Abstract
In steady-state hematopoiesis, G-CSF (granulocyte-colony stimulating factor) regulates the level of neutrophils in the bone marrow and blood. In this study, we have exploited the availability of G-CSF–deficient mice to evaluate the role of G-CSF in steady-state granulopoiesis and the release of granulocytes from marrow into circulation. The thymidine analogue bromodeoxyuridine (BrdU) was used to label dividing bone marrow cells, allowing us to follow the release of granulocytes into circulation. Interestingly, the labeling index and the amount of BrdU incorporated by blast cells in bone marrow was greater in G-CSF–deficient mice than in wild-type mice. In blood, 2 different populations of BrdU-positive granulocytes, BrdUbright and BrdUdim, could be detected. The kinetics of release of the BrdUbright granulocytes from bone marrow into blood was similar in wild-type and G-CSF–deficient mice; however, BrdUdim granulocytes peaked earlier in G-CSF–deficient mice. Our findings suggest that the mean transit time of granulocytes through the postmitotic pool is similar in G-CSF–deficient and control mice, although the transit time through the mitotic pool is reduced in G-CSF–deficient mice. Moreover, the reduced numbers of granulocytes that characterize G-CSF–deficient mice is primarily due to increased apoptosis in cells within the granulocytic lineage. Collectively, our data suggest that at steady state, G-CSF is critical for the survival of granulocytic cells; however, it is dispensable for trafficking of granulocytes from bone marrow into circulation.
Introduction
G-CSF is a growth factor that promotes the production and maturation of myeloid cells and, in particular, the proliferation and differentiation of neutrophil progenitors both in vitro and in vivo.1 The importance of G-CSF in neutrophil production has been clearly demonstrated by the generation of mice deficient in either G-CSF or the G-CSF receptor. These mice are chronically neutropenic, but in blood they have morphologically recognizable mature neutrophils, albeit reduced in number.2 3 However, in the bone marrow, there is no accumulation of immature precursor neutrophils, suggesting that there is no differentiation blockade in the absence of G-CSF.
In an emergency situation, such as infection with pathogenic organisms, a component of a typical host response includes increased production of neutrophils for tissue restoration by eliminating invading organisms and removing damaged cells. This response involves not only sequestration of cells from circulation to the sites of infection, but also mobilization of neutrophils from bone marrow into peripheral blood. The mechanisms governing the trafficking of neutrophils between bone marrow and blood in steady-state as well as during emergency hematopoiesis, and the eventual suppression of these processes following resolution of the infection, remain poorly understood. It is generally believed that this process requires alterations in the adhesive interactions between the myeloid and stromal cells and the subsequent transmigration of neutrophils through the subendothelial basal lamina and the endothelial cell layer.4 Various factors have been implicated in playing a role in mobilization of neutrophils from bone marrow to blood. There is a body of evidence indicating that in normal steady-state hematopoiesis, G-CSF not only regulates the production of neutrophils, but also is involved in their release from bone marrow into circulation.1 In addition, the administration of G-CSF to healthy subjects or experimental animals stimulates a significant neutrophilia, with peripheral neutrophils reaching 10 to 15 times normal levels. Moreover, the newly produced mature cells are released into the circulation significantly earlier than under normal unperturbed conditions.5 However, G-CSF does not seem to have a measurable impact on the survival of blood polymorphonuclear neutrophils (PMN) or their distribution between the marginal and circulating pools.6
To establish whether G-CSF plays an indispensable role in the release of neutrophils from bone marrow into peripheral blood, we have employed bromodeoxyuridine labeling to track neutrophil migration in wild-type and G-CSF–deficient mice. Surprisingly, at steady state, the kinetics of neutrophil mobilization in G-CSF–deficient and control mice is identical; thus G-CSF is dispensable for neutrophil trafficking. Our data indicate that a primary role for G-CSF at steady-state hematopoiesis is to support the survival of myeloid progenitors and cells within the neutrophil lineage.
Materials and methods
Mice
The mice, 8- to 10-week-old G-CSF–deficient (G-CSF−/−)7 and wild type, were housed in micro-isolator cages. The mice of mixed C57BL/6 and 129/OLA background were used for all studies. Bromodeoxyuridine (BrdU) solution was prepared in endotoxin-free saline; furthermore, no endotoxin could be detected in the final solution with an endotoxin detection assay (detection limit 10 pg/mL). Mice were injected via the lateral tail vein with BrdU at a dose of 10 mg/mL in a volume of 0.1 mL. All experiments were conducted in accordance with the Guidelines of the National Health and Medical Research Council, Australia, and the experimental protocols were approved by the Animal Ethics Committee of the Royal Melbourne Hospital Campus and Ludwig Institute for Cancer Research (Melbourne, Australia).
Leukocyte counts
Groups of 3 mice of each genoytype were bled through retro-orbital puncture and then killed at various time points after BrdU injection (as mentioned in “Results”). At each of the time points studied, control (uninjected) mice of each genotype were also bled and killed by cervical dislocation. Femurs were excised and bone marrow cells were harvested in phosphate buffered saline (PBS) containing 5% fetal calf serum (FCS). An aliquot of blood was taken, diluted 1:4 in PBS containing 2 mg/mL of EDTA, and the cells were counted using a blood cell counter Sysmex K-1000 (Toa Medicals, Tokyo, Japan).
FACScan detection of BrdU-labeled granulocytes and estimation of bone marrow transit times
Red blood cells (RBCs) in blood were lysed using RBC lysis buffer, and then the cells were washed once with PBS containing 5% FCS. Both the bone marrow and the blood cells were then transferred to a fluorescence-activated cell sorter (FACS) tube, centrifuged and resuspended in 0.5 mL of 0.5% cold paraformaldehyde. The cells were incubated on ice for 5 minutes and for a further 30 minutes at room temperature. They were then centrifuged, and the pellet was resuspended in 0.5 mL of 2 M HCl-0.5% Tween 20. The cells were incubated at 37°C for 30 minutes, pelleted and resuspended in 0.5 mL of 0.1 M sodium borate. The cells were immediately centrifuged and the pellet was resuspended in PBS containing 1% Tween-20 and then stained sequentially with fluorescein conjugated anti-BrdU (BrdU-FITC) antibody (Becton Dickinson, San Jose, CA) at room temperature for 30 minutes, followed by phycoerythrin-conjugated Gr-1 (Gr-1-PE) antibody (Pharmingen, San Diego, CA) on ice for 30 minutes. The cells were then washed and resuspended in PBS containing 0.25% FCS and analyzed by FACScan using Cell Quest software (Becton Dickinson).
Gr-1 is a 21-25 kDa glycosylphosphatidylinositol (GPI)–anchored cell surface protein that is primarily expressed on cells within the myeloid lineage and in particular within the neutrophil series.8 In the bone marrow, the level of Gr-1 expression is directly correlated with granulocytic differentiation and maturation;9 in the periphery, anti–Gr-1 antibodies specifically recognize peripheral neutrophils.8-10 Granulocytes (cells positive for Gr-1) were classified into 2 populations, depending on the fluorescence intensity corresponding to BrdU labeling as detected by anti–BrdU-FITC antibody using an arbitrarily designated grading system into weakly positive (BrdUdim) and strongly positive (BrdUbright) granulocytes (the mean geometric fluorescence of BrdUbright granulocytes being at least 5-fold higher than that of BrdUdim granulocytes). This grading system was designed to evaluate the transit time of the myeloid cells that were at different stages of the cell cycle in the mitotic pool following exposure to BrdU. This method of calculating the transit time of granulocytes through the different pools in the marrow is based on the study by Maloney and Patt,11 who have used tritiated thymidine to label dividing cells in the bone marrow (BM) of dogs.
To study the cycling cells, bone marrow cells were stained with anti–BrdU-FITC antibody as described above and then further stained with propidium iodide (PI, final concentration 5 μg/mL). The cells were then analyzed by FACScan, using Cell Quest Software.
Analysis of apoptosis of bone marrow cells
Bone marrow cells were dispersed into single-cell suspension in PBS containing 5% FCS. The cells were stained with mercocyanine 540 dye (MC540; Molecular Probes, Eugene, OR) as described by Reid et al.12 Briefly, cells were resuspended in 100 μL PBS to which 4 μL of 10 μg/mL MC540 stock was added. The cells were incubated in the dark with the MC540 dye at room temperature for 20 minutes. At the end of the incubation period, the Fc receptors on the cell surface were blocked with anti-CD32/CD16 antibodies (Pharmingen). The cell and antibody solution was incubated at room temperature for 5 minutes. Thereafter, cells were stained with FITC-conjugated anti–Gr-1. The cells were then analyzed with FACScan, using Cell Quest software.
Calculation of labeling index and cycling time of bone marrow cells and half-life of peripheral neutrophils
Under steady-state conditions, for a population of proliferating cells, the labeling index (IL) represents:5
To make the comparisons of the cell-cycle parameters between wild-type and G-CSF–deficient mice, we have assumed an average control value of 5 hours for ts in mice.5
The half-life (t1/2) of the peripheral neutrophils is calculated from the rate of loss of labeled cells in the blood, using the formula n = No e-λt, where No = peak no. of cells, n = number of cells at time t, and λ = decay constant.
Cycling status of hematopoietic progenitor cells
The proportion of granulocyte-macrophage (GM)–progenitors undergoing DNA synthesis (S-phase of the cell cycle) was estimated as reported previously.13 Bone marrow cells from individual mice (5 × 106cells in 1 mL of Dulbecco modified Eagle medium (DMEM)) were incubated in paired tubes at 37°C for 30 minutes. Tritiated thymidine (3H-TdR; [99.9 × 10−5 Ci (37 MBq)], specific activity 35 Ci/mmole [12.9 × 105 MBq]) was added to one tube of each pair, and an equal volume of medium was added to the second tube. At the end of the incubation period, the tubes were placed in ice to prevent further incorporation of isotope. Cells were washed twice in DMEM containing cold thymidine (100 μg/mL) and assayed for GM-CFC. Colonies were assayed in culture by plating 5 × 104 bone marrow cells per mL in methylcellulose media (Stem Cell Technologies, Vancouver, BC, Canada). GM-CSF was added to the cultures at a final concentration of 10 ng/mL. The cultures were established in triplicate and were incubated at 37°C in a fully humidified atmosphere of 10% CO2. The colonies (> 50 cells) were counted after 7 days of culture. For determining the number of cells per colony, after counting colonies, cells from the entire plate were harvested and counted using a hemocytometer to determine the total number of cells recovered and the average number of cells per colony.
Statistical analysis
Data are presented as mean ± SEM unless otherwise stated. Comparisons were made using 2-tailed unpaired Student t test and Mann-Whitney signed rank test as appropriate. A P value of < .05 was considered significant.
Results
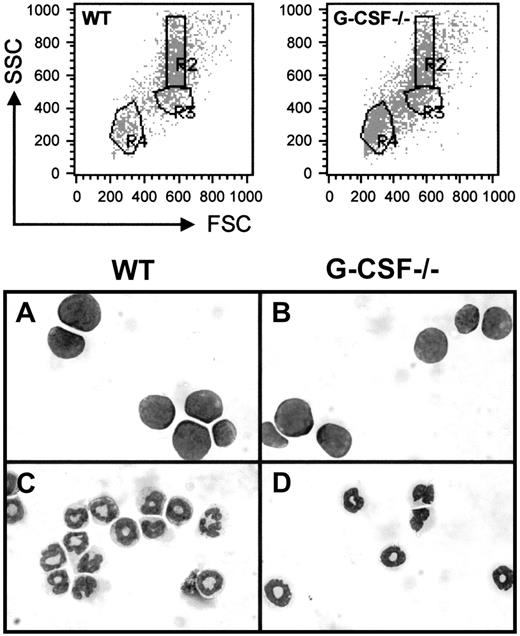
In the present study, we have used the fluorescence intensity of labeled cells, following staining with Gr-1 antibody conjugated with FITC, and their position in a forward scatter (FSC) and side scatter (SSC) profile to identify cells within the granulocytic lineage both in bone marrow and blood. A dot blot representation of FSC versus SSC of bone marrow cells from wild-type and G-CSF–deficient mice is shown in Figure 1; cells in region R4 and R3 represent lymphocytes and blast cells, respectively, and cells with relatively high side scatter in region R2 represent granulocytes. The morphological analysis of the May-Grünwald stained cytospin preparation of the sorted cells in regions R2 and R3 has confirmed our definition of cell types in these regions. Further staining with the Gr-1 antibody has shown that the cells in region R3 (blast cells) are largely Gr-1 negative, whereas those in region R2 (granulocytes) are mostly Gr-1 positive (> 98%) (data not shown).
Dot blot presentation of forward scatter (FSC) versus side scatter (SSC) of bone marrow cells from wild-type and G-CSF–deficient mice.
The bone marrow cells were fixed with paraformaldehyde and permeabilized using acidified Tween-20 as described in “Materials and methods” and then analyzed using a FACScan. RBCs and cellular debris have been gated out. Regions were set on the white blood cell (WBC) population to define lymphocytes (R4), blasts (R3), and granulocytes (R2). Cells in regions R2 and R3 were sorted, and cytospin preparations of these cells were stained with May-Grünwald-Giemsa. The morphology of these cells is shown in the bottom panel. Panels A and B represent cells in region R3, and panels C and D represent cells in region R2 from wild-type and G-CSF–deficient mice, respectively.
Dot blot presentation of forward scatter (FSC) versus side scatter (SSC) of bone marrow cells from wild-type and G-CSF–deficient mice.
The bone marrow cells were fixed with paraformaldehyde and permeabilized using acidified Tween-20 as described in “Materials and methods” and then analyzed using a FACScan. RBCs and cellular debris have been gated out. Regions were set on the white blood cell (WBC) population to define lymphocytes (R4), blasts (R3), and granulocytes (R2). Cells in regions R2 and R3 were sorted, and cytospin preparations of these cells were stained with May-Grünwald-Giemsa. The morphology of these cells is shown in the bottom panel. Panels A and B represent cells in region R3, and panels C and D represent cells in region R2 from wild-type and G-CSF–deficient mice, respectively.
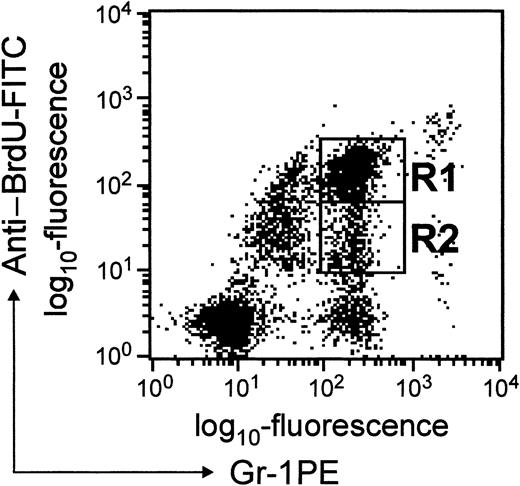
A convenient means of studying the transit time of PMNs through different pools in the marrow and their subsequent trafficking in peripheral blood is to exploit the fact that cells in mitosis incorporate BrdU into replicating DNA.14 Typically, BrdU incorporation has been detected by in-situ microscopy. In the present study, we have exploited anti-BrdU antibody conjugated to FITC to detect BrdU-labeled cells by FACScan. BrdU-labeled granulocytes could be derived from either myelocytes that incorporated BrdU during their last division or from myeloid progenitors/precursors that may have divided since incorporating BrdU. Since during each division (after BrdU incorporation) the label is diluted (50%), using this scheme cell divisions could be followed precisely. By following the changes in relative fluorescence intensity, it is possible to unambiguously distinguish granulocytes derived from myelocytes from granulocytes derived from myeloid cells that have undergone one or more divisions since incorporating BrdU. Cells in region R1 (BrdUbright) correspond to granulocytes that had incorporated BrdU in their last division, whereas cells in region R2 (BrdUdim) correspond to granulocytes derived from cells that had undergone few divisions since incorporating BrdU (Figure2). Compared to BrdUdimgranulocytes, BrdUbright granulocytes could be detected in blood as early as 24 hours after BrdU challenge. In this study, FL-1 relates to fluorescence corresponding to Gr-1 expression, and FL-2 is fluorescence corresponding to BrdU uptake and will be referred to as such in the “Results” and “Discussion” sections.
Dot blot presentation of BrdU-labeled cells in blood.
WBCs were stained with anti–BrdU-FITC and anti–Gr-1-PE antibodies as described in “Materials and methods.” The fluorescence intensity corresponding to anti-BrdU staining is shown on the y-axis, and that due to Gr-1 staining is shown on the x-axis. Cells in regions R1 and R2 represent BrdUbright and BrdUdim granulocytes, respectively.
Dot blot presentation of BrdU-labeled cells in blood.
WBCs were stained with anti–BrdU-FITC and anti–Gr-1-PE antibodies as described in “Materials and methods.” The fluorescence intensity corresponding to anti-BrdU staining is shown on the y-axis, and that due to Gr-1 staining is shown on the x-axis. Cells in regions R1 and R2 represent BrdUbright and BrdUdim granulocytes, respectively.
Bone marrow
In preliminary experiments, using the criteria described in the previous section, we found that in the marrow, the proportion of Gr-1lo cells was 15% versus 14% and the proportion of Gr-1high cells was 36% versus 17% in wild-type and G-CSF−/− mice, respectively. Morphological analysis of the cytospin preparations of bone marrow cells from wild-type and G-CSF−/− mice showed a similar distribution of early (promyelocyte and myelocyte) and late (metamyelocyte, band and segmented) forms of neutrophils in the 2 groups of mice. The Gr-1lo population is likely to represent immature neutrophils, whereas the Gr-1highrepresents a population of mature neutrophils (Table1). Administration of BrdU into the mice had no effect on the differential composition of bone marrow cells as similar results were obtained from mice that had not been injected with BrdU (data not shown).
Proportion of Gr-1+ cells and neutrophils in the bone marrow as determined by FACScan and morphological analysis, respectively
| Genotype of mice . | Bone marrow cellularity, × 107 . | Percentage of total Gr-1+ cells . | Percentage of Gr-1lo* cells . | Percentage of Gr-1high* cells . | Percentage of neutrophils, total . | Percentage of promyelocytes + myelocytes . | Percentage of metamyelocyte + bands + polymorphs . |
|---|---|---|---|---|---|---|---|
| Wild type | 1.85 ± 0.3 | 52 ± 3.4 | 15 ± 2.3 | 36 ± 0.5 | 45 ± 3.0 | 15 ± 2.5 | 30 ± 2 |
| G-CSF−/− | 1.5 ± 0.5 | 32 ± 3.6 | 14 ± 1.4 | 17 ± 1.2 | 29 ± 4.6 | 15 ± 3.8 | 14 ± 2 |
| Genotype of mice . | Bone marrow cellularity, × 107 . | Percentage of total Gr-1+ cells . | Percentage of Gr-1lo* cells . | Percentage of Gr-1high* cells . | Percentage of neutrophils, total . | Percentage of promyelocytes + myelocytes . | Percentage of metamyelocyte + bands + polymorphs . |
|---|---|---|---|---|---|---|---|
| Wild type | 1.85 ± 0.3 | 52 ± 3.4 | 15 ± 2.3 | 36 ± 0.5 | 45 ± 3.0 | 15 ± 2.5 | 30 ± 2 |
| G-CSF−/− | 1.5 ± 0.5 | 32 ± 3.6 | 14 ± 1.4 | 17 ± 1.2 | 29 ± 4.6 | 15 ± 3.8 | 14 ± 2 |
Gr-1lo and Gr-1high represent cells expressing low and high levels of Gr-1, respectively.
Values are represented as mean ± SD; n = 6 for each group.
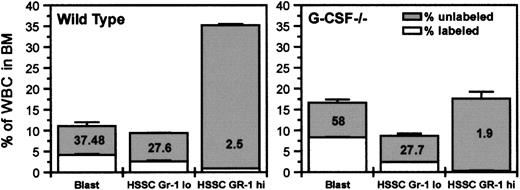
The blastlike cells (as defined on the basis of their position on FSC versus SSC profile) were mostly Gr-1 negative, and the proportion of these cells in bone marrow was found to be similar in both G-CSF–deficient and wild-type mice. However, by 2 hours after BrdU challenge, the proportion of BrdU-labeled cells in the blast compartment was higher in G-CSF–deficient mice than in wild-type mice (58% vs 37%) (Figure 3). In addition to an increased proportion of labeled blastlike cells, the uptake of BrdU/cell was also higher in G-CSF–deficient mice compared to wild-type mice (Table 2). Using an average ts of 5 hours in wild-type mice,5 the relative cycle time is given by tc = 5/IL. Therefore, in wild-type mice, for blast cells with IL = 0.37 the mean relative cycle time is 13.5 hours. There was not any significant difference in the synthesis time (ts) between wild-type and G-CSF–deficient mice (5.0 hours vs 4.9 hours). However, with an increase in labeling index in G-CSF–deficient mice compared to wild-type mice (58.7 vs 37), the relative cycling time tc is reduced to 8.5 hours (Table 2). However, this phenomenon was not observed with Gr-1high(high SSC) and Gr-1lo (low SSC) cells: the uptake of BrdU in these 2 populations was comparable in G-CSF−/− and wild-type mice (Figure 3). These findings suggest that in bone marrow, in the absence of G-CSF, the most significant difference is in the blast cell compartment. An increased proportion of these cells is in S-phase; moreover, they are cycling faster compared to their counterparts in wild-type mice.
Initial BrdU uptake by various subpopulations of bone marrow cells and their distribution in bone marrow.
Wild-type and G-CSF–deficient mice were injected with BrdU (intraperitoneally), and after 2 hours they were killed. The bone marrow cells were fixed and permeabilized and then labeled with anti–BrdU-FITC antibody followed by Gr-1-PE staining. The total height of each column represents the percentage of total cells. The unshaded portions represent labeled cells (cells in DNA synthesis). BrdU uptake by blast cells from G-CSF–deficient mice was significantly higher than that from wild-type mice (P < .05). HSSC represents cells with high side scatter in FSC versus SSC FACS profile. Data are represented as mean ± SEM (n = 3) and are a representative plot of 1 of the 3 independent experiments.
Initial BrdU uptake by various subpopulations of bone marrow cells and their distribution in bone marrow.
Wild-type and G-CSF–deficient mice were injected with BrdU (intraperitoneally), and after 2 hours they were killed. The bone marrow cells were fixed and permeabilized and then labeled with anti–BrdU-FITC antibody followed by Gr-1-PE staining. The total height of each column represents the percentage of total cells. The unshaded portions represent labeled cells (cells in DNA synthesis). BrdU uptake by blast cells from G-CSF–deficient mice was significantly higher than that from wild-type mice (P < .05). HSSC represents cells with high side scatter in FSC versus SSC FACS profile. Data are represented as mean ± SEM (n = 3) and are a representative plot of 1 of the 3 independent experiments.
Bone marrow labeling and calculated relative cell-cycle parameters
| Type of cell . | Genotype . | IL* . | FL† . | DNA-S time ts (h) . | Cycle time tc (h) . |
|---|---|---|---|---|---|
| Blast | WT | 37.0 ± 2.9‡ | 183.0 ± 27.0 | 5.02-153 | 13.5 ± 1.1 |
| G-CSF−/− | 58.7 ± 2.7 | 192.0 ± 33.0 | 4.9 ± 0.9 | 8.5 ± 2.1 | |
| HSSC Gr-1low | WT | 27.8 ± 3.6 | 147.0 ± 13.0 | 5.02-153 | 18.0 ± 0.8 |
| G-CSF−/− | 24.3 ± 0.3 | 174.0 ± 2.4 | 4.4 ± 0.2 | 18.0 ± 1.2 |
| Type of cell . | Genotype . | IL* . | FL† . | DNA-S time ts (h) . | Cycle time tc (h) . |
|---|---|---|---|---|---|
| Blast | WT | 37.0 ± 2.9‡ | 183.0 ± 27.0 | 5.02-153 | 13.5 ± 1.1 |
| G-CSF−/− | 58.7 ± 2.7 | 192.0 ± 33.0 | 4.9 ± 0.9 | 8.5 ± 2.1 | |
| HSSC Gr-1low | WT | 27.8 ± 3.6 | 147.0 ± 13.0 | 5.02-153 | 18.0 ± 0.8 |
| G-CSF−/− | 24.3 ± 0.3 | 174.0 ± 2.4 | 4.4 ± 0.2 | 18.0 ± 1.2 |
IL represents index of labeling.
FL represents fluorescence intensity.
Data is presented as mean ± SD of 3 mice.
HSSC represents cells with high side scatter. The HSSC cells that express low levels of Gr-1 have been represented as Gr-1low.
Adopted from Lord et al.5
Cell cycle analysis of bone marrow cells
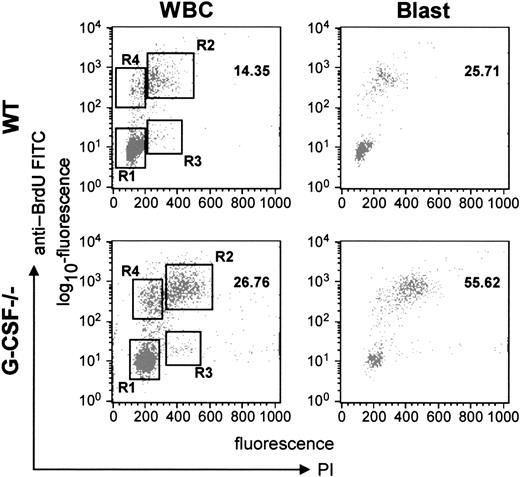
To address this issue further, we analyzed the cycling status of bone marrow cells by harvesting the bone marrow cells 2 hours after BrdU challenge and then labeling the cells with anti-BrdU antibody and propidium iodide. As shown in Figure4, it was evident that in G-CSF–deficient mice a greater proportion of cells were in the S- (region R2) and G2/M (region R3) phases compared to wild-type mice. These data were further analyzed for different subpopulations of cells (on the basis of FSC and SSC). From this analysis, it was evident that this effect was most prominent in blast cells; in G-CSF–deficient mice a significantly greater proportion of blast cells were in S-phase (57.3 vs 26) and the G2/M phase (1.6 vs 0.5) compared to wild-type mice (Table 3). This confirmed our earlier finding that a greater proportion of blast cells were in cycle and were cycling faster. However, the cycling status of lymphocytes was comparable in both wild-type and G-CSF–deficient mice (data not shown).
Cell-cycle analysis of bone marrow cells from wild-type and G-CSF–deficient mice.
Mice of each genotype were injected with BrdU intraperitoneally. After 2 hours they were killed, and the bone marrow cells were harvested. The bone marrow cells were then fixed and permeabilized and labeled with anti–BrdU-FITC antibody. The cells were further stained with PI and then analyzed in a FACScan machine using Cell Quest software. The relative DNA content of a cell as measured by PI uptake is represented on the x-axis, and the cells in S-phase as determined by BrdU uptake are represented on the y-axis. The left-hand panel shows the plot for total WBCs, and the right-hand panel represents the plot for blast cells.
Cell-cycle analysis of bone marrow cells from wild-type and G-CSF–deficient mice.
Mice of each genotype were injected with BrdU intraperitoneally. After 2 hours they were killed, and the bone marrow cells were harvested. The bone marrow cells were then fixed and permeabilized and labeled with anti–BrdU-FITC antibody. The cells were further stained with PI and then analyzed in a FACScan machine using Cell Quest software. The relative DNA content of a cell as measured by PI uptake is represented on the x-axis, and the cells in S-phase as determined by BrdU uptake are represented on the y-axis. The left-hand panel shows the plot for total WBCs, and the right-hand panel represents the plot for blast cells.
Distribution of blast cells in different stages of cell cycle in wild-type and G-CSF−/− mice
| Genotype . | G0/G1 . | S . | G2/M . | G0/G13-150 . |
|---|---|---|---|---|
| WT | 69.6 ± 2.8 | 26 ± 1.7 | 0.5 ± 0.03 | 4.3 ± 0.7 |
| G-CSF−/− | 30.4 ± 5.0 | 57.3 ± 2.9‡ | 1.6 ± 0.43-151 | 9.6 ± 2.5 |
| Genotype . | G0/G1 . | S . | G2/M . | G0/G13-150 . |
|---|---|---|---|---|
| WT | 69.6 ± 2.8 | 26 ± 1.7 | 0.5 ± 0.03 | 4.3 ± 0.7 |
| G-CSF−/− | 30.4 ± 5.0 | 57.3 ± 2.9‡ | 1.6 ± 0.43-151 | 9.6 ± 2.5 |
Cells that were in S-phase at the time of BrdU challenge and were in G0/G1 stage at the time of analysis (2 hours after challenge).
P < .05 (G-CSF−/− versus wild type).
Cycling rates of GM-progenitors
Blast cells are a heterogeneous population of cells comprising lympho-, erythro-, mono-, and myeloblasts. In this study, we have analyzed blast cells with relatively higher side scatter to include mostly myeloblasts (blast cells with relatively higher side-scatter due to the presence of primary granules). To confirm that the increased cycling of the blast cells in G-CSF–deficient mice reflected an increase number of myeloid progenitors in cycle, we further evaluated the cycling rates of GM-progenitors in G-CSF–deficient and wild-type mice. Bone marrow cells were harvested and the proportion of GM-progenitors in the S-phase of the cell cycle was estimated using a suicide assay employing high specific activity tritiated thymidine. As shown in Table 4, pulse exposure of bone marrow cells to tritiated thymidine led to a significantly greater reduction in the number of colonies that developed in response to GM-CSF in G-CSF–deficient mice compared to wild-type mice. This observation demonstrates that, indeed, a significantly greater proportion of GM-progenitors is in S-phase of cell cycle in G-CSF–deficient mice compared to wild-type mice. However, both the total number of colonies obtained in response to GM-CSF as well as the average number of cells per colony was lower in G-CSF–deficient mice compared to wild-type mice (Table 4).
GM-progenitors from wild-type and G-CSF-deficient mice
| Genotype . | Total no. of GM-CFC/50 000 BM cells . | No. of GM-CFC/50 000 BM cells after pulse exposure of BM cells to3H-TdR4-150 . | Percent of GM-progenitors in S-phase . | No. of cells/colony . |
|---|---|---|---|---|
| Wild-type | 86.0 ± 5.5 | 63.8 ± 8.0 | 25.8 ± 1.6 | 1 259 ± 77 |
| G-CSF−/− | 45.0 ± 7.34-151 | 22.3 ± 5.0 | 50.6 ± 2.84-151 | 434 ± 644-151 |
| Genotype . | Total no. of GM-CFC/50 000 BM cells . | No. of GM-CFC/50 000 BM cells after pulse exposure of BM cells to3H-TdR4-150 . | Percent of GM-progenitors in S-phase . | No. of cells/colony . |
|---|---|---|---|---|
| Wild-type | 86.0 ± 5.5 | 63.8 ± 8.0 | 25.8 ± 1.6 | 1 259 ± 77 |
| G-CSF−/− | 45.0 ± 7.34-151 | 22.3 ± 5.0 | 50.6 ± 2.84-151 | 434 ± 644-151 |
BM cells were pulse exposed to 3H-TdR prior to plating on methylcellulose medium in the presence of 10 ng/mL GM-CSF as described in “Materials and methods.” Data are represented as mean ± SEM (n = 6).
P < .05 (G-CSF−/− versus wild type).
Appearance of labeled neutrophils in blood
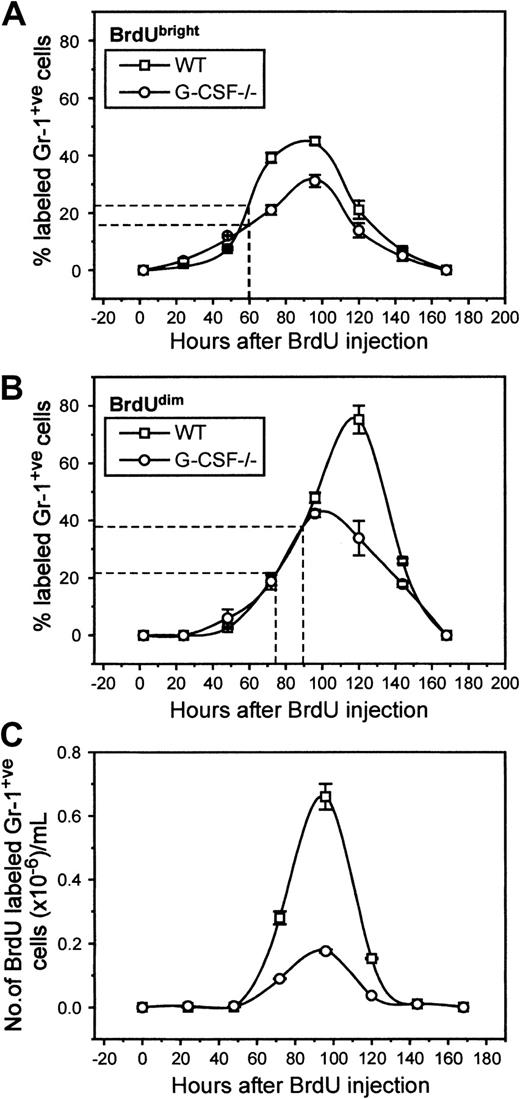
In the blood of BrdU-injected G-CSF−/−mice, 8% of cells were Gr-1 positive compared to 24% in wild-type mice (data not shown). These values are similar to those seen in unchallenged mice, suggesting again that BrdU challenge itself does not promote changes in the cellular composition of blood. Both in G-CSF–deficient and wild-type mice, BrdU-labeled Gr-1–positive cells began to appear 24 hours after challenge (2% of total granulocytes). There was a gradual increase in numbers of BrdU-labeled Gr-1–positive cells in blood, with peak levels of labeling being reached simultaneously in G-CSF−/− and wild-type mice (96 hours), indicating that the kinetics of mature neutrophil release from marrow in these mice is very similar (Figure5A). However, at peak labeling, only 32% of granulocytes cells were labeled in G-CSF–deficient mice compared to 48% in wild-type mice. The mean appearance time of BrdUbright granulocytes was 60 hours both in wild-type and G-CSF–deficient mice.
Kinetics of appearance of BrdU-labeled granulocytes in circulation of wild-type and G-CSF–deficient mice.
The top (A) and the middle (B) panels show the proportion of granulocytes that are BrdUbright and BrdUdim in the blood of wild-type and G-CSF–deficient mice, respectively. The bottom panel (C) shows the number of BrdU-positive granulocytes in the blood of wild-type and G-CSF–deficient mice. The average time of appearance of BrdUbright and BrdUdimgranulocytes in wild-type and G-CSF–deficient mice is marked on the x-axes in panels (A) and (B). This is a representative plot of 3 independent experiments showing similar results. Data are represented as mean ± SEM (n = 3).
Kinetics of appearance of BrdU-labeled granulocytes in circulation of wild-type and G-CSF–deficient mice.
The top (A) and the middle (B) panels show the proportion of granulocytes that are BrdUbright and BrdUdim in the blood of wild-type and G-CSF–deficient mice, respectively. The bottom panel (C) shows the number of BrdU-positive granulocytes in the blood of wild-type and G-CSF–deficient mice. The average time of appearance of BrdUbright and BrdUdimgranulocytes in wild-type and G-CSF–deficient mice is marked on the x-axes in panels (A) and (B). This is a representative plot of 3 independent experiments showing similar results. Data are represented as mean ± SEM (n = 3).
A second population of BrdU-labeled granulocytes was defined on the basis of their FL-2 intensity. These cells had lower FL-2 (corresponding to BrdU labeling) and are presumed to be the progeny of cells that have undergone more postlabeling maturational divisions before release into circulation. These cells were studied separately, and Figure 5B illustrates the emergence of these cells in circulation. It is evident that there was a shift in the peak appearance of this population of cells in G-CSF−/− and wild-type mice; in G-CSF–deficient mice the peak was observed at 96 hours after BrdU challenge, whereas in wild-type mice this was found to be 120 hours after BrdU challenge. The mean appearance time in blood of this population was 72 hours versus 90 hours in G-CSF–deficient and wild-type mice, respectively.
The half-life of the neutrophils in circulation is calculated from the rate of loss of labeled cells in the blood, using the formula n = No e-λt. From Figure 4C, for wild-type mice, No = 6.6 × 105 at 96 hours and n = 1.53 × 105 at 120 hours. Therefore, λ = 0.0609, and t1/2 is given by 1 = 2e-0.0609t½ = 11.4 hours.6Similarly for granulocytes from G-CSF−/−mice, No = 1.76 × 105 at 96 hours and n = 0.37 × 105 at 120 hours. Therefore, λ = 0.065 and t1/2 = 10.7 hours. These values are similar to the published half-lives of human and mice neutrophils.5 6
Apoptosis of Gr-1–positive cells
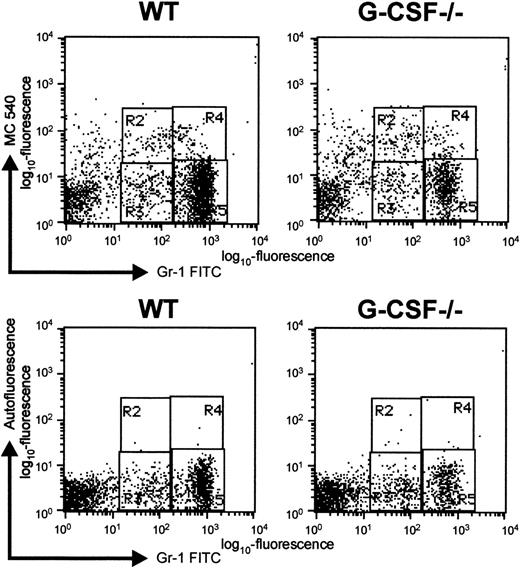
Although a greater proportion of blast cells are in cycle in G-CSF–deficient mice compared to wild-type mice, the number of mature neutrophils in bone marrow is significantly lower in the bone marrow of G-CSF–deficient mice. Finding that in the absence of G-CSF, the mobilization of mature granulocytes from bone marrow to blood was unaffected suggests that there is a greater loss of cells within the granulocytic lineage of G-CSF–deficient mice. Since it is well established that neutrophils are preprogrammed to undergo apoptosis,15 we determined the proportion of granulocytic cells undergoing apoptosis in the bone marrow of G-CSF–deficient and wild-type mice. To evaluate apoptosis, BM cells from G-CSF−/− and wild-type mice were stained with mercocyanine 540 (MC540) dye in addition to staining with antibodies specific to Gr-1. The MC540 dye detects apoptotic cells by associating with altered lipid moieties in the outer layer of the plasma membrane.12 Changes in the lipid composition of the outer leaflet of the plasma membrane represent one of the earliest stages of apoptotic cell death.16,17 Binding of MC540 dye detects cells that have just initiated an apoptotic program. This method is most suitable for studying apoptosis in cells freshly isolated from an experimental animal, since late-stage apoptotic cells are most likely to have already been cleared by phagocytic cells.18 The Gr-1–positive bone marrow cells were analyzed for MC540 staining, and a representative dot blot is shown in Figure6. The percentage of Gr-1loapoptotic cells (region R2, Figure 6) found in the BM of G-CSF–deficient mice was significantly greater than the percentage found in the same population from wild-type mice: 6.7 ± 0.9% versus 3.5 ± 0.2%, P < .001, n = 5. Thus, in the absence of G-CSF, a greater proportion of early granulocytic cells are undergoing apoptosis.
Representative dot blot to show apoptosis of Gr-1–positive cells as detected by MC-540 staining.
Bone marrow cells were harvested from G-CSF–deficient and wild-type mice and immediately stained with MC-540 dye and Gr-1 FITC antibody. The cells were analyzed by 2-color flow cytometry using Cell Quest software. Regions R2 and R4 represent apoptotic cells in Gr-1lo and Gr-1bright populations, respectively. Regions R3 and R5 represent nonapoptotic cells in Gr-1lo and Gr-1bright populations, respectively. In the bottom panel, cells were stained only with Gr-1-FITC antibody, and their red autofluorescence under the same settings was determined. The regions were adjusted to take into account background autofluorescence.
Representative dot blot to show apoptosis of Gr-1–positive cells as detected by MC-540 staining.
Bone marrow cells were harvested from G-CSF–deficient and wild-type mice and immediately stained with MC-540 dye and Gr-1 FITC antibody. The cells were analyzed by 2-color flow cytometry using Cell Quest software. Regions R2 and R4 represent apoptotic cells in Gr-1lo and Gr-1bright populations, respectively. Regions R3 and R5 represent nonapoptotic cells in Gr-1lo and Gr-1bright populations, respectively. In the bottom panel, cells were stained only with Gr-1-FITC antibody, and their red autofluorescence under the same settings was determined. The regions were adjusted to take into account background autofluorescence.
Discussion
Granulopoiesis is a dynamic process that supplies and replenishes mature neutrophils. The maintenance of neutrophil numbers is an important feature in hematopoiesis, both during steady state and in response to inflammatory stimuli.19 At steady state, bone marrow is the major site of production of hematopoietic cells, which are subsequently released into peripheral blood. This is achieved, in part, by an alteration in the expression of adhesion molecules, triggered by cytokines/chemokines. Earlier studies have indicated a multifunctional role for G-CSF, including proliferation, differentiation, survival, and chemotaxis for cells within the granulocytic lineage.20 There have been few studies that have investigated and reported the role of G-CSF in granulopoiesis. The most conclusive evidence has come from gene-targeting studies involving G-CSF gene from our laboratory7 or G-CSF receptor gene.3 Both G-CSF–deficient and G-CSF-receptor–deficient mice have defective granulopoiesis with chronic neutropenia both peripheral and in bone marrow as well as a decrease in mature myeloid elements in their bone marrow. These studies have confirmed unambiguously the hypothesis in the literature that G-CSF is a major regulator of granulopoiesis. Although initially the studies were performed in mixed genetic background (C57BL/6 × 129Ola G-CSF–deficient and C57BL/6 × 129 Sv G-CSF receptor–deficient), we have very recently generated G-CSF–deficient mice in C57BL/6 background and have found that the neutropenia in these mice is comparable to G-CSF–deficient mice in mixed background, conclusively proving that the differences in peripheral neutrophil counts between G-CSF–deficient and wild-type mice is due solely to the absence and presence of G-CSF in these mice, respectively (S.B. and A.R.D., unpublished observation, April 2001). In addition to its role in neutrophil production, G-CSF has also been shown to facilitate the mobilization of neutrophils from bone marrow into blood.5However, this observation has been made by injecting G-CSF into healthy human volunteers and experimental animals. It remains possible that some of the observed effects may be indirect. In the present study, we have analyzed the cycling of bone marrow cells and neutrophil trafficking from bone marrow into circulation at steady state and evaluated the importance of G-CSF in these processes.
It was interesting to find that in the bone marrow, although the proportion of blastlike cells was similar in G-CSF–deficient and wild-type mice, the proportion of these cells labeled with BrdU was significantly higher in G-CSF–deficient mice. Increased labeling index coupled with the fact that there was also a slight increase in fluorescence intensity suggested that the mean cycle time of the blastlike cells in G-CSF–deficient mice is significantly reduced compared to wild-type mice. The cell-cycle study further established that at a given time point, a greater proportion of blast cells are in the S and G2/M phases in G-CSF–deficient mice compared to wild-type mice. Intriguingly, our studies show that a greater proportion of myeloid (GM)-progenitors is in cycle in G-CSF–deficient mice compared to wild-type mice. One possible explanation for this observation is that the rapid proliferation of blast cells in the bone marrow of G-CSF–deficient mice is a compensatory pathway that is activated in the absence of G-CSF. The cytokine(s) presumed to be responsible for activating this pathway might be a known factor or a novel factor. Alternatively, in steady-state granulopoiesis, major amplification occurs at the blast stage; in this scheme, proliferation of this population of cells might be regulated by a feedback mechanism controlled by neutrophil numbers. Reduced numbers of mature granulocytes in G-CSF–deficient mice generates a positive feedback signal leading to an increase in the proliferation of blast cells.
The transit time of granulocytes through the mitotic and postmitotic pools in the bone marrow is represented by the mean appearance time of weakly labeled granulocytes in blood. The mean appearance time of BrdUbright granulocytes in blood was similar in wild-type and G-CSF–deficient mice. This demonstrated that the transit time of the granulocytes through the postmitotic pool is similar in wild-type and G-CSF–deficient mice. However, since the mean appearance time of BrdUdim granulocytes in blood was reduced in G-CSF–deficient mice compared to wild-type mice, it suggests that this is mainly due to a shortening of the transit time through the mitotic pool in G-CSF–deficient mice. Therefore, the difference in kinetics of appearance of weakly labeled (BrdUdim) granulocytes in blood (wild-type versus G-CSF–deficient mice) most likely reflects the difference in the cycling rate of blast cells and may be a direct consequence of this phenomenon.
Despite a greater proportion of blast cells being in cycle, there is a significant reduction in the mature granulocyte pool in the bone marrow of G-CSF–deficient mice. One possible explanation for this phenomenon is the early release of mature granulocytes from bone marrow into the circulation of G-CSF–deficient mice compared to wild-type mice. However, in the present study, we have demonstrated that the transit time of the granulocytes through the postmitotic pool in bone marrow is similar in G-CSF–deficient and wild-type mice. Therefore, the smaller pool of mature granulocytes in G-CSF–deficient mice is more likely due to reduced amplification of promyelocytes and myelocytes and/or a greater loss of cells in the neutrophil lineage. The mature pool of granulocytes comprises chiefly nonproliferating cells that are mainly generated through the proliferation and differentiation of granulocytic precursor/progenitors. The mean fluorescence intensity and coefficient of variance of BrdUbright granulocytes in the blood were similar in wild-type and G-CSF–deficient mice (data not shown). This clearly indicates that the number of cell divisions this population of granulocytes has undergone before being released into blood is independent of G-CSF. However, the percentage of BrdUbrightgranulocytic cells is different between the G-CSF–deficient and wild-type mice. Our studies have shown that at steady state, a greater proportion of granulocytic lineage cells within the bone marrow are undergoing apoptosis in G-CSF–deficient mice, indicating that G-CSF is a key contributor to survival of these cells. Moreover, although a greater proportion of GM-progenitors in the bone marrow are in S-phase, the number of GM-progenitor cells (colonies on day 7) was fewer in G-CSF–deficient mice compared to wild-type mice, suggesting a greater loss of progenitor cells. These findings imply that reduced survival rather than decreased amplification may be the factor responsible for the reduced pool of mature granulocytes in G-CSF–deficient mice. Thus, in steady-state hematopoiesis, an important role of G-CSF is to support survival of myeloid progenitors and cells within the neutrophil lineage.
Earlier studies have suggested that the G-CSF–mediated expansion of PMN occurs mainly at the promyelocyte and myelocyte stage II.21 In the present study, we have demonstrated that in the absence of G-CSF, in the bone marrow, only the cycling of blast cells is affected; no significant difference was observed in the cycling status of Gr-1lo and Gr-1hi cells. In healthy humans, the circulating levels of G-CSF are generally undetectable (< 30 pg/mL) and even when detected have always been < 100 pg/mL.22,23 The current view of the role of G-CSF in the proliferation of cells of the neutrophil lineage is based on various in vitro and in vivo studies. However, most of these studies have used doses of G-CSF much higher than those found in unperturbed healthy individuals. Although the neutropenic state of G-CSF- and G-CSF-receptor–deficient mice is consistent with a role for G-CSF in the proliferation of cells of the neutrophil lineage, our present findings would suggest that the neutropenia in these mice at steady state is most likely due to enhanced apoptosis rather than to reduced proliferation. G-CSF does play a role in stimulating cellular proliferation, but this role is manifested under stress conditions (when G-CSF levels are significantly elevated compared to steady state). We have reported earlier that G-CSF–deficient mice challenged with the pathogen Candida albicans have significantly elevated numbers of GM-progenitors in the bone marrow and develop neutrophilia and monocytosis, whereas in wild-type mice, GM-progenitors are not significantly elevated and they develop only neutrophilia.24 This difference in the expansion of different populations of myeloid cells in wild-type and G-CSF–deficient mice in response to Candida infection most likely reflects differences at a stage at which the myeloid mitotic pool is being amplified. As reported earlier, during stress, G-CSF levels increase dramatically23 and thus in response to candida infection, the neutrophilia in wild-type mice is most likely driven by G-CSF (a lineage-specific growth factor), which expands marrow mitotic cells distal to colony-forming unit–granulocyte macrophage (CFU-GM), primarily at the promyelocyte and myelocyte stages.6,25 Since in the absence of G-CSF expansion of neutrophil lineage cells is unlikely to be initiated at the neutrophil precursor stage (in G-CSF–deficient mice), the expansion most likely occurs at the CFU-GM stage. This hypothesis is supported by the finding that 7 days following candida challenge, neutrophilia in wild-type mice consists predominantly of mature segmented neutrophils, whereas in G-CSF–deficient mice, neutrophilia is predominantly comprised of immature neutrophils.24 It also remains possible that candida infection of G-CSF–deficient mice promotes expression of one or more cytokines, whose action serves to retard apoptosis, thereby permitting the expansion of myeloid cells in the bone marrow.
Taken together with our findings, it appears that G-CSF plays a dual role in granulopoiesis; at steady state it functions primarily as a survival factor, whereas during an emergency situation, it can function as both a proliferative stimulus and as a survival factor. While these findings support the previously defined functions of G-CSF, they provide new insights into the different roles of G-CSF at steady-state and emergency hematopoiesis in a physiological setting.
This study is dedicated to the memory of Dr George Hodgson, a dear friend and highly valued colleague. We are grateful to Drs G. Bradford, I. Bertoncello, A. Ward, and T. Paul for useful discussions. We are thankful to T. Helman, B. Morrow, and E. Richardson for assistance in the animal house; to J. Strickland for assistance with the artwork; and to Professor A. W. Burgess for critically reviewing the manuscript.
Supported in part by a research grant funded by NHMRC.
George Hodgson died on March 20, 2000.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Sunanda Basu, Molecular Biology Laboratory, Ludwig Institute for Cancer Research, PO Box 2008, Royal Melbourne Hospital, Victoria 3050, Australia; e-mail: sunanda.basu@ludwig.edu.au.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal