Methotrexate (MTX) and mercaptopurine (MP) are widely used antileukemic agents that inhibit de novo purine synthesis (DNPS) as a mechanism of their antileukemic effects. To elucidate pharmacodynamic differences among children with acute lymphoblastic leukemia (ALL), DNPS was measured in leukemic blasts from newly diagnosed patients before and after therapy with these agents. Patients were randomized to receive low-dose MTX (LDMTX: 6 oral doses of 30 mg/m2) or high-dose MTX (HDMTX: intravenous 1 g/m2) followed by intravenous MP; or intravenous MP alone (1 g/m2), as initial therapy. At diagnosis, the rate of DNPS in bone marrow leukemia cells was 3-fold higher in patients with T-lineage ALL compared with those with B-lineage ALL (769 ± 189 vs 250 ± 38 fmol/nmol/h;P = .001). DNPS was not consistently inhibited following MP alone but was markedly inhibited following MTX plus MP (median decrease 3% vs 94%; P < .001). LDMTX plus MP and HDMTX plus MP produced greater antileukemic effects (percentage decrease in circulating leukocyte counts) compared with MP alone (−50% ± 4%, −56% ± 3%, and − 20% ± 4%, respectively;P < .0001). Full DNPS inhibition was associated with greater antileukemic effects compared with partial or no inhibition (−63% ± 4% vs −37% ± 4%; P < .0001) in patients with nonhyperdiploid B-lineage and T-lineage ALL. HDMTX plus MP yielded 2-fold higher MTX polyglutamate concentrations than LDMTX plus MP (2148 ± 298 vs 1075 ± 114 pmol/109 cells;P < .01) and a higher percentage of patients with full DNPS inhibition (78% vs 53%; P < .001). Thus, the extent of DNPS inhibition was related to in vivo antileukemic effects, and a single dose of intravenous MP produced minimal DNPS inhibition and antileukemic effects, whereas MTX plus MP produced greater antileukemic effects and DNPS inhibition, with full inhibition more frequent after HDMTX.
Introduction
Methotrexate (MTX) and mercaptopurine (MP) are antimetabolites that form the cornerstone of continuation therapy for childhood acute lymphoblastic leukemia (ALL).1 Both of these agents can inhibit de novo purine synthesis (DNPS), which is postulated as a mechanism of their antileukemic effects and a rationale for using them in combination.2 MTX is converted by folylpolyglutamate synthase to methotrexate polyglutamates (MTXPGs).3 MTXPGs inhibit dihydrofolate reductase, thereby decreasing the amount of reduced folates, the one-carbon donor for the purine ring formation in DNPS,3 and MTXPGs also directly inhibit phosphoribosylpyrophosphate (PRPP) amidotransferase,4 glycinamide ribonucleotide (GAR) transformylase, and 5-aminoimidazole-4-carboxamide ribonucleotide (AICAR) transformylase,5 key enzymes in the DNPS pathway. MP is metabolized by the purine salvage enzyme, hypoxanthine-guanine phosphoribosyltransferase, to thioinosine monophosphate and subsequently metabolized to thioguanine nucleotides or alternatively metabolized by thiopurine methyltransferase to methylthioinosine monophosphate, an inhibitor of PRPP amidotransferase.6,7The consequence of DNPS inhibition by these antimetabolites is purine deprivation, leading to inhibition of DNA synthesis, decreased cell proliferation, and cytotoxicity.8
Although in vitro studies have demonstrated that DNPS inhibition by MTX and/or MP contributes to their cytotoxic effects6,8 and that MTX putatively acts synergistically with MP by enhancing the accumulation of PRPP, a necessary cofactor for MP activation,9 10 these mechanisms have not been established in primary leukemia cells in vivo.
In the present investigation, we determined the effects of a single dose of MP alone or in combination with low-dose or high-dose MTX on DNPS in bone marrow leukemia cells from patients with newly diagnosed ALL. These studies revealed significant lineage differences in DNPS rates at diagnosis of ALL and a significant relation between the extent of DNPS inhibition and antileukemic effects of these medications.
Patients and methods
Patients and treatment
Children aged 18 or younger with newly diagnosed ALL were enrolled on the Total Therapy XIIIB protocol from 1994 to 1998. The study was approved by the Institutional Review Board at St Jude Children's Research Hospital. Signed informed consent was obtained from parents or legal guardians before enrollment in the protocol. The diagnosis of ALL was based on morphology, cytochemical staining properties, and immunophenotyping of blast cells for classification as B lineage or T lineage, as previously described.11 Ploidy was determined based on DNA index (ratio of DNA content in leukemic cells versus normal diploid G0/G1 cells) and classified as nonhyperdiploid or hyperdiploid.12 Mature B-cell ALL cases were excluded.
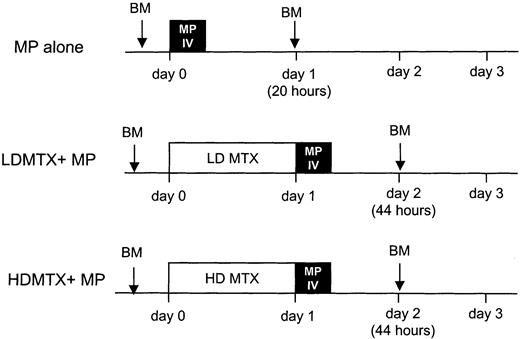
After stratification for age, leukocyte count, immunophenotype, and sex, patients were randomized to receive 1 of 3 upfront therapies (Figure 1): intravenous MP alone (intravenous MP: 1 g/m2 over 6 hours consisting of 200 mg/m2 over 20 minutes and 800 mg/m2 over 5 hours 40 minutes); low-dose oral MTX (LDMTX: 30 mg/m2 every 6 hours for a total of 6 doses) followed by intravenous MP (same dose as above); or high-dose intravenous MTX (HDMTX: 1 g/m2 over 24 hours consisting of 200 mg/m2 intravenous push and 800 mg/m2 over 24 hours) immediately followed by intravenous MP (same as above). Intravenous MTX and oral MTX were purchased from Lederle Laboratories (Pearl River, NY). Intravenous MP was supplied by the National Cancer Institute, as a lyophilized powder of sodium salt of MP (0.5 mg per vial). MP salt was reconstituted with sterile water for injection. Patients received hydration with intravenous dextrose 5%/0.25 normal saline with 40 mEq NaHCO3 per liter. NaHCO3 was given as needed to maintain urine pH at least 6.5 but less than 8.0. All patients treated with MTX received leucovorin rescue (10 mg/m2 orally or intravenously every 6 hours for 5 doses starting 48 hours from the start of MTX). Leucovorin rescue was continued until the plasma MTX concentration was below 0.1 μM.
Treatment schema.
Patients with newly diagnosed ALL were randomized to 1 of 3 treatments: intravenous MP (1 g/m2) over 6 hours (MP); oral MTX (30 mg/m2 every 6 hours, 6 doses) followed at 24 hours by intravenous MP (1 g/m2) over 6 hours (LDMTX plus MP); or intravenous MTX (1 g/m2) over 24 hours followed by intravenous MP (1 g/m2) over 6 hours (HDMTX plus MP). DNPS rates were measured in leukemia cells from bone marrow (BM) aspirates obtained at diagnosis and at 20 hours after the start of MP infusion (corresponding to 44 hours after the start of MTX therapy). MTXPG concentrations were measured in bone marrow leukemia cells obtained at 44 hours after initiation of chemotherapy in patients randomized to MTX plus MP.
Treatment schema.
Patients with newly diagnosed ALL were randomized to 1 of 3 treatments: intravenous MP (1 g/m2) over 6 hours (MP); oral MTX (30 mg/m2 every 6 hours, 6 doses) followed at 24 hours by intravenous MP (1 g/m2) over 6 hours (LDMTX plus MP); or intravenous MTX (1 g/m2) over 24 hours followed by intravenous MP (1 g/m2) over 6 hours (HDMTX plus MP). DNPS rates were measured in leukemia cells from bone marrow (BM) aspirates obtained at diagnosis and at 20 hours after the start of MP infusion (corresponding to 44 hours after the start of MTX therapy). MTXPG concentrations were measured in bone marrow leukemia cells obtained at 44 hours after initiation of chemotherapy in patients randomized to MTX plus MP.
To prevent or treat hyperuricemia and tumor lysis syndrome, urate oxidase13 (Uricozyme; Sanofi-Synthelabo, Paris, France) or its recombinant analog (Rasburicase; Sanofi-Synthelabo) was administered intravenously if required. In case of contraindication to Uricozyme and Rasburicase (glucose 6-phosphate dehydrogenase deficiency, asthma, and history of atopic allergy), allopurinol (Zyloprim; Glaxo-Wellcome, Research Triangle Parks, NJ) was given orally.
Bone marrow collection and processing
A bone marrow aspirate (5-10 mL in a syringe containing 800 units heparin) was obtained at 20 hours after initiation of the MP infusion. It was kept on ice, diluted with HHH solution (Hanks, heparin, and HEPES), and bone marrow lymphoblasts were isolated on a Ficoll density gradient, as previously described.14
Evaluation of response to chemotherapy
Circulating leukocyte counts were measured before therapy (day 0) and at day 3, prior to the administration of other antileukemic agents. Leukocyte counts were determined with a Coulter counter (model F+STKR; Coulter, Hialeah, FL). The percentage change in leukocyte count 3 days after beginning chemotherapy was determined as the day 3 count minus the day 0 count, divided by the day 0 count, and multiplied by 100.
Determination of bone marrow purine bases, rate of DNPS, and MTXPG concentrations
The concentration of purine bases from hydrolyzed nucleotides and the rate of DNPS in ALL blasts were simultaneously determined by quantifying unlabeled and radiolabeled purine bases (adenine and guanine) after acid hydrolysis of a 2-hour ex vivo incubation of 5 × 106 lymphoblasts with 14C-formate, as previously described.14,15 The concentrations of unlabeled adenine and guanine were determined from peak areas of UV chromatograms against a linear calibration curve of standards (1-75 nmol on column) prepared in phosphate-buffered saline. Newly synthesized purines were determined by the concentration of 14C-adenine and14C-guanine, which were calculated on the basis of the total disintegrations per minute (dpm) corresponding to the relevant peaks of the chromatograms, with a specific activity of 50 dpm/pmol. The detection limit for the radiolabeled purines was 0.02 pmol on column. The rate of de novo adenine plus guanine synthesis (DNPS) was calculated as the femtomoles of newly synthesized adenine plus guanine per nanomole of total intracellular adenine plus guanine per hour of incubation (fmol/nmol/h). Under conditions used, the rate of DNPS (14C incorporation from formate) remained linear for incubation times of 30 to 120 minutes in human CEM-CCRF (ATCC, Manassas, VA) leukemia cells (linear correlation coefficient 0.986 ± 0.009, mean of 3 experiments). Similarly, the rate of de novo adenine or de novo guanine synthesis was calculated as the femtomoles of newly synthesized adenine or guanine per nanomole of total intracellular adenine plus guanine per hour of incubation. The percentage change in DNPS 20 hours after initiation of MP was determined by comparison with the initial DNPS (subtraction of the 20 hours of DNPS from the pretherapy DNPS, dividing by the pretherapy DNPS, and multiplying by 100). Intracellular purine concentrations are expressed as nanomoles per 106 cells. Inhibition of DNPS was classified as full (> 90%), partial (10%-89%), or no (< 10%) inhibition. The high-performance liquid chromatography separation and measurement of MTX and 6 polyglutamated metabolites (MTXPG2 to MTXPG7) were performed as previously described.16
Statistical analyses
The distributions of sex, immunophenotypes, ploidy, type of uricolytic therapy received in the 72 hours preceding key time points (bone marrow aspirates at diagnosis and after chemotherapy), as well as the patterns of DNPS inhibition were assessed with either the χ2 or exact χ2 test as appropriate. Quantitative variables and demographic characteristics were compared among the 3 treatment arms with the exact Kruskal-Wallis test or compared with the exact Wilcoxon rank sum test17 when only 2 treatment groups were considered. Change between any 2 time points was assessed with the exact Wilcoxon signed rank test. No adjustments have been made for multiple testing. Results are expressed as mean ± SE unless otherwise indicated.
Results
Patients
Between August 1994 and July 1998, 233 children with newly diagnosed ALL were enrolled on the St Jude Total Therapy XIIIB protocol and randomized to receive 1 of 3 initial treatments: MP alone (76 patients), LDMTX plus MP (83 patients), or HDMTX plus MP (74 patients). There were no significant differences in demographic characteristics (Table 1) or the frequency of uricolytics administered among the 3 groups of patients or between patients randomized to MP alone versus MTX plus MP.
Demographics of patients randomized to MP alone or LDMTX plus MP or HDMTX plus MP
| Demographic . | MP alone (n = 76) . | LDMTX plus MP (n = 83) . | HDMTX plus MP (n = 74) . | Intergroup comparison, P . |
|---|---|---|---|---|
| Age, y (range) | 5.9 | 5.9 | 5.9 | .74 |
| (0.1-18.6) | (0.5-17.1) | (0.3-18.8) | ||
| WBC day 0, × 109/L (range) | 6.3 | 7.5 | 7.4 | .83 |
| (1-225) | (1-399) | (1-276) | ||
| Sex | ||||
| Male | 42 | 52 | 40 | .49 |
| Female | 34 | 31 | 34 | |
| Immunophenotype | ||||
| T lineage | 14 | 13 | 11 | |
| B lineage | 61 | 70 | 62 | .82 |
| Nonhyperdiploid | 38 | 44 | 43 | |
| Hyperdiploid | 23 | 26 | 19 | |
| Unknown | 1 | 0 | 1 | |
| No. of patients with uricolytics administered within 72 h prior to bone marrow diagnosis | ||||
| Urate oxidase | 1 | 1 | 1 | .22 |
| Allopurinol alone or with urate oxidase | 8 | 2 | 6 | |
| None | 67 | 80 | 67 | |
| No. of patients with uricolytics administered within 72 h of posttreatment bone marrow sample | ||||
| Urate oxidase | 45 | 52 | 37 | .44 |
| Allopurinol alone or with urate oxidase | 5 | 5 | 9 | |
| None | 26 | 26 | 28 |
| Demographic . | MP alone (n = 76) . | LDMTX plus MP (n = 83) . | HDMTX plus MP (n = 74) . | Intergroup comparison, P . |
|---|---|---|---|---|
| Age, y (range) | 5.9 | 5.9 | 5.9 | .74 |
| (0.1-18.6) | (0.5-17.1) | (0.3-18.8) | ||
| WBC day 0, × 109/L (range) | 6.3 | 7.5 | 7.4 | .83 |
| (1-225) | (1-399) | (1-276) | ||
| Sex | ||||
| Male | 42 | 52 | 40 | .49 |
| Female | 34 | 31 | 34 | |
| Immunophenotype | ||||
| T lineage | 14 | 13 | 11 | |
| B lineage | 61 | 70 | 62 | .82 |
| Nonhyperdiploid | 38 | 44 | 43 | |
| Hyperdiploid | 23 | 26 | 19 | |
| Unknown | 1 | 0 | 1 | |
| No. of patients with uricolytics administered within 72 h prior to bone marrow diagnosis | ||||
| Urate oxidase | 1 | 1 | 1 | .22 |
| Allopurinol alone or with urate oxidase | 8 | 2 | 6 | |
| None | 67 | 80 | 67 | |
| No. of patients with uricolytics administered within 72 h of posttreatment bone marrow sample | ||||
| Urate oxidase | 45 | 52 | 37 | .44 |
| Allopurinol alone or with urate oxidase | 5 | 5 | 9 | |
| None | 26 | 26 | 28 |
All results are median (range) for quantitative variables.
WBC indicates white blood count.
Effect of chemotherapy among treatment regimens and immunophenotypes
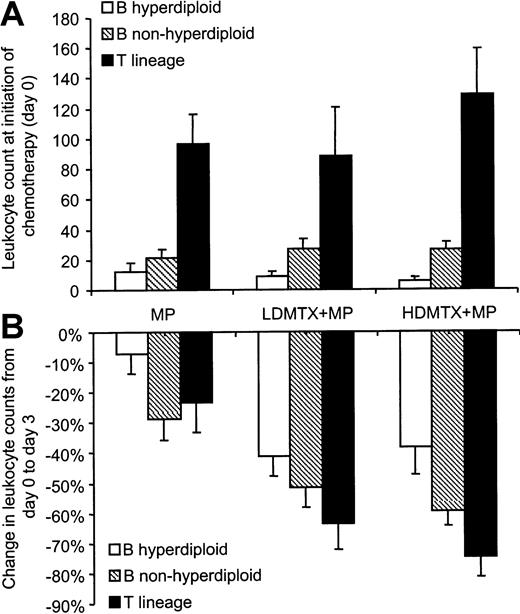
At initiation of chemotherapy, leukocyte counts were similar among the 3 treatment groups (P = .83; n = 233), whereas within each treatment arm patients presenting with T-lineage ALL had higher leukocyte counts than those with B-lineage ALL (P < .001). As depicted in Figure2A, patients with nonhyperdiploid B-lineage ALL presented with higher leukocyte counts than those with hyperdiploid B-lineage ALL (P < .001).
Effect of chemotherapy on circulating leukocyte counts.
(A) Initial leukocyte counts (leukemia burden) were similar among treatment arms, but within each treatment arm patients with T-lineage ALL (MP: n = 14; LDMTX plus MP: n = 13; HDMTX plus MP: n = 11) had higher leukocyte counts than those with B-lineage ALL (MP: n = 61; LDMTX plus MP: n = 70; HDMTX plus MP: n = 62;P < .001), and those with nonhyperdiploid B-lineage ALL (MP: n = 38; LDMTX plus MP: n = 44; HDMTX plus MP: n = 43) had higher leukocyte counts than those with hyperdiploid B-lineage ALL (MP: n = 23; LDMTX plus MP: n = 26; HDMTX plus MP: n = 19;P < .001). (B) Within each treatment arm, chemotherapeutic regimens produced significant decreases in circulating leukocyte counts (MP: n = 51; LDMTX plus MP: n = 77; HDMTX plus MP: n = 67) (P < .01), but the combination of MTX plus MP produced greater antileukemic effects than MP alone (P < .001). In patients randomized to receive MTX plus MP, those with T-lineage ALL (n = 22) had a greater percentage decrease in circulating leukocyte counts than those with B-lineage ALL (n = 121; P = .07), and those with nonhyperdiploid B-lineage ALL (n = 80) had a greater percentage decrease of leukocyte counts than those with hyperdiploid B-lineage ALL (n = 41;P < .01).
Effect of chemotherapy on circulating leukocyte counts.
(A) Initial leukocyte counts (leukemia burden) were similar among treatment arms, but within each treatment arm patients with T-lineage ALL (MP: n = 14; LDMTX plus MP: n = 13; HDMTX plus MP: n = 11) had higher leukocyte counts than those with B-lineage ALL (MP: n = 61; LDMTX plus MP: n = 70; HDMTX plus MP: n = 62;P < .001), and those with nonhyperdiploid B-lineage ALL (MP: n = 38; LDMTX plus MP: n = 44; HDMTX plus MP: n = 43) had higher leukocyte counts than those with hyperdiploid B-lineage ALL (MP: n = 23; LDMTX plus MP: n = 26; HDMTX plus MP: n = 19;P < .001). (B) Within each treatment arm, chemotherapeutic regimens produced significant decreases in circulating leukocyte counts (MP: n = 51; LDMTX plus MP: n = 77; HDMTX plus MP: n = 67) (P < .01), but the combination of MTX plus MP produced greater antileukemic effects than MP alone (P < .001). In patients randomized to receive MTX plus MP, those with T-lineage ALL (n = 22) had a greater percentage decrease in circulating leukocyte counts than those with B-lineage ALL (n = 121; P = .07), and those with nonhyperdiploid B-lineage ALL (n = 80) had a greater percentage decrease of leukocyte counts than those with hyperdiploid B-lineage ALL (n = 41;P < .01).
Antileukemic response was evaluable in 195 patients (38 were not evaluable because leukocyte count was not determined on day 3) based on the decrease in circulating leukocytes over the initial 3 days of treatment. As depicted in Figure 2B, the average percentage decrease in leukocyte counts was significantly less in patients randomized to MP alone (−20% ± 4%, n = 51) compared with patients randomized to LDMTX plus MP (−49% ± 4%, n = 77) or HDMTX plus MP (−56% ± 4%, n = 67) (P < .001). Patients in the HDMTX plus MP group tended to have a greater percentage decrease in leukocyte counts than those in the LDMTX plus MP group, but the difference was not statistically significant (P = .33). Similarly, when the analysis was restricted to each of the 3 leukemic subtypes (hyperdiploid or nonhyperdiploid B-lineage ALL or T-lineage ALL), MTX plus MP produced greater antileukemic effects than MP alone (P < .004), but no significant difference in the antileukemic response was observed between the 2 MTX treatment regimens (P > .24).
In patients randomized to MP alone, a similar percentage decrease in leukocyte counts was observed among the 3 leukemic subtypes (P = .137) (Figure 2B). In patients who received MTX (high dose or low dose) plus MP, those with T-lineage ALL had a greater percentage decrease in leukocyte counts (−69% ± 6%, n = 22) than those with nonhyperdiploid B-lineage ALL (−55 ± 4%, n = 80;P = .075) or hyperdiploid B-lineage ALL (−40% ± 5%, n = 41; P < .001). Also, patients with nonhyperdiploid B-lineage ALL had a greater percentage decrease in leukocyte counts than those with hyperdiploid B-lineage ALL (P = .004).
DNPS and intracellular purine concentrations
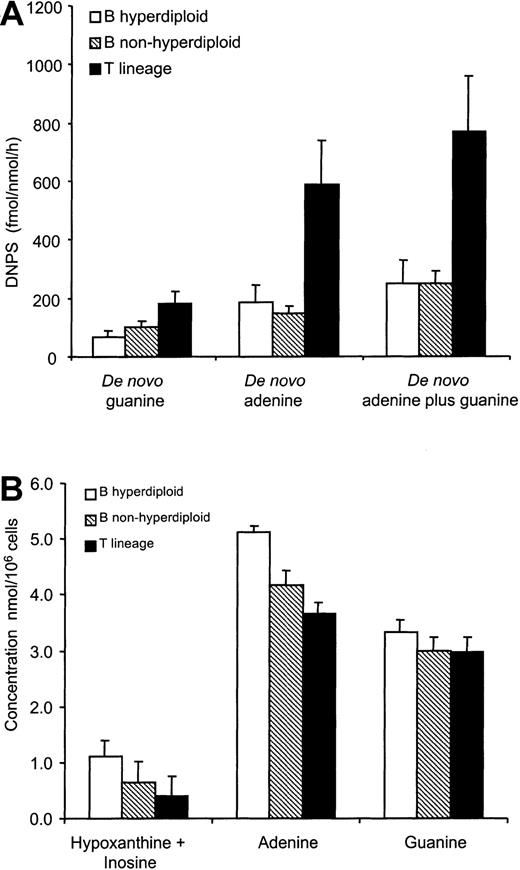
At diagnosis, bone marrow DNPS rates and intracellular purine concentrations were evaluable in 194 patients (40 were not evaluable because of poor yields of the bone marrow aspirate). No significant effect of uricolytics (within 72 hours prior to the bone marrow aspirate) was observed on de novo adenine, guanine, or adenine plus guanine synthesis rates (P > .20). De novo adenine, guanine, and de novo adenine plus guanine synthesis rates were higher in T-lineage ALL (n = 32) than B-lineage ALL (n = 161), with no difference between hyperdiploid B-lineage ALL (n = 55) and nonhyperdiploid B-lineage ALL (n = 106) (P > .60) (Figure 3A). Patients with T-lineage ALL had on average 4-fold and 2-fold higher de novo adenine and de novo guanine synthesis rates when compared with B-lineage ALL (P = .0007 and P = .010 respectively), resulting in a 3-fold higher total DNPS rate (adenine plus guanine) in T-lineage ALL compared with B-lineage ALL (769 ± 189 vs 250 ± 38 fmol/nmol/h; P = .001) (Figure 3A).
DNPS and intracellular purine concentrations at diagnosis.
(A) De novo guanine, de novo adenine, and total de novo purine (adenine plus guanine) synthesis rates were higher in patients with T-lineage ALL (n = 32) compared with patients with hyperdiploid (n = 55) or nonhyperdiploid B-lineage ALL (n = 106; P < .01). (B) Intracellular hypoxanthine-plus-inosine and adenine concentrations were higher in patients with hyperdiploid B-lineage ALL (n = 42) compared with patients with nonhyperdiploid B-lineage ALL (n = 93;P < .01), and both B-lineage subtypes (n = 135) had higher hypoxanthine-plus-inosine and adenine levels compared with patients with T-lineage ALL (n = 27; P < .01). Intracellular guanine concentrations were similar among lineages.
DNPS and intracellular purine concentrations at diagnosis.
(A) De novo guanine, de novo adenine, and total de novo purine (adenine plus guanine) synthesis rates were higher in patients with T-lineage ALL (n = 32) compared with patients with hyperdiploid (n = 55) or nonhyperdiploid B-lineage ALL (n = 106; P < .01). (B) Intracellular hypoxanthine-plus-inosine and adenine concentrations were higher in patients with hyperdiploid B-lineage ALL (n = 42) compared with patients with nonhyperdiploid B-lineage ALL (n = 93;P < .01), and both B-lineage subtypes (n = 135) had higher hypoxanthine-plus-inosine and adenine levels compared with patients with T-lineage ALL (n = 27; P < .01). Intracellular guanine concentrations were similar among lineages.
Bone marrow hypoxanthine plus inosine concentrations were higher in hyperdiploid B-lineage ALL (1.10 ± 0.29 nmol/106 cells, n = 42) than in nonhyperdiploid B-lineage ALL (0.65 ± 0.12 nmol/106 cells, n = 93) and higher in hyperdiploid B-lineage ALL than T-lineage ALL (0.41 ± 0.20 nmol/106cells, n = 27) (P < .001). Furthermore, adenine concentrations were higher in hyperdiploid B-lineage ALL (5.10 ± 0.37 nmol/106 cells, n = 51) than in nonhyperdiploid B-lineage ALL (4.16 ± 0.26 nmol/106cells, n = 99) and higher than in nonhyperdiploid B-lineage ALL compared with T-lineage ALL (3.64 ± 0.24 nmol/106 cells, n = 27) (P = .007). However, intracellular guanine concentrations were similar among the 3 leukemic subtypes (P = .52) (Figure 3B).
Effect of treatment regimens on DNPS
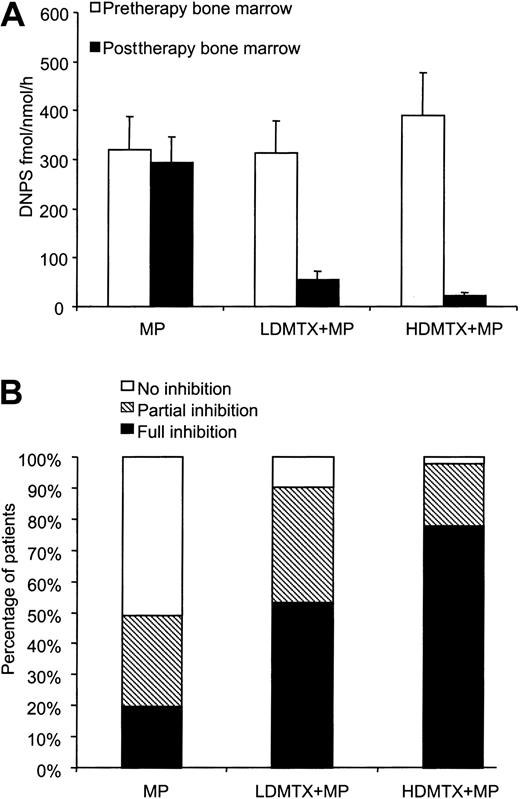
The change in DNPS rate from initiation of chemotherapy to 20 or 44 hours after chemotherapy was evaluable in 158 patients (51 patients randomized to MP, 62 patients to LDMTX plus MP, and 45 to HDMTX plus MP). The remaining 75 patients were not evaluated because there were insufficient cells to measure DNPS at diagnosis or after treatment. In patients randomized to MP alone, there was no significant inhibition of DNPS rate at 20 hours (median change of −3%, n = 51;P = .34). In contrast, as depicted in Figure4A, there was inhibition of DNPS in patients randomized to receive LDMTX plus MP (median change of −94%, n = 62; P < .001) or HDMTX plus MP (median change of −99%, n = 45; P < .001). These differences were similar when either de novo adenine or de novo guanine synthesis rates were compared (not shown).
Effect of treatment regimens on DNPS.
(A) In patients randomized to MP (n = 51), the DNPS rate was not significantly inhibited (P = .34), whereas inhibition was evident in patients randomized to LDMTX (P < .01; n = 62) or HDMTX (P < .01; n = 45). (B) A lower frequency of full DNPS inhibition was achieved in patients who received MP compared with MTX plus MP (20% vs 68%; P < .01). HDMTX with MP produced full inhibition in a greater percentage of patients, compared with LDMTX plus MP (78% vs 53%;P = .03).
Effect of treatment regimens on DNPS.
(A) In patients randomized to MP (n = 51), the DNPS rate was not significantly inhibited (P = .34), whereas inhibition was evident in patients randomized to LDMTX (P < .01; n = 62) or HDMTX (P < .01; n = 45). (B) A lower frequency of full DNPS inhibition was achieved in patients who received MP compared with MTX plus MP (20% vs 68%; P < .01). HDMTX with MP produced full inhibition in a greater percentage of patients, compared with LDMTX plus MP (78% vs 53%;P = .03).
Patients with hyperdiploid B-lineage ALL exhibited less inhibition of DNPS (median change of −84%, n = 43) compared with those with nonhyperdiploid B-lineage ALL (median change −94%, n = 85;P = .004) or T-lineage ALL (median change −94%, n = 30; P = .017). However, no difference in the inhibition of DNPS rate was observed between T-lineage ALL and nonhyperdiploid B-lineage ALL (P = .93).
Among 158 evaluable patients, full DNPS inhibition occurred in 78 patients (49%), partial inhibition in 47 patients (30%), and no inhibition in 33 patients (21%). The percentage of patients having full DNPS inhibition was significantly lower after MP alone (20%) compared with LDMTX plus MP (53%; P < .001) or HDMTX plus MP treatment (78%; P < .001). Furthermore, full DNPS inhibition was achieved in a higher percentage of patients randomized to HDMTX plus MP compared with LDMTX plus MP (P = .027) (Figure 4B).
Effects of MTXPGs on DNPS
In 106 patients treated with MTX plus MP, MTXPG concentrations were measured in leukemia cells from bone marrow aspirates obtained 44 hours after the start of MTX treatment. Patients randomized to HDMTX plus MP had on average 2-fold higher MTXPG concentrations (MTXPG1-7: 2148 ± 298 pmol/109 cells, n = 47) than those randomized to LDMTX plus MP (1075 ± 114 pmol/109 cells, n = 59) (P = .0027). Similarly, HDMTX plus MP treatment produced 1.7-fold higher long-chain MTXPG concentrations (MTXPG4-7: 1316 ± 159 pmol/109 cells, n = 47) compared with LDMTX plus MP (818 ± 88 pmol/109 cells, n = 59) (P = .0141).
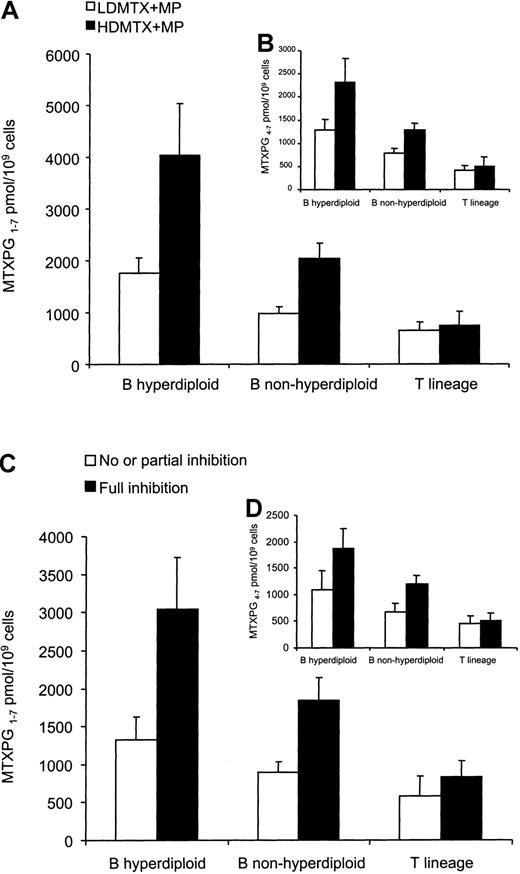
As depicted in Figure 5A, patients with T-lineage ALL had significantly lower MTXPG1-7concentrations (691 ± 146 pmol/109 cells, n = 23) than patients with nonhyperdiploid B-lineage ALL (1467 ± 161 pmol/109 cells, n = 61; P < .001), and patients with nonhyperdiploid B-lineage ALL had lower MTXPG1-7 concentrations than those with hyperdiploid B-lineage ALL (2684 ± 499 pmol/109 cells, n = 22;P = .001). Similar results were observed when long-chain MTXPG4-7 concentrations were compared (Figure 5B) or when lineage and ploidy were assessed within the LDMTX plus MP or the HDMTX plus MP groups. The administration of HDMTX plus MP resulted in 2.1-fold higher MTXPG1-7 concentrations compared with LDMTX plus MP administration in patients with hyperdiploid B-lineage ALL (4033 ± 1005 pmol/109 cells, n = 9, vs 1751 ± 308 pmol/109 cells, n = 13; P < .01) or nonhyperdiploid B-lineage ALL (2043 ± 284 pmol/109cells, n = 28, vs 978 ± 127 pmol/109 cells, n = 33;P < .01). However, in patients with T-lineage ALL, the administration of HDMTX plus MP did not produce higher MTXPG1-7 concentrations when compared with LDMTX plus MP (749 ± 274 pmol/109 cells, n = 10, vs 646 ± 161 pmol/109 cells, n = 13, respectively;P = .49) (Figure 5A).
MTXPGs and effects on DNPS.
(A,B) MTXPGs were measured in bone marrow aspirates at 44 hours after the start of MTX (n = 106). (A) In patients with hyperdiploid (LDMTX plus MP: n = 9; HDMTX plus MP: n = 13) or nonhyperdiploid B-lineage ALL (LDMTX plus MP: n = 28; HDMTX plus MP: n = 33), total MTXPG concentrations (MTXPG1-7) were higher in those randomized to HDMTX plus MP compared with LDMTX plus MP (P < .01). In contrast, HDMTX plus MP did not achieve higher MTXPG1-7 concentrations in patients with T lineage (P = .49). (B) Similar trends were observed with long-chain MTXPG concentrations (MTXPG4-7). (C,D) Full DNPS inhibition was associated with higher MTXPG1-7concentrations in ALL blasts compared with partial or no inhibition of DNPS (C). Lower MTXPG1-7 concentrations were required to achieve DNPS inhibition in patients with T-lineage (n = 14) compared with those with B-lineage ALL (n = 38) (P < .01). (D) Similar trends were observed with long-chain MTXPG4-7concentrations.
MTXPGs and effects on DNPS.
(A,B) MTXPGs were measured in bone marrow aspirates at 44 hours after the start of MTX (n = 106). (A) In patients with hyperdiploid (LDMTX plus MP: n = 9; HDMTX plus MP: n = 13) or nonhyperdiploid B-lineage ALL (LDMTX plus MP: n = 28; HDMTX plus MP: n = 33), total MTXPG concentrations (MTXPG1-7) were higher in those randomized to HDMTX plus MP compared with LDMTX plus MP (P < .01). In contrast, HDMTX plus MP did not achieve higher MTXPG1-7 concentrations in patients with T lineage (P = .49). (B) Similar trends were observed with long-chain MTXPG concentrations (MTXPG4-7). (C,D) Full DNPS inhibition was associated with higher MTXPG1-7concentrations in ALL blasts compared with partial or no inhibition of DNPS (C). Lower MTXPG1-7 concentrations were required to achieve DNPS inhibition in patients with T-lineage (n = 14) compared with those with B-lineage ALL (n = 38) (P < .01). (D) Similar trends were observed with long-chain MTXPG4-7concentrations.
There were significantly higher MTXPG1-7 concentrations in leukemia cells of patients with full inhibition of DNPS (1803 ± 236 pmol/109 cells, n = 52) compared with patients with partial or no inhibition (939 ± 124 pmol/109 cells, n = 31) (P = .0172) (Figure 5C). Similar results were observed when only patients with hyperdiploid or nonhyperdiploid B-lineage ALL were considered (P < .025); however, within patients with T-lineage ALL there was not a statistically significant difference, although the trend was similar (P = .35) (Figure 5C). Significantly lower MTXPG1-7 concentrations were required for full DNPS inhibition in patients with T-lineage ALL (831 ± 215 pmol/109 cells, n = 14) compared with those with nonhyperdiploid B-lineage ALL (1845 ± 303 pmol/109cells, n = 28) or hyperdiploid B-lineage ALL (3044 ± 674 pmol/109 cells, n = 10) (P = .001). Similar differences were observed with long-chain MTXPG4-7 (Figure5D) or when the analysis was restricted to the LDMTX plus MP or the HDMTX plus MP group (data not shown).
Effect of treatment regimen on DNPS and relationship to response to chemotherapy
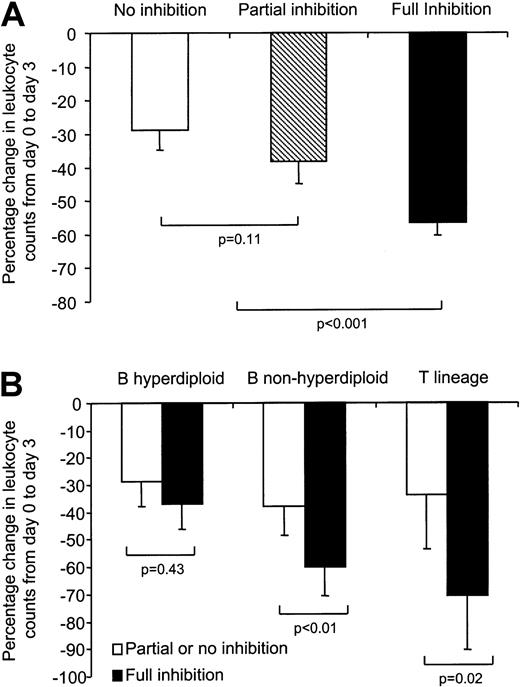
Among the 158 patients with evaluable DNPS inhibition, 135 (85%) were also evaluable for initial response to chemotherapy. As depicted in Figure 6A, full DNPS inhibition was associated with a significantly greater percentage decrease in circulating leukocytes (−57% ± 4%, n = 71) compared with partial inhibition (−38% ± 7%, n = 38; P = .008) or no inhibition (−29% ± 6%, n = 26; P < .001). However, there was no significant difference in the percentage decrease in circulating leukocytes between patients having partial or no inhibition (P = .11). Therefore, these 2 groups (47% of patients) were combined and compared with the group of patients (53%) having full DNPS inhibition.
Effect of DNPS inhibition on antileukemic effects.
(A) Full DNPS inhibition (n = 71) was associated with a greater percentage decrease in circulating leukocytes compared with partial inhibition (n = 38) or no inhibition (n = 26) (P < .01). There was no difference in the percentage decrease in circulating leukocytes between patients exhibiting partial or no inhibition (P = .11). (B) Full DNPS inhibition was associated with a greater percentage decrease in circulating leukocyte counts at day 3 compared with pretherapy counts in patients with nonhyperdiploid B-lineage and T-lineage ALL but not in those with hyperdiploid B-lineage ALL. Within patients having full DNPS inhibition (n = 71), those with nonhyperdiploid B-lineage (n = 39) and T-lineage ALL (n = 15) had a greater percentage decrease in circulating leukocyte counts compared with those with hyperdiploid B-lineage ALL (n = 17) (P < .01). In contrast, among patients with partial or no inhibition of DNPS (nonhyperdiploid B lineage, n = 36; hyperdiploid B lineage, n = 20; T lineage, n = 8), no difference in antileukemic effect was observed among ALL lineages (P = .39).
Effect of DNPS inhibition on antileukemic effects.
(A) Full DNPS inhibition (n = 71) was associated with a greater percentage decrease in circulating leukocytes compared with partial inhibition (n = 38) or no inhibition (n = 26) (P < .01). There was no difference in the percentage decrease in circulating leukocytes between patients exhibiting partial or no inhibition (P = .11). (B) Full DNPS inhibition was associated with a greater percentage decrease in circulating leukocyte counts at day 3 compared with pretherapy counts in patients with nonhyperdiploid B-lineage and T-lineage ALL but not in those with hyperdiploid B-lineage ALL. Within patients having full DNPS inhibition (n = 71), those with nonhyperdiploid B-lineage (n = 39) and T-lineage ALL (n = 15) had a greater percentage decrease in circulating leukocyte counts compared with those with hyperdiploid B-lineage ALL (n = 17) (P < .01). In contrast, among patients with partial or no inhibition of DNPS (nonhyperdiploid B lineage, n = 36; hyperdiploid B lineage, n = 20; T lineage, n = 8), no difference in antileukemic effect was observed among ALL lineages (P = .39).
In patients with nonhyperdiploid B-lineage ALL or T-lineage ALL, those having full inhibition of DNPS had a greater percentage decrease in circulating leukocytes compared with those with partial or no inhibition (B-lineage: −59.9% ± 5.0%, n = 39, vs −37.8% ± 6.2%, n = 36, P = .004; T-lineage: −70.6% ± 7.2%, n = 15, vs −33.8% ± 10.6%, n = 8,P = .02, respectively). In contrast, no such difference was evident in patients with hyperdiploid B-lineage ALL (−36.8% ± 7.8%, n = 17, vs −28.8% ± 7.0%, n = 20 for full vs partial/no inhibition, respectively; P = .43) (Figure 6B). In patients having partial or no DNPS inhibition, no differences in the percentage decrease of circulating leukocytes were observed among lineage or ploidy subgroups (P = .40). In contrast, in patients having full DNPS inhibition, nonhyperdiploid B-lineage and T-lineage ALL had a greater percentage decrease in circulating leukocyte counts compared with patients with hyperdiploid B-lineage ALL (P < .01).
Within patients randomized to MTX plus MP, those with hyperdiploid B-lineage ALL did not exhibit a significantly greater percentage decrease in circulating leukocyte counts with full DNPS inhibition versus partial or no inhibition of DNPS (−36% ± 8%, n = 16, vs −52% ± 9%, n = 9, respectively; P = .22). These findings were similar within the LDMTX plus MP or the HDMTX plus MP groups (data not shown).
Within patients with nonhyperdiploid B-lineage or T-lineage ALL randomized to LDMTX plus MP (n = 42), those with full DNPS inhibition had a greater percentage decrease in leukocyte counts (−67% ± 6%, n = 22) compared with those with partial or no inhibition (−37% ± 11%, n = 20) (P = .005). In contrast, within the HDMTX plus MP treatment group (n = 31), the percentage decrease in circulating leukocyte count did not differ in patients with full versus partial or no DNPS inhibition (−72% ± 11%, n = 26, vs −67% ± 4%, n = 5; P = .55), suggesting additional or alternative mechanisms of cytotoxicity with HDMTX. Interestingly, within the largest lineage and ploidy group (patients with nonhyperdiploid B-lineage ALL, about 70% of childhood ALL), in patients with partial or no DNPS inhibition, HDMTX plus MP produced a greater percentage decrease (P = .032) in leukocyte counts (−72% ± 11%, n = 5) than LDMTX plus MP (−36% ± 13%, n = 16). However, when there was full DNPS, there was no difference in antileukemic effects between LDMTX plus MP and HDMTX plus MP (−65% ± 5%, n = 18, with HDMTX plus MP, vs −61% ± 8%, n = 16, with LDMTX plus MP; P = .98).
Discussion
We have investigated in a randomized clinical trial the in vivo antileukemic effects of MP alone or in combination with LDMTX or HDMTX in children with ALL. These 2 agents are among the most widely used medications in the curative therapy of childhood ALL, yet their mechanisms and optimal doses remain to be fully elucidated in different ALL subtypes. Previous studies suggested that high-dose intravenous MP plus HDMTX improves outcome in childhood ALL.18 19However, the therapeutic effect of high-dose MP alone has not been evaluated. The objective of the current research was to determine whether MP alone or in combination with MTX inhibits DNPS and how this relates to the antileukemic effects of these medications.
Purine nucleotides are synthesized by the salvage pathway and by the de novo pathway, the latter being specifically measured in the current study. We found that leukemic cells from patients with T-lineage ALL have on average 4-fold and 2-fold higher de novo adenine and de novo guanine synthesis rates at diagnosis, resulting in a 3.0-fold higher total DNPS rate when compared with patients with B-lineage ALL. In addition, patients with T-lineage ALL had lower intracellular concentrations of inosine, hypoxanthine, and adenine but similar guanine concentrations compared with those with B-lineage ALL. These new findings are consistent with prior studies reporting constitutive differences in purine enzyme activities in T-lineage ALL compared with B-lineage ALL.20,21 Therefore, T-lineage ALL may be more dependent on the de novo pathway for purine synthesis (especially adenine) compared with B-lineage ALL, a finding consistent with previous in vitro data in cell lines9 and with recent evidence22-24 that methylthioadenosine phosphorylase (an adenine salvage gene) is more frequently deleted in T-lineage than in B-lineage ALL, because of its close genomic proximity to the tumor suppressors P16INK4A/P15INK4B. In addition, hypoxanthine inhibits DNPS by feedback mechanisms,25 26and the lower intracellular hypoxanthine plus inosine concentrations in T-lineage ALL compared with B-lineage ALL suggest that lower purine recycling and salvage activity may be compensated for by increased DNPS in T-lineage ALL.
In vitro, MTX produces DNPS inhibition through folate depletion as well as direct inhibition by MTXPGs of key DNPS enzymes, amidophosphoribosyltransferase, AICAR, and GAR transformylases.3-5 In contrast, MP is known to inhibit DNPS only through inhibition of amidophosphoribosyltransferase by methylthioinosine nucleotides.7 Our data establish that a single high-dose of MP does not consistently inhibit DNPS in ALL cells in vivo, whereas the combination of MTX and MP does. It is unclear whether inhibition of DNPS is due predominantly to MTX, but this is likely because MP had little effect alone and HDMTX produced greater inhibition than LDMTX. In this regard, our data support the use of HDMTX in childhood ALL, to more consistently achieve full DNPS inhibition, compared with LDMTX, because full DNPS inhibition produced greater antileukemic effects compared with partial or no inhibition.
Partial DNPS inhibition was not associated with significantly greater antileukemic effects when compared with no DNPS inhibition, suggesting that full DNPS inhibition is required to trigger cell death. This is consistent with the notion that leukemia cells rely more on the de novo pathway than on salvage mechanisms to synthesize purines and that near complete inhibition of purine synthesis may be required to produce a purineless state, leading to cell death. However, DNPS inhibition is only one mechanism of action shared by MTX and MP, and a component of the antileukemic effects of these 2 agents may be mediated via alternative mechanisms, such as inhibition of thymidylate synthase by MTXPGs or incorporation of deoxythioguanosine into DNA following MP. MTXPGs inhibit other targets in a concentration-dependent manner, and the current work further establishes that HDMTX achieves higher MTXPGs concentrations in ALL blasts in vivo compared with LDMTX.
Interestingly, the relationship of DNPS inhibition to antileukemic effect was dissimilar among leukemic subtypes. In patients with nonhyperdiploid B-lineage and T-lineage ALL, full DNPS inhibition produced greater antileukemic effects than partial or no inhibition, whereas in patients with hyperdiploid B-lineage ALL, no relationship was evident between the extent of DNPS inhibition and antileukemic response. This suggests that DNPS inhibition may be a more important mechanism for antileukemic effects in patients with nonhyperdiploid B-lineage or T-lineage ALL than hyperdiploid B-lineage ALL. Unexpectedly, patients with T-lineage ALL had a greater decrease in circulating leukocytes than those with B-lineage ALL following MTX. It is plausible that T-lineage ALL is more susceptible to DNPS inhibition than B-lineage ALL, because T-ALL blasts rely more on DNPS than on the purine salvage pathway.24,27-29 In contrast, more efficient purine salvage or purine recycling mechanisms in hyperdiploid B-lineage (consistent with greater hypoxanthine and inosine concentrations at diagnosis) may explain their lower sensitivity to DNPS inhibition compared with nonhyperdiploid B-lineage ALL. A good initial response during the first week of chemotherapy has been associated with a good overall treatment outcome in patients with ALL (ie, response to steroids).30 In that regard, results in the present study appear paradoxical, because patients with hyperdiploid B-lineage ALL exhibited lower initial response to chemotherapy compared with patients with T-lineage ALL, yet, overall, patients with hyperdiploid B-lineage ALL have a better event-free survival than most other ALL subtypes. However, it is possible that the initial decrease in leukocyte counts following MTX and MP (over 72 hours in our study) does not have the same prognostic value as the steroid response over 7 days.30 In addition, the improved event-free survival in patients with hyperdiploid B-lineage ALL compared with patients with T-lineage ALL might reflect their sensitivity to other chemotherapeutic agents given during 2.5 to 3 years of therapy (eg, l-asparaginase and cytarabine).31
In the LDMTX plus MP group, patients with nonhyperdiploid B-lineage or T-lineage ALL had greater antileukemic response when full DNPS inhibition was achieved. In contrast, within the HDMTX plus MP treatment group, the antileukemic response did not differ in patients with full versus partial or no DNPS inhibition. In addition, among patients with nonhyperdiploid B-lineage ALL, HDMTX plus MP produced a greater percentage decrease in leukocyte counts than LDMTX plus MP in patients with partial or no inhibition of DNPS, and similar differences were observed between the 2 MTX arms in case of full inhibition. Taken together, these findings support the rationale for HDMTX in childhood ALL, because HDMTX plus MP is more likely to produce complete DNPS inhibition and because HDMTX plus MP produces greater antileukemic effects in the absence of complete DNPS inhibition, consistent with additional mechanisms of action for HDMTX versus LDMTX. Going forward, it will be important to determine the optimal dosage and schedule of HDMTX in the major lineage and genetic subtypes of childhood ALL to maximize the efficacy of this widely used antileukemic agent.
We thank our clinical staff for scrupulous attention to patient care and management of blood sampling; our research nurses, Sheri Ring, Lisa Walters, and Terri Kuehner; and the patients and their parents for their participation in this study. We also thank Eve Su, YaQin Chu, May Chung, Kathryn Brown, Margaret Needham, Emily Melton, and Anatoli Lenchik for technical assistance and Nancy Kornegay for her computer and database expertise.
Prepublished online as Blood First Edition Paper, May 17, 2002; DOI 10.1182/blood-2002-02-0495.
Supported by grants CA 36401, CA 78224, CA21765, and CA51001 from the National Institutes of Health; a Center of Excellence grant from the State of Tennessee; and American Lebanese Syrian Associated Charities.
T.D. and T.L.B. contributed equally to this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
William E. Evans, Department of Pharmaceutical Sciences, St Jude Children's Research Hospital, 332 N Lauderdale, Memphis, TN 38105; e-mail: william.evans@stjude.org.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal