Abstract
Identification of the molecular mechanisms that can promote human hematopoietic stem cell amplification is a major goal in experimental and clinical hematology. Recent data indicate that a variety of regulatory molecules active in early development may also play a role in the maintenance of hematopoietic stem cells with repopulating activity. One important class of early developmental genes determining hematopoietic development are homeobox transcription factors. Here, we report that retrovirally mediated expression of the homeobox geneHOXB4 rapidly triggers an increase in the number of human hematopoietic cord blood cells with stem cell and progenitor cell properties detected both by in vitro and in vivo assays. This growth enhancement extended across primitive myeloid-erythroid and B-lymphoid progenitors but did not lead to alterations in the balance of lymphomyeloid reconstitution in vivo, suggesting that HOXB4does not affect control of end-cell output. These findings revealHOXB4 as a novel, positive regulator of the primitive growth activity of human hematopoietic progenitor cells and underline the relevance of early developmental factors for stem cell fate decisions.
Introduction
The remarkable proliferative capacities of primitive undifferentiated cells within the hematopoietic system provide for the lifelong maintenance of blood cell production and an extensive regenerative capacity. Identification of extrinsic and intrinsic regulators that control the self-renewal, lineage commitment and initial differentiation processes within these cells remains a key goal of experimental and clinical hematology.1-3 Molecular regulators influencing hematopoietic stem cell expansion have attracted particular attention because of the importance of these cells for a variety of clinical applications, including hematopoietic stem cell–based gene therapy and rescue of the hematopoietic system after myeloablative therapeutic strategies. Indeed, considerable progress has been made in delineating and exploiting the properties of multiple hematopoietic cytokines to achieve expansion of primitive hematopoietic cells in vitro.4-8 Expansion of both murine and human hematopoietic stem cells (HSCs) with long-term repopulating ability, however, is still limited. Recent findings intriguingly point to the possibility that regulatory molecules known for their growth-promoting roles in early developmental processes may also affect HSC activity. These include growth factors such as bone morphogenetic protein (BMP), which plays a pivotal role in the patterning of the embryonic ventral mesoderm and in inducing hematopoiesis from mesodermal tissue, and sonic hedgehog (SHH), a key segment polarity and patterning gene in the embryo.9-12
Homeobox transcription factors, first recognized as an evolutionarily conserved gene family critical to the control of embryonic development, have emerged as another important class of developmental genes determining early hematopoietic development.13,14 Multiple HOX family members are expressed in the most primitive hematopoietic stem cell–enriched populations, whereas their expression is consistently down-regulated to undetectable levels in terminally differentiating CD34− cells.15 This has suggested that Hox genes participate in the self-renewal and growth mechanisms operative at the earliest stages of hematopoiesis. In support of this concept, engineered overexpression of several different members of the clustered Hox gene family has been shown to have major effects on the proliferation and differentiation programs exhibited by many early hematopoietic progenitor cell types of both murine and human origin.16-22 Importantly,HOXB4, unlike other Hox genes studied, has been shown to induce a significant enhancement of the growth of primitive murine hematopoietic cells both in vivo and in vitro without apparent deleterious effects on their subsequent differentiation or regulation of mature progeny output.21 22 Although these data are intriguing, they are based on murine experimental systems and the degree to which they reflect functional properties of homeobox genes in the human system is unclear. Here, we have now directly investigated the possibility that extended HOXB4 expression in human hematopoietic cells would enhance their primitive growth activity. Retroviral-mediated gene transfer was used to force the expression ofHOXB4 in primitive human cord blood (CB) cells and their progeny and the biologic effects on subsequent hematopoiesis both in vitro and in vivo were then analyzed. We now show that constitutive expression of HOXB4 rapidly increased the number of cells displaying functional properties of very primitive human hematopoietic cells suggesting a retardation in the exit of cells from this compartment or the reactivation within more differentiated progenitors of a stem cell state.
Materials and methods
Retroviral constructs
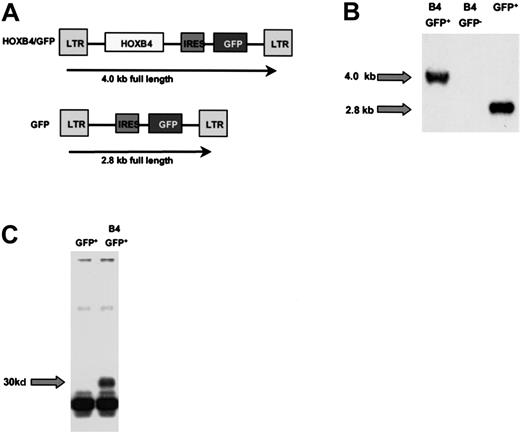
A human HOXB4 complementary DNA (cDNA) containing the complete HOXB4 open reading frame was cloned as an EcoRI fragment and inserted upstream of the internal ribosomal entry site (IRES) into a murine stem cell virus (MSCV) 2.1 vector (from R. Hawley, Rockville, MD) containing an IRES-green fluorescent protein (GFP) cassette originally provided by P. Leboulch (Boston, MA; Figure 1A). As a control, the MSCV vector carrying the IRES-GFP cassette alone was used (GFP virus). High-titer, helper-free recombinant retrovirus was generated by transfecting the amphotropic Phoenix packaging cell line and subsequently transducing PG13 packaging cells for pseudotyping with the gibbon ape leukemia virus envelope as previously described.19 The presence of full-length provirus in the PG13 producer cells and transduced primary CB cells was confirmed by Southern blot analysis using standard techniques and a 32P-labeled full-length GFP probe.19 (Figure 1B). HOXB4 transcripts in primary CB cells were demonstrated by reverse transcription–polymerase chain reaction (RT-PCR) and Southern hybridization using aHOXB4-specific probe (data not shown). For proof of protein expression, the HOXB4 cDNA was subcloned in frame 3′ to the FLAG-site of the pSC plasmid (Clontech, Palo Alto, CA). Protein expression of HOXB4 was tested as described previously.23In brief, protein-lysate of 293 cells was incubated with 0.5 μL of an anti-FLAG M2-antibody (Santa Cruz Biotechnology, Santa Cruz, CA) and 20 μL protein G-Sepharose (Pharmacia, Baie d'Urfe, QC, Canada) for 4 hours at 4°C on a slowly rotating wheel. Samples were subjected to gel electrophoresis on a 15% gel. Following sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE), proteins were transferred to a nitrocellulose membrane and immunoblotted. Membranes were subsequently incubated overnight with anti-FLAG M2 antibody (Santa Cruz Biotechnology) and Western blots were developed using horseradish peroxidase–conjugated secondary antibody (Sigma, St Louis, MO) and enhanced chemiluminescence (Amersham, Baie d'Urfe, QC; Figure 1C).
Structure and expression of the HOXB4 retrovirus.
(A) Schematic representation of the MSCV-HOXB4-IRES-GFP (B4-GFP) construct and the MIG (GFP) control vector. (B) Southern blot analysis of CB cells after 4 weeks in vitro culture hybridized with a GFP probe. (C) Immunoblotting of 293 cells transfected with the FLAG-tagged B4-GFP virus.
Structure and expression of the HOXB4 retrovirus.
(A) Schematic representation of the MSCV-HOXB4-IRES-GFP (B4-GFP) construct and the MIG (GFP) control vector. (B) Southern blot analysis of CB cells after 4 weeks in vitro culture hybridized with a GFP probe. (C) Immunoblotting of 293 cells transfected with the FLAG-tagged B4-GFP virus.
Isolation and transduction of human cells
Low-density cells (< 1.077 g/mL) were isolated by centrifugation of CB obtained with informed consent from mothers undergoing cesarean delivery of healthy, full-term infants and cryopreserved prior to thawing and removal of lineage marker–positive (lin+) cells using a StemSep column (Stem Cell Technologies, Vancouver, BC) as described by the manufacturer. This resulted in a cell suspension containing 59% ± 13% CD34+ cells. These were then transduced as previously described.19 24 Briefly, cells at 2 × 105/mL were prestimulated for 48 hours in Iscoves modified Dulbecco medium (IMDM) containing a serum substitute (BIT, Stem Cell Technologies), 10−4 M mercaptoethanol (Sigma), and 40 μg/mL low-density lipoproteins (Sigma) supplemented with the following recombinant human cytokines: 100 ng/mL Flt-3 ligand (FL; Immunex, Seattle, WA), 100 ng/mL Steel factor (SF, prepared and purified in the Terry Fox Laboratory), 20 ng/mL interleukin-3 (IL-3; Novartis, Basel, Switzerland), 20 ng/mL IL-6 (Cangene, Mississauga, ON, Canada), and 20 ng/mL granulocyte colony-stimulating factor (G-CSF; Stem Cell Technologies). After 48 hours, cells were resuspended in filtered virus-containing medium (VCM) supplemented with the same cytokine combination and protamine sulfate (5 μg/mL) on Petri dishes that had been precoated with 5 μg/cm2 fibronectin (Sigma) or on tissue culture dishes, both preloaded twice with VCM, each time for 30 minutes (Corning, Acton, MA). This procedure was repeated on the next 2 consecutive days for a total of 3 infections. For in vitro studies, aliquots of these cells were transferred to fresh serum-free medium plus the same additives and cytokines and then incubated for an additional 48 hours prior to being stained with Cy5-labeled anti-CD34 antibody (Becton Dickinson, San Jose, CA) and isolation of the GFP+/CD34+ cells on a 3 laser FACStar Plus (Becton Dickinson). For in vivo studies, transduced cells were injected into nonobese diabetic/severe combined immunodeficiency (NOD/SCID) mice immediately after transduction (< 24 hours after the last exposure to fresh VCM) without preselection.
In vitro progenitor assays
In vitro colony-forming cell (CFC) activity was assessed by plating aliquots of cells in methylcellulose medium (GF H4434, Stem Cell Technologies) supplemented with 50 ng/mL human SF, 20 ng/mL each of human IL-3, IL-6, granulocyte-macrophage colony-stimulating factor (GM-CSF; (Novartis, Basel, Switzerland), G-CSF, and 3 U/mL erythropoietin (Stem Cell Technologies) and counting erythroid, myeloid, and mixed erythroid-myeloid colonies after 2 weeks of incubation at 37°C. Secondary CFC assays were performed by replating aliquots of cells obtained by harvesting complete 14-day-old primary CFC cultures.19 Cell morphology was assessed on Wright-Giemsa–stained smears of individually plucked colonies.
Six-week long-term culture-initiating cell (LTC-IC) assays were carried out using pre-established irradiated murine fibroblasts genetically engineered to produce human IL-3, G-CSF, and SF as feeder layers.25 Both bulk assays and limiting dilution assays, initiated 2 days and 2 to 7 days after termination of transduction, respectively, were performed. In the latter case, cell numbers ranging from 1 to 12 800 GFP+ control or HOXB4-GFP+transduced cells were sorted into 96-well plates (Nunc, Naperville, IL) and LTC-IC frequencies were calculated using Poisson statistics and the method of maximum likelihood with the assistance of the L-calc software (Stem Cell Technologies). To set up liquid suspension cultures, transduced GFP+ cells were placed in the same cytokine-supplemented serum-free medium described above and then aliquots removed at the times indicated for CFC assays. B-cell progenitor activity was assessed by plating 2 × 104GFP+CD34+ cells on murine MS-5 cells in RPMI 1640 with 10% fetal calf serum (FCS) and 5% human AB serum with either 50 ng/mL SF, 10 ng/mL IL-2, and 10 ng/mL IL-15 (R & D Systems, Minneapolis, MN) or with these same factors plus 10 ng/mL IL-7 (R & D Systems), 100 ng/mL FL, and 50 ng/mL thrombopoietin (TPO; Genentech, San Francisco, CA), conditions permissive for B-lymphoid development in addition to varying degrees of myeloid cell development. After 3 or 6 weeks, both adherent and nonadherent cells were collected and analyzed by FACS for the expression of CD34, CD38, myeloid (CD15, CD33), lymphoid (CD3, CD19, CD20), erythroid (glycophorin A [GlyA], CD71), and megakaryocytic (CD41) and natural killer (NK) cell (CD56) markers (see below).
In vivo assays
The NOD/LtSz-scid/scid (NOD/SCID) mice were bred and maintained in the animal facility of the British Columbia Cancer Research Centre (Vancouver, BC, Canada) in microisolator cages containing autoclaved food and water. Test cells were injected intravenously into sublethally irradiated mice (350 cGy from a137Cs source given at 6-12 weeks of age) and marrow cell aspirates performed 3, 6, and 10 weeks later.19 Mice were killed 6 to 18 weeks after transplantation, and the cells from both tibiae and femurs of each mouse collected for additional analyses. The absolute number of cells in the marrow of each mouse was calculated assuming that the contents of both femurs and both tibiae represent 25% of the total marrow.
Flow cytometry
Cells were suspended in cold Hanks balanced salt solution, supplemented with 5% pooled normal human serum (HBSS, Stem Cell Technologies) and were then incubated with an antimouse Fc receptor antibody 2.4G2 to block nonspecific antibody binding. The percent positive cells was determined after excluding nonviable (propidium iodide [PI]+) cells and at least 99.9% of cells labeled with isotype control antibodies. Separate aliquots of cells were stained for 30 minutes at 4°C, with the antihuman CD45-phycoerythrin (PE; Becton Dickinson) and antihuman CD71-PE antibodies (OKT9) to quantitate the total number of human cells present (CD45+/71+), with antihuman CD34 8G12-Cy5, antihuman CD19-PE, and antihuman CD20-PE (Becton Dickinson) to quantitate the number of human B cells (CD34−CD19+) present, and with antihuman CD15-PE (Becton Dickinson) to quantitate the number of human myeloid cells present. A detection limit of more than 20 CD45+human cells per 2 × 104 cells analyzed and at least 5 human B cells plus at least 5 human myeloid cells per 2 × 104 cells analyzed was used to identify positively engrafted and lymphomyeloid-engrafted mice, respectively.19 Additional antibodies used for certain analyses included antihuman GlyA-PE, 10F7 antihuman CD33-PE (Becton Dickinson), antihuman CD41a-PE (Pharmacia Biotech, QC, Canada), and antihuman CD38-PE (Becton Dickinson).
Frequencies of lymphomyeloid stem repopulating (referred to as competitive repopulating units or CRUs)6 7 were calculated from the proportions of mice in a given experiment, or set of identical experiments, that were negative for lymphomyeloid engraftment using Poisson statistics and the method of maximum likelihood with the assistance of the L-calc software (Stem Cell Technologies).
Statistical analysis
Statistical tests were performed using the Student ttest (software STATISTICA 5.1, StatSoft, Tulsa, OK).
Results
Retroviral transduction of HOXB4 in human Lin−CB
The Lin− CB cells were transduced withHOXB4 virus-conditioned medium using a 5-day transduction protocol previously optimized for transduction of the control GFP vector used here.24 In the present experiments (n = 14), a mean transduction efficiency of 48% (20%-80%) and 23% (8%-56%) was achieved with the GFP and B4-GFP virus, respectively, with an equivalent frequency of CD34+ cells in the GFP+fraction in both cases (mean, 12%).
HOXB4 expression increases the production of secondary CFCs both in semisolid and liquid suspension cultures
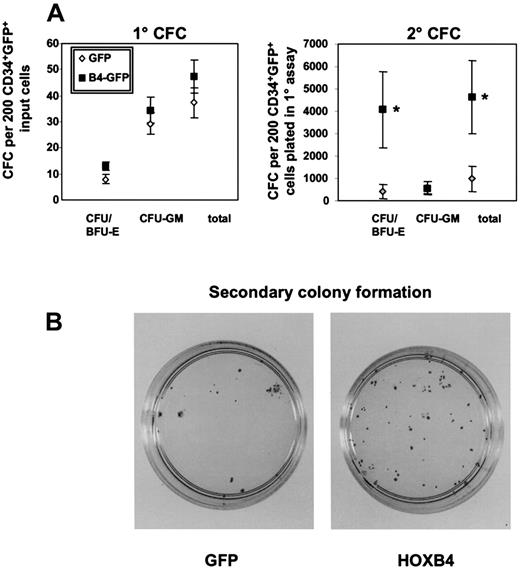
As an initial test of possible HOXB4 effects on human hematopoietic cell proliferative potential, we examined the ability of transduced (GFP+) CD34+ CB cells to generate colonies of erythroid and myeloid colonies in standard methylcellulose assays. Primary assays did not show any significant differences in either the total number or type of colonies detected after 14 days (48 ± 6 versus 37 ± 6 CFC/200 cells plated from theHOXB4 and control cells, respectively). However, replating of the cells harvested from these primary cultures into secondary CFC assays revealed 5-fold more CFC for HOXB4 cells compared with controls (mean of 4600 ± 1600 secondary CFC/200 initially plated cells versus 983 ± 580 in the controls; n = 6;P = .03; Figure 2A). Analysis of the types of cells present in the secondary colonies derived from the HOXB4-transduced cells further showed a selective increase in erythroid colonies (P = .03) compared with the control (n = 6; Figure 2B). Notably, in 3 of 6 experiments, there were no detectable secondary erythroid CFC colonies in the control cultures, whereas high numbers were observed in the cultures of HOXB4-transduced cells (> 103) per 200 initially plated cells. Interestingly, both erythroid and myeloid secondary colonies produced showed a normal morphology indistinguishable from those obtained from the cells transduced with the control GFP vector based on microscopic analysis of single plucked Wright-Giemsa–stained colonies and immunophenotyping. Furthermore, enforced HOXB4 expression did not induce formation of secondary blast colonies in any of the experiments in contrast to previous findings with HOXA1019 (data not shown). Thus, constitutive expression of HOXB4 significantly increased the proliferative capacity of transduced human CB cells without apparently blocking terminal differentiation, once it was initiated.
Primary and secondary colony formation in methylcellulose.
Methylcellulose dishes of primary CFC cultures were killed on day 14 and replated in secondary methylcellulose cultures. Secondary colony formation was assessed on day 14. Mean numbers of primary and secondary colonies for 6 independent experiments with 6 different CB samples are indicated by bars (A). The morphology and increased number of macroscopic colonies with a clear erythroid component in replated methylcellulose dishes are shown from one representative experiment compared with the GFP control (B). *P < .03.
Primary and secondary colony formation in methylcellulose.
Methylcellulose dishes of primary CFC cultures were killed on day 14 and replated in secondary methylcellulose cultures. Secondary colony formation was assessed on day 14. Mean numbers of primary and secondary colonies for 6 independent experiments with 6 different CB samples are indicated by bars (A). The morphology and increased number of macroscopic colonies with a clear erythroid component in replated methylcellulose dishes are shown from one representative experiment compared with the GFP control (B). *P < .03.
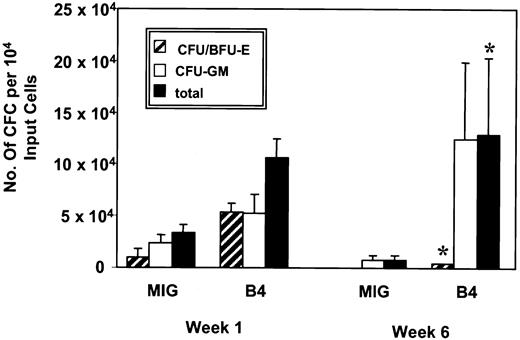
Similar results were obtained when CFC assays were performed on transduced cells maintained in serum-free liquid suspension cultures. These showed no significant difference after 1 week in the number of total nucleated cells present in cultures of HOXB4 or control GFP-transduced cells (3.1 × 105 ± 0.8 and 3.4 × 105 ± 0.3 at week 1, respectively). However, the cultures of HOXB4-transduced cells were found to contain significantly greater numbers of CFC (compared with cultures of GFP+ control cells, P < .01, with a difference ranging from 2-fold after 1 week to 14-fold at 6 weeks Figure 3, n = 3). This was associated with a net increase in the number of CFCs present after 6 weeks in the cultures of HOXB4-transduced cells, whereas in the GFP control arm, the total number of CFCs present declined about 3-fold during the same time period (Figure 3). This increase in CFC numbers compared with the control included myeloid as well as erythroid progenitors throughout the 6-week duration of the experiments (2.2- versus 5.7-fold increases after 1 week, respectively; 14-fold increase in myeloid CFCs and 4.2 × 103 erythroid CFCs forHOXB4 versus none for the control after 6 weeks; Figure 3).
Yield of clonogenic progenitors in liquid expansion culture.
At weeks 1 and 6 the number of granulocyte-macrophage and erythrocyte colony-forming units generated by B4-GFP– and GFP-transduced (MIG) progenitors cultured in serum-free medium supplemented with IL-3, IL-6, G-CSF, FL, and SF were quantified by plating aliquots of cells into methylcellulose and determining the number and morphology of colonies after 14 days (n = 3). *P < .01.
Yield of clonogenic progenitors in liquid expansion culture.
At weeks 1 and 6 the number of granulocyte-macrophage and erythrocyte colony-forming units generated by B4-GFP– and GFP-transduced (MIG) progenitors cultured in serum-free medium supplemented with IL-3, IL-6, G-CSF, FL, and SF were quantified by plating aliquots of cells into methylcellulose and determining the number and morphology of colonies after 14 days (n = 3). *P < .01.
HOXB4 expression amplifies the number of cells with LTC-IC activity
The GFP+ CD34+ cells were also assayed for LTC-IC activity to evaluate potential effects of HOXB4expression on more primitive hematopoietic cells. Bulk LTC-ICs for the detection of clonogenic progenitor cell output were initiated 2 days after termination of transduction (n = 6; Figure4). HOXB4 LTCs contained nearly 10-fold more CFCs after 6 weeks than the control cultures and more than 90% of the CFCs obtained under LTC conditions were granulopoietic in both experimental arms (data not shown). To determine whether the increased CFC output by HOXB4-transduced cells was due to the number of cells detectable as LTC-IC or to an enhanced output of CFCs per LTC-IC, LTC-IC frequencies were determined by limiting dilution analysis after 4 to 7 days in vitro culture of CD34+/GFP+ or CD34+/HOXB4-GFP+cells. As shown in Figure 4, the initial LTC-IC frequency ofHOXB4–transduced CB cells was significantly increased (∼5-fold; P < .001) compared with the control, whereas the progenitor yield per LTC-IC was essentially unchanged. Thus, the major impact of HOXB4 overexpression was a rapid increase in the number of cells with the primitive functional capacity of LTC-ICs.
Clonogenic progenitor output, frequency of LTC-ICs, and production of CFC/LTC-ICs from week 6 LTC-ICs.
GFP- and HOXB4-GFP–transduced CD34+ were cultured in LTC-IC assays for 6 weeks. (A) The clonogenic progenitor output per 104 cells was determined in bulk LTC-IC assays (n = 6). The frequency of LTC-IC/106 cells (B) and the output of CFC/LTC-ICs (C) were determined by limit dilution analysis (n = 3).
Clonogenic progenitor output, frequency of LTC-ICs, and production of CFC/LTC-ICs from week 6 LTC-ICs.
GFP- and HOXB4-GFP–transduced CD34+ were cultured in LTC-IC assays for 6 weeks. (A) The clonogenic progenitor output per 104 cells was determined in bulk LTC-IC assays (n = 6). The frequency of LTC-IC/106 cells (B) and the output of CFC/LTC-ICs (C) were determined by limit dilution analysis (n = 3).
HOXB4 promotes the development of B cells in vitro
To evaluate whether the growth-promoting effects ofHOXB4 might extend to the lymphoid pathway, we examined its effect on the generation of B-lineage cells in vitro. Transduced-positive CD34+ CB cells were cultured for 6 weeks on MS-5 fibroblast feeders in media with 2 different growth factor cocktails supportive of B-lymphoid development (SF, IL-2, and IL-15 with or without IL-7, TPO, and FL, respectively) and then assessed by FACS for the number of CD19+ cells present in addition to cells of myeloid and NK lineages using a range of markers. Both conditions also supported a significant level of myeloid cell growth and these constituted the dominant cell type in cultures examined at 3 or 6 weeks with no significant difference observed in either total cell number or CD15+ myeloid cells or CD56+ NK cells. However, as shown in Table1, under both conditions, the proportion and absolute number of CD19+ B cells present was significantly higher in the cultures of HOXB4-transduced cells (∼2- to 8-fold after 3 weeks and 3- to 20-fold after 6 weeks;P < .04). When we analyzed the effect of constitutiveHOXB4 expression on generation of CD34+/CD19+ B-cell precursors, the difference was even more pronounced: at week 3, HOXB4induced a 31-fold (P < .02) and 16-fold (P < .0005) increase with 3 and 6 cytokines, respectively. Thus, in culture conditions permissive for B lymphopoiesis, constitutive expression of HOXB4 promoted production of B cells in sharp distinction to effects observed withHOXA10.19
HOXB4 enhances production of B cells in vitro
| . | . | Total CD19+ B cells GFP (× 103) . | Total CD19+ B cells B4 (× 103) . | Total CD34+CD19+ B-cell precursors GFP (× 103) . | Total CD34+CD19+ B-cell precursors B4 (× 103) . |
|---|---|---|---|---|---|
| 3 GFs | 3 wk | 55.5 ± 39 | 94 ± 45 | 0.09 ± 0.09 | 3 ± 0.5 |
| (9.5 ± 6.4%)* | (31.3 ± 15.1%)* | ||||
| 6 wk | 1.6 ± 0.2 | 31.2 ± 3.5† | 0 | 0.5 ± 0.5† | |
| (0.7 ± 0.4%)* | (11.3 ± 2.2%)* | ||||
| 6 GFs | 3 wk | 8.6 ± 7.2 | 67.8 ± 13.4† | 0.2 ± 0.06 | 4 ± 0.09† |
| (0.2 ± 0.1%)* | (1.4 ± 0.2%)* | ||||
| 6 wk | 1.6 ± 0.7 | 4.3 ± 0.1† | 0 | 0 | |
| (0.1 ± 0.03%)* | (0.3 ± 0.01%)* |
| . | . | Total CD19+ B cells GFP (× 103) . | Total CD19+ B cells B4 (× 103) . | Total CD34+CD19+ B-cell precursors GFP (× 103) . | Total CD34+CD19+ B-cell precursors B4 (× 103) . |
|---|---|---|---|---|---|
| 3 GFs | 3 wk | 55.5 ± 39 | 94 ± 45 | 0.09 ± 0.09 | 3 ± 0.5 |
| (9.5 ± 6.4%)* | (31.3 ± 15.1%)* | ||||
| 6 wk | 1.6 ± 0.2 | 31.2 ± 3.5† | 0 | 0.5 ± 0.5† | |
| (0.7 ± 0.4%)* | (11.3 ± 2.2%)* | ||||
| 6 GFs | 3 wk | 8.6 ± 7.2 | 67.8 ± 13.4† | 0.2 ± 0.06 | 4 ± 0.09† |
| (0.2 ± 0.1%)* | (1.4 ± 0.2%)* | ||||
| 6 wk | 1.6 ± 0.7 | 4.3 ± 0.1† | 0 | 0 | |
| (0.1 ± 0.03%)* | (0.3 ± 0.01%)* |
HOXB4-GFP and GFP-transduced CB cells were seeded on MS-5 murine fibroblasts and cultured with 3 (SCF, IL-2, and IL-15) or 6 (SCF, IL-2, IL-15, IL-7, FL, and TPO) growth factors permissive for B lymphoid in addition to variable degrees of myeloid cell growth (6 GFs > 3 GFs for myeloid growth). Hematopoietic differentiation into the lymphoid lineage was quantified by CD19 expression or CD34/CD19 coexpression (n = 2).
GF indicates growth factor.
Proportion of CD19+ B cells expressed as percentage of all cells in the culture. Nonlymphoid cells were predominantly of myeloid phenotype as assessed by FACS using a range of lineage markers (data not shown).
Statistically significant P < .03.
HOXB4 expression increases the number of cells with repopulating activity detectable in NOD/SCID mice
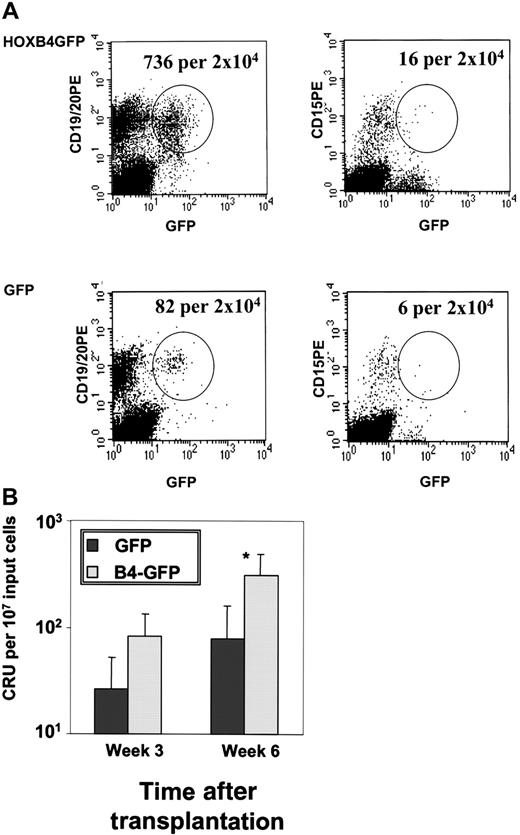
Because we had seen that constitutive expression ofHOXB4 significantly amplified the number of cells with LTC-IC activity, we assessed whether HOXB4 would also expand long-term repopulating stem cells using the CRU assay and the xenograft NOD/SCID mouse model. The CRU frequency was determined by limit dilution analysis in 3 cohorts of mice (n = 5) transplanted with CB infected either with the HOXB4 or the GFP virus. Mice were injected with the CB progeny of an original input of lin− cells containing 2.5 × 105CD34+ cells, one fourth or one eighth of this cell number, respectively, after 24 hours or less in vitro culture after infection. There was no significant difference in the proportion of CD34+GFP+ cells after transduction between the control and B4-GFP transduced CB cells and cells were injected without any preselection of CD34+ GFP+cells. The CRU frequency was calculated by Poisson statistics for the same starting number of GFP+ cells for both experimental arms at the time point of transplantation taking into account the number of lymphomyeloid-engrafted mice per dilution. Figure5A shows the FACS analysis of a representative mouse from the HOXB4 and GFP cohort after injection of 2.5 × 105 CD34+ cells 6 weeks after transplantation with positive lymphomyeloid engraftment. Strikingly, HOXB4-GFP–transduced CB had a 3-fold increase and a 4-fold increase in the CRU frequency as determined at weeks 3 and 6 after transplantation, respectively (P = .02; Figure 5B).
Lymphomyeloid repopulation in NOD-SCID mice transplanted with HOXB4-GFP– and GFP-transduced CB cells.
Three cohorts of NOD/SCID mice were injected with different dilutions of CB cells infected either with the HOXB4 or the GFP virus. (A) FACS analysis of a representative lymphomyeloid-engrafted mouse of the HOXB4 and the GFP cohort after injection of 2.5 × 105CD34+ cells 6 weeks after transplantation. The number of human transduced lymphoid (GFP+/CD19+) and myeloid (GFP+/CD15+) cells is shown. Human lymphomyeloid engraftment was defined as at least 5 GFP+/CD19+ and GFP+/CD15+ cells/2 × 104events.19 (B) CRU frequency among HOXB4-GFP– and GFP-transduced CB cells in NOD/SCID mice. Analysis of BM aspirates and total BM was performed 3 and 6 weeks after transplantation and analyzed with Poisson statistics. *P = .02.
Lymphomyeloid repopulation in NOD-SCID mice transplanted with HOXB4-GFP– and GFP-transduced CB cells.
Three cohorts of NOD/SCID mice were injected with different dilutions of CB cells infected either with the HOXB4 or the GFP virus. (A) FACS analysis of a representative lymphomyeloid-engrafted mouse of the HOXB4 and the GFP cohort after injection of 2.5 × 105CD34+ cells 6 weeks after transplantation. The number of human transduced lymphoid (GFP+/CD19+) and myeloid (GFP+/CD15+) cells is shown. Human lymphomyeloid engraftment was defined as at least 5 GFP+/CD19+ and GFP+/CD15+ cells/2 × 104events.19 (B) CRU frequency among HOXB4-GFP– and GFP-transduced CB cells in NOD/SCID mice. Analysis of BM aspirates and total BM was performed 3 and 6 weeks after transplantation and analyzed with Poisson statistics. *P = .02.
In contrast to its effect on the number of primitive repopulating hematopoietic cells, constitutive expression of HOXB4 (as documented by the continued expression of the linked GFPgene at readily detectable levels through the observation period [Figure 5A]) did not alter the normal differentiation program of human progenitor cells in vivo; proportions of engrafted lymphoid (CD19+/CD45+), myeloid (CD15+/CD45+), megakaryocytic (CD41+/CD45+), and erythroid (glycophorin+/CD45+) human cells were determined at weeks 3, 6, and 10 after transplantation by femoral bone marrow aspiration and again at 18 weeks when the mice were killed (n = 3). Constitutive expression of HOXB4 did not alter the proportions of the different lineages at any time point compared to the nontransduced compartment of the HOXB4 mice and the transduced and nontransduced compartment of the control mice. This was confirmed when absolute cell numbers were calculated after sacrificing the animals (data not shown). Thus, enhanced and extended expression ofHOXB4 significantly augmented the number of primitive cells with repopulating activity, but did not alter differentiation in vivo.
Discussion
The characterization of molecular mechanisms to expand human stem cells has major medical implications with respect to therapeutic strategies based on stem cell transplantation. Earlier studies have shown that cytokine-induced proliferation of progenitor-enriched populations is characterized by induction of lineage commitment and terminal differentiation accompanied by a rapid loss of stem cell activity.2 8 An intriguing alternative to use of extrinsic growth factors is to harness the function of intrinsic regulators that may be upstream of cytokine receptor–mediated signal transduction pathways. We now show that constitutive expression of the clustered homeobox gene HOXB4 can rapidly lead to increased numbers in vitro of cells detectable at the level of NOD/SCID repopulating cells, LTC-ICs, and committed clonogenic progenitors. Furthermore, enforced expression did not block terminal differentiation or changed lineage distribution in vivo. These data thus reveal HOXB4 as a novel growth stimulatory regulator of primitive human hematopoietic cells.
Intriguingly, constitutive expression of HOXB4 induced a rapid and significant increase in the number of cells with LTC-IC activity because cells were plated into the LTC-IC detection assay as soon as 48 hours as well as 7 days after transduction. This rapid onset of a HOXB4 effect on the number of human primitive hematopoietic cells was confirmed in the CRU assay in which HOXB4-expressing cells were injected into NOD/SCID mice within 24 hours after transduction and by this time CRU numbers were increased by some 4-fold over control transduced cells, as determined by limit dilution assay. Constitutive expression of HOXB4 might influence the growth kinetics of primitive progenitor cells, for example, by shortening the doubling time, or enhance self-renewal of HSCs. However, these data also suggest the interesting possibility of recruitment of cells, which have already left the primitive progenitor compartment, thus enhancing the number of cells detectable as LTC-ICs or CRUs. Both explanations are consistent with previous findings in the murine system, in which constitutive expression of HOXB4 shortened the doubling time of hematopoietic progenitor cells and induced an accelerated regeneration of CRUs in lethally irradiated recipient mice (eg, 25% of normal CRU level versus 0.2% in the control 2 weeks after transplantation).22 The striking effect on CRU numbers observed after only 24 hours after infection culture will also make it of interest to further assess in future experiments, the potential to achieve significant in vitro expansion of human CRUs with more extended culture periods as has recently been demonstrated in the murine model.26
The impact of the 3′-located Antennapedia-likeHOXB4 on early human hematopoietic development contrasts with effects so far described for other HOX genes on human hematopoietic progenitor cells. Thus, constitutive expression of the 5′-located Abdominal-B–like Hox geneHOXA10 resulted in competitive growth advantage of myeloid cells and blockage of differentiation with formation of blast colonies in vitro and ex vivo.19 The differential impact ofHOXB4 and HOXA10 is furthermore highlighted in their opposite effects on B-cell differentiation and erythropoiesis with HOXA10 overexpression impairing B-cell development, whereas HOXB4 induced a marked enhancement of B lymphopoiesis.19 The mechanisms that lead to the differential gene effect are not known, but data point to a pivotal role of the TALE homeobox genes and Hox cofactors Meis1 and Pbx1 for the specification of Hox gene effects in the hematopoietic system. Importantly, HOXB4 cannot interact directly with Meis1 but only with Pbx1 in contrast to HOXA10, which can interact directly with both proteins.13,18,HOXA5overexpression, also in contrast to HOXB4, has been shown to impair erythroid differentiation and enhance formation of colonies with undifferentiated blasts.27 Furthermore, HOXB7overexpression has been associated with the induction of persistent proliferation of a blast population in vitro, but without modifying the total number of hematopoietic progenitor cells.28
The effect of HOXB4 is somewhat reminiscent of effects recently reported for BMP-4 or SHH, which were originally described for their essential role in early developmental cell fate decisions9,11; both factors are able to maintain or increase human stem cells with repopulating capacity in vitro without altering lineage differentiation.10-12 Interestingly, both genes are linked to homeobox genes: SHH induces expression of Bmp-4 and of the 5′-located Hox genes Hoxd-11 as well as Hoxd-13, while Hoxd-12 regulates SHH expression in a positive feedback loop.29,30 BMP-4 regulates Hoxc-8 expression31 and acts together with the homeobox gene Mix.1 in inducing embryonic hematopoiesis.9
Our data characterize HOXB4 as a potentially powerful positive mediator of the maintenance and expansion of human stem cells and provide a new avenue to manipulate and further elucidate the basis for human hematopoietic stem cell fate decisions. These in vitro and in vivo models will facilitate the dissection of the molecular mechanisms underlying the HOXB4-induced stem cell proliferation in the human cellular milieu. Furthermore, they will allow tests of whether stem cell amplification by HOXB4 can be further augmented by mutating distinct motifs of the gene such as the PBX YPWM interacting motif as reported previously in the murine system.32
The expert assistance of Ms Patty Rosten for technical support and Ms Colleen MacKinnon in the preparation of the manuscript is gratefully acknowledged.
Prepublished online as Blood First Edition Paper, April 30, 2002; DOI 10.1182/blood-2002-01-0220.
Supported by the National Cancer Institute of Canada with funds from the Canadian Cancer Society and the Terry Fox Run; and the National Institutes of Health (grants no. HL65430 and DK48642). C.B. was supported by a grant from the Deutsche Forschungsgesellschaft (DFG), Bonn, Germany, and M.F.-B. by a grant from the Deutsche Krebshilfe, Bonn, Germany.
C.B. and M.F.-B. contributed equally to this article.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
R. Keith Humphries, Terry Fox Laboratory, 601 W 10th Ave, Vancouver, BC, V5Z 1L3, Canada; e-mail:khumphri@bccancer.bc.ca.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal