for future ZAP-70–based gene transfer strategies.
This report demonstrates that MLV-based retroviral-mediated gene correction of lin− BM cells restores T-lymphocyte development and function in a murine model of ZAP-70 deficiency with no detectable adverse effects in other hematopoietic lineages. These findings, together with our previous results showing that transduced CD4+ T cells from ZAP-70–deficient patients have a growth advantage over their noncorrected counterparts,12 suggest that stem cell gene transfer may successfully treat this disease. The ensemble of these data provide the basis for developing a clinical protocol for ZAP-70 deficiency.
We are indebted to Al Singer for providing the ZAP-70 knock-out mice as well as for his critical insights and assistance in making this study possible. The precious SCID-hu experiments of K. Weijer and H. Spits are very much appreciated. We are grateful to Remy Bosselut for his scientific input. We thank Christophe Duperray for his expertise and assistance with FACS sorting. Dr Ikunoshin Kato and Setsuko Yoshimura of Takara Shuzo are generously acknowledged for providing the recombinant fibronectin fragment and for their continuing assistance. V. DiBartolo generously provided an αZAP-70 antibody. F. Bernard, M. Burdjanadze, V. Dardalhon, S. Jaleco, S. Kinet, C. Mongellaz, and M. Perreau all helped in their own way. In honor of his retirement, this work is dedicated to Prof Philippe Jeanteur, Director of the Institut de Génétique Moléculaire de Montpellier, whose unwavering support has been paramount.
Prepublished online as Blood First Edition Paper, April 30, 2002; DOI 10.1182/blood-2002-01-0247.
M.O. and M.S. contributed equally to this work and are listed in alphabetical order.
Supported by the JPS Research Fellowship for Japanese Biomedical and Behavioral Researchers at NIH, Fundacion YPF, Association France-Israel pour la Recherche en Science et Technologie (AFIRST), and March of Dimes (M.O., M.S., P.M., and N.N., respectively). Also supported by funding from the Association Française contre les Myopathies, March of Dimes grant 6-FY99-406, Association France-Israel pour la Recherche en Science et Technologie, Immune Deficiency Foundation, Association pour la Recherche sur le Cancer (ARC), INSERM and Centre National de la Recherche Scientifique (CNRS) (N.T.), and NIH (F.C.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Naomi Taylor, Institut de Génétique Moléculaire de Montpellier, 1919 Route de Mende, 34293 Montpellier, Cedex 5, France; e-mail: taylor@igm.cnrs-mop.fr.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal