Abstract
Murine dendritic cells (DCs) can be classified into at least 2 subsets, “myeloid-related” (CD11bbright, CD8α−) and “lymphoid-related” (CD11bdull, CD8α+), but the absolute relationship between the 2 remains unclear. Methods of generating DCs from bone marrow (BM) precursors in vitro typically employ granulocyte-macrophage colony-stimulating factor (GM-CSF) as the principal growth factor, and the resultant DCs exhibit a myeloidlike phenotype. Here we describe a flt3-ligand (FL)–dependent BM culture system that generated DCs with more diverse phenotypic characteristics. Murine BM cells cultured at high density in recombinant human FL for 9 days developed into small lymphoid-sized cells, most of which expressed CD11c, CD86, and major histocompatibility complex (MHC) class II. The CD11c+ population could be divided into 2 populations on the basis of the level of expression of CD11b, which may represent the putative myeloid- and lymphoid-related subsets. The FL in vitro–derived DCs, when treated with interferon-α or lipopolysaccharide during the final 24 hours of culture, expressed an activated phenotype that included up-regulation of MHC class II, CD1d, CD8α, CD80, CD86, and CD40. The FL-derived DCs also exhibited potent antigen-processing and antigen-presenting capacity. Neutralizing anti–interleukin-6 (IL-6) antibody, but not anti–GM-CSF, significantly reduced the number of DCs generated in vitro with FL, suggesting that IL-6 has a role in the development of DCs from BM precursors. Stem cell factor, which exhibits some of the same bioactivities as FL, was unable to replace FL to promote DC development in vitro. This culture system will facilitate detailed analysis of murine DC development.
Introduction
Dendritic cells (DCs) capture and process antigens for presentation to naive T cells and B cells.1 Although they are relatively rare, DCs are found in a broad range of lymphoid and nonlymphoid tissues and are considered to be essential for the rapid, efficient initiation of an immune response. DCs are considered to arise from either myeloid-committed or lymphoid-committed progenitors2; however, the specific stages of development within these lineages are poorly defined, largely owing to a lack of understanding of which growth factors regulate this process. More recent studies have demonstrated that at least 2 cytokines, flt3 ligand (FL) and granulocyte-macrophage colony-stimulating factor (GM-CSF), induce DC expansion in vivo.3-6 Administration of FL to mice results in large increases in vivo of 2 populations of DCs, which appear to represent myeloid- and lymphoid-related subsets in normal lymphoid tissues.3 In contrast, GM-CSF administration favors in vivo expansion of only the myeloid-related subset.4 Studies in gene knockout (KO) mice support the notion that these cytokines play different roles in normal DC development. FL-deficient mice have reduced numbers of lymphoid-tissue DCs.7 In contrast, mice lacking GM-CSF have normal numbers of DCs in lymphoid tissues,8 but are functionally deficient at initiating B-cell and T-cell responses.9 10
The role of cytokines in DC development from hematopoietic precursors may be more easily examined with in vitro culture systems. Inaba et al11 have shown that murine bone marrow (BM) cells cultured in GM-CSF for 6 to 8 days generate large numbers of mature DCs. These GM-CSF–derived DCs can be further activated and enriched by including interleukin (IL)–4.12,13 DCs derived in GM-CSF plus IL-4 express cell-surface antigens typically associated with DCs, including DEC205, major histocompatibility complex (MHC) class II, CD80, and CD86, and demonstrate potent allo-stimulatory activity.13 On the basis of their expression of myeloid cell-surface antigens such as CD11b, 33D1, and F4/80, as well as the lack of CD8α expression, these GM-CSF–derived cells are considered to be the progeny of cells of the myeloid lineage. Lymphoid-related DCs can be generated by culturing thymus-derived CD4lowprecursors in a cytokine combination that includes stem cell factor (SCF), IL-1, tumor necrosis factor α (TNF-α), IL-3, and IL-7, but not GM-CSF.14 These DCs express variable levels of CD11b and high levels of MHC class II, as well as CD80 and CD86, but fail to express CD8α normally found on a subset of DCs from lymphoid tissues of mice.15
Here we describe a novel method of generating large numbers of DCs in vitro from BM cells cultured in FL alone. Upon activation with lipopolysaccharide (LPS) or interferon-α (IFN-α), these FL-derived DCs share morphological characteristics and phenotypic cell-surface antigens found on normal lymphoid-tissue–derived DC subsets. This culture system will allow us to investigate endogenous factors that contribute to DC development in the mouse and to directly compare the effects of exogenous cytokines on DC development and differentiation.
Materials and methods
Mice
Female BALB/c, DBA/2, and C57BL/6 mice (8 to 12 weeks of age) were obtained from Taconic (Germantown, NY). The D011.10 mice were a kind gift from Dr Mark Jenkins (University of Minnesota). The C57BL/6 IL-6 gene KO and control mice were purchased from The Jackson Laboratory (Bar Harbor, ME). All mice were housed under specific pathogen-free conditions.
Cell preparations
BM cells were isolated by flushing femurs with 2 mL phosphate-buffered saline (PBS) supplemented with 2% heat-inactivated fetal bovine serum (FBS) (Gibco BRL Life Technologies, Grand Island, NY). The BM cells were centrifuged once and then resuspended in tris-ammonium chloride at 37°C for 2 minutes to lyse red blood cells. The cells were centrifuged again and then resuspended in culture medium (CM) consisting of McCoy's medium supplemented with essential and nonessential amino acids, 1 mmol/L sodium pyruvate, 2.5 mmol/L Hepes buffer pH 7.4, vitamins, 5.5 × 10−5 mol/L 2-mercaptoethanol (2-ME), 100 U/mL penicillin, 100 μg/mL streptomycin, 0.3 mg/mL L-glutamine (PSG), and 10% FBS (all media reagents from Gibco).
DC cultures
BM cells were cultured in CM containing 200 ng/mL (180 units/mL) human FL (Immunex, Seattle, WA) for 9 days at 1 × 106/mL, in 6-well plates (Costar Corning, Cambridge, MA) unless otherwise noted. Cultures were incubated at 37°C in a humidified atmosphere containing 5% CO2 in air. DCs were harvested from the cultures by vigorously pipetting and removing nonadherent cells, then washing each well 2 times with room temperature PBS without Ca++ or Mg++ to remove loosely adherent cells, which were pooled with the nonadherent fraction.
DCs were also generated in BM cultures supplemented with GM-CSF plus IL-4 as described.16 Briefly, BM was processed as described above, including lysis of red blood cells. BM cells were cultured at 4 × 105/mL in CM containing 20 ng/mL murine GM-CSF (Immunex) and 20 ng/mL murine IL-4 (Immunex) in 6-well plates. On days 3 and 5 of culture, plates were swirled gently before two thirds of the conditioned medium was removed with the nonadherent cells. Fresh GM-CSF and IL-4–containing medium were added back to the cultures. These cultures were harvested for the nonadherent fraction after vigorous pipetting of DC clusters on day 7, and consisted of DCs and monocytes.
Activation of the DCs from FL-supplemented cultures was accomplished by the addition of 10 ng/mL recombinant murine GM-CSF, 1000 U/mL human IFN-α A/D (Genzyme, Cambridge, MA), or 1 μg/mL Escherichia coli (E coli)(0217:B8)–derived LPS (Difco, Detroit, MI) during the final 24 hours of culture unless otherwise noted.
To distinguish between the presence of soluble and membrane-bound factors produced by the BM cells, 6-well transwell plates (0.4-μm filter; Costar) were used. Various concentrations of feeder cells (whole BM) were added to the upper chamber, and the lower chamber contained 6 × 105 BM cells at 2 × 105/mL (low–cell-density BM). The DCs were harvested from the lower chamber after 9 days of culture. In some experiments, FL was excluded or replaced with 100 ng/mL (160 units/mL) murine SCF (Immunex). The bioactivity of FL and SCF was determined using the WWF7 and MO7E cell lines, respectively.
Cultures for cytokine neutralization studies were established as above, and neutralizing antibodies were included at the initiation of culture. Rat anti–murine IL-6 monoclonal antibody (mAb) (MP5-20F3) (Pharmingen, San Diego, CA) and the rat immunoglobulin (Ig)–G1 isotype control (Immunex) were used at 1 μg/mL. Rabbit anti–murine GM-CSF polyclonal antiserum (Immunex) was used at a dilution of 1:400, which was sufficient to neutralize 10 ng/mL of recombinant murine GM-CSF (data not shown).
Splenic-derived DCs
Adult BALB/c or C57/BL/6 female mice were administered 10 μg/d FL for 10 days in endotoxin-free PBS (Gibco) via intraperitoneal injections.3 At 24 hours after the last injection, the spleens were harvested and the DCs enriched as previously described.17 Briefly, spleens were digested with 100 U/mL collagenase (Worthington Biochemical Corp, Freehold, NJ), in Hanks' buffered salt solution (HBSS, Gibco) with Ca++ and Mg++ and 1% FBS for 30 minutes at 37°C. The spleens were minced and large debris was filtered out before washing the single-cell suspension in HBSS without Ca++ and Mg++ and 10 mmol/L EDTA. For functional assays (mixed lymphocyte reaction [MLR] and stimulation of ovalbumin [OVA]–specific T cells), DCs were enriched by being run over a Nycodenz discontinuous gradient. Cells within the interface were collected and washed twice before being used in antigen-presenting cell (APC) function assays. There were fewer than 1% contaminating T cells or B cells from the DC-enriched interface.
Cytologic assays
Harvested cells were centrifuged at room temperature onto slides at 30 000 to 40 000 cells per slide. Slides were air-dried and stained with LeukoStat (Fisher Diagnostics, Pittsburgh, PA) for morphological analysis. Phase-contrast observations of cultures were made by means of an inverted microscope (Nikon, Los Angeles, CA) at 400 × magnification.
Flow cytometric analysis
DCs from harvested cultures were centrifuged once and resuspended in cell-staining medium (SM) consisting of PBS supplemented with 2% heat-inactivated calf serum (Gibco), 2% heat-inactivated mouse serum (Biocell, Rancho Dominguez, CA), 10 μg/mL 2.4G2 anti-Fc receptor mAb (ATCC, Rockville, MD), and 0.02% sodium azide (Sigma, St Louis, MO). Cells were blocked with SM at 4°C for 20 minutes before incubation with mAbs. Cells were incubated with directly conjugated mAbs for 25 minutes at 4°C at 3 to 5 × 105 cells per sample in a 50-μL volume. All mAbs were purchased from Pharmingen, except where noted. The following mAbs (clone name given in parentheses) were used: CD1d (1B1), CD8α (53-6.7), CD11b (M1/70), CD11c (HL3), CD14 (rmC5-3), CD25 (7D4), CD40 (HM40-3), CD80 (16-10A1), CD86 (GL-1), IAb (AF6-120.1), Gr-1 (RB6-8C5), Ly6c (AL-21), Ly6A/E (also known as Sca-1) (D7), and c-kit (ACK45). Phycoerytherin-conjugated F4/80 (CI:A3-1) mAb was purchased from Caltag Laboratories (Burlingame, CA). Biotinylated 33D1 (ATCC) and DEC205 (NLDC145; Accurate Chemical and Scientific Corp, Westbury, NY) binding were detected with streptavidin-phycoerytherin (Molecular Probes, Eugene, OR). Propidium iodide (Boehringer Mannheim, Indianopolis, IN) at 2 μg/mL was added to exclude dead cells from analysis. We collected 10 000 events per sample. Flow cytometric analysis was performed on a FACSCalibur (Becton Dickinson, Mountain View, CA) with CELLQuest software (Becton Dickinson). Gates were determined with the use of appropriate isotype controls. Results are given as the percentage positive minus background from appropriate isotype controls.
Generation of DCs from sorted progenitor cells
BM cells from adult female C57BL/6 mice were processed as above, but resuspended in SM after lysis of red blood cells. Depletion of lineage-positive cells was accomplished by incubating cells with unconjugated mAb to CD11b, CD122 (TMb1), B220 (RA3-6B2), Gr-1, NK1.1 (PK136), TER-119, and F4/80, for 25 minutes at 4°C. All antibodies were from Pharmingen, except F4/80 which was purchased from Caltag Laboratories. Labeled BM cells were washed and then incubated with sheep-antirat IgG–conjugated magnetic beads (Dynal Inc, Oslo, Norway) per instructions. Nonadherent cells were collected and washed, then stained with phycoerythrin-labeled anti-flt3 (A2F10.1) mAb and pooled fluorescein-isothiocyanate–labeled mAb to the following lineage markers (Pharmingen) for 25 minutes at 4°C: CD3ε (500A2), CD11b, CD11c, B220, Gr-1, and Ly6c. The flt3+, lineage-negative population was sorted on the FACsVantage (Becton-Dickinson). Collected cells were cultured in a 200 μL vol per well at high density (approximately 2.5 × 105 cells per milliliter) in U-well 96-well plates (Costar). Media consisted of CM supplemented with 200 ng/mL FL and 10% conditioned medium from cultured spleen cells. Spleen-cell–conditioned medium, a source of DC-promoting activity,18 was prepared by mincing spleens from adult C57BL/6 mice in PBS, pipetting them vigorously to break up clumps, and then washing and resuspending cells in CM. Spleen cells were then cultured at 3 × 106 cells per milliliter in T-75 flasks (Costar) for 10 to 14 days before collecting the conditioned media. After sorted progenitor cells were cultured for 2 days, each well was transferred to another well in a 24-well plate (Costar) containing FL-and 10%-conditioned media from spleen cells as above. Cultures were stimulated with 1 μg/mL E coli–derived LPS on day 8 and harvested for cell counts and flow cytometric analysis on day 9.
MLR assay
DCs were generated as described above. Briefly, BM cells from C57BL/6 mice were cultured for 9 days in media containing FL alone, in cultures containing FL for 9 days to which LPS or IFN-α was added during the final 24 hours of culture, or in cultures containing GM-CSF plus IL-4 for 7 days. Additionally, DCs were enriched from the spleens of mice treated with FL for 10 days. T cells were enriched from spleen and peripheral lymph nodes (LNs) of DBA/2 mice by depletion of non–T cells with mAbs against B220 and CD11b, followed by removal of mAb-coated cells with sheep-antirat IgG–conjugated magnetic beads (Dynal). DBA/2 T cells at 1 × 105 cells per well were seeded into U-well 96-well plates (Costar) and cultured with varying numbers of allogeneic C57BL/6-derived DCs in 200 μL per well CM. Cultures were maintained for 5 days in CM at 37°C in a humidified atmosphere containing 5% CO2 in air. Alamar blue (Biosource, Camarillo, CA) at 20 μL per well was added during the final 24 hours of culture.19 The optical density of the assay plates was read at a wavelength of 570 to 600 nm on a Molecular Devices (Sunnyvale, CA) plate reader with the use of SoftMax software (Molecular Devices).
Preparation and purification of antigen-specific CD4 T cells
CD4+ T cells recognizing a peptide of chicken OVA in the context of IAd were isolated from the spleens and peripheral LN of D011.10 TCR transgenic BALB/c mice.20Single-cell suspensions were incubated for 30 minutes at 4°C with anti-CD8α (53-6.7), MHC class II (IAd, AMS-32.1), and B220 (RA3-6B2) (Pharmingen). CD4+ T cells were enriched by depleting mAb-coated cells with sheep-antirat IgG–conjugated magnetic beads (Dynal).
Antigen-presentation assay
OVA presentation assays were performed in 96-well U-well plates (Costar). Naive D011.11 OVA-specific CD4+ T cells at 1 × 105 cells per well were incubated either with a constant number of DCs (2 × 104 cells) per well and titrated OVA protein (Calbiochem, San Diego, CA), or in a constant amount of OVA protein (300 μg/mL) with varying numbers of DCs per well. DCs were generated as described above. Briefly, BM cells from female BALB/c mice were cultured for 9 days in media containing FL alone, or for 9 days in cultures containing FL to which LPS or IFN-α was added during the final 8 hours of culture, or in cultures containing GM-CSF plus IL-4 for 7 days. Additionally, DCs were enriched from the spleens of mice treated with FL for 10 days. Cells were cultured in 200 μL per well in Dulbecco modified Eagle medium containing 10% FBS, 2-ME, and PSG, at 37°C in a humidified atmosphere containing 10% CO2 in air. Cells were cultured for 5 days and then pulsed with 0.5 μCi3H-thymidine for 8 hours, and the cells were then harvested onto glass fiber sheets for counting in a gas-phase beta counter.
In vitro IL-12 production assay
Myeloid- and lymphoid-type DCs from FL-supplemented BALB/c BM cultures were sorted and separated by their expression of CD11b and CD11c by means of the Epics Elite (Beckman Coulter, Brea, CA). Sorted cells were cultured in CM supplemented with 20 ng/mL GM-CSF (Immunex), 20/ng/mL IFN-γ (PBL, New Brunswick, NJ), and 50 μg/mL Pansorbin (Calbiochem) at 1 × 106 cells per milliliter for 40 hours. Culture supernatants were assayed for murine IL-12 p70 by means of an enzyme-linked immunosorbent assay (ELISA) Quantikine kit (R & D Systems, Minneapolis, MN). Limit of detection was 8 pg/mL.
Statistical analysis
Statistical analyses were performed by means of the unpaired 2-tailed Student t test.
Results
Generation of CD11c+ DCs from FL-supplemented BM cultures
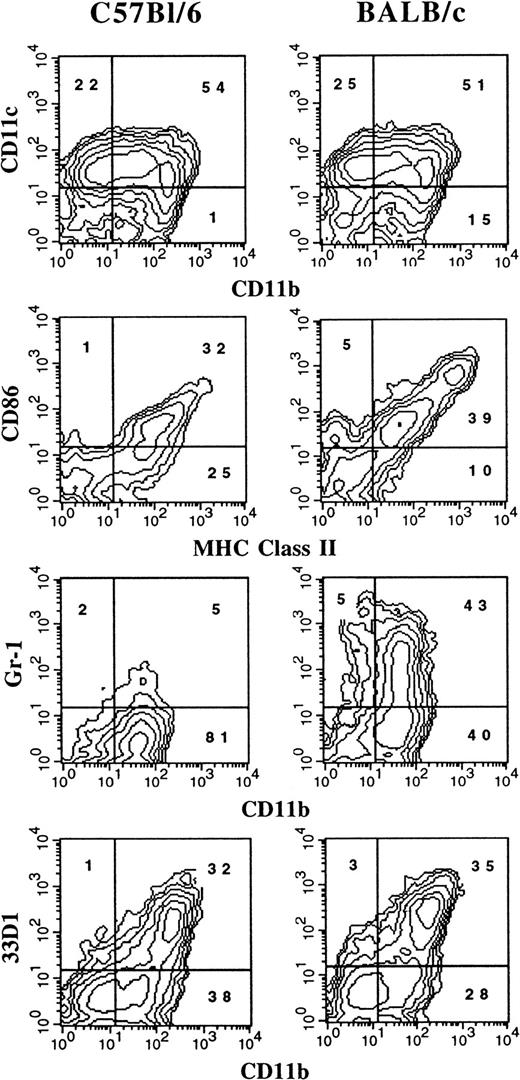
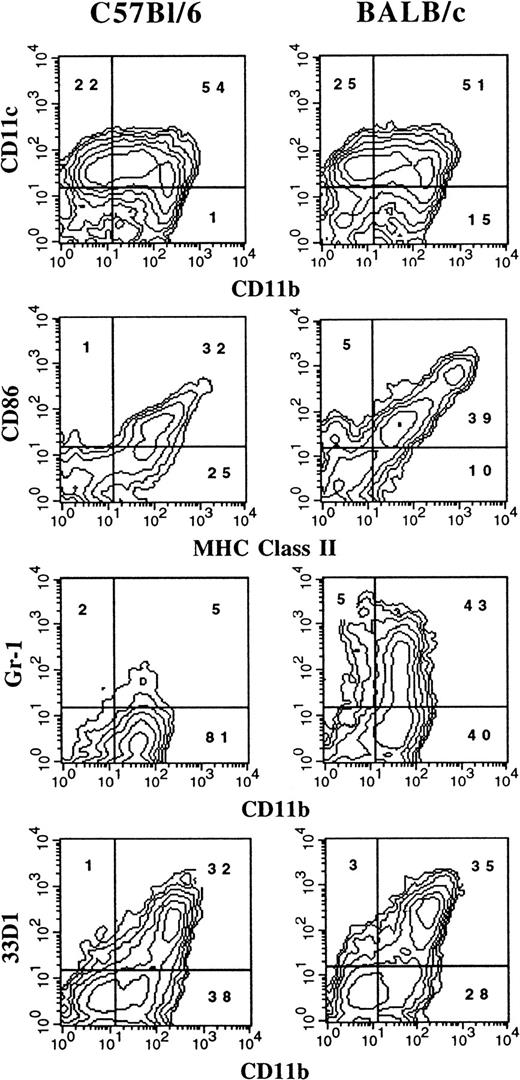
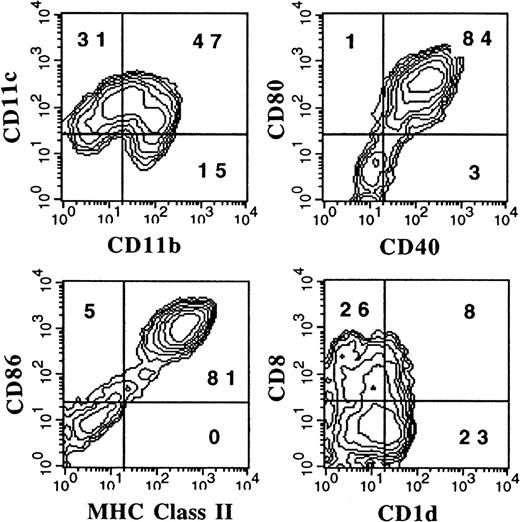
It has previously been reported that the administration of FL to mice generates large numbers of DCs in vivo.3 We were interested in determining whether culture conditions could be established in which FL, as a single factor, could induce DC development in vitro. Murine BM cells were cultured at high density (1 × 106 cells per milliliter) in the presence of 100 ng/mL FL. After 9 days, cultures contained small lymphoid-sized cells that grew in clusters associated with adherent macrophagelike cells. Cells derived from the FL-supplemented cultures of BM cells from BALB/c or C57BL/6 mice expressed variable levels of CD11b, CD11c, 33D1, CD86, MHC class II, and Gr-1 (Figure 1). However, fewer than 1% of the cells were positive for CD19 (B cells), CD3ε (T cells), NK1.1 (NK cells), or TER-119 (erythroid cells) on the basis of flow cytometric analysis (data not shown). The absence of lineage-specific, cell-surface antigens for T, B, NK, and erythroid cells, and expression of CD11c and 33D1, as well as low levels of MHC class II and CD86, were suggestive of immature DC-lineage committed cells. We detected 2 populations of DCs defined by CD11b expression. One population expressed high levels of CD11b (CD11bbright), while the other population expressed little to no CD11b (CD11bdull). Most of the cells from FL-supplemented BM cultures were positive for CD11c (76%), with approximately 50% expressing CD86 or MHC class II. Expression of 33D1, a marker of marginal zone DCs,21 was restricted to the CD11bbright population. Gr-1, a marker of granulocytes, was expressed on half of the CD11b+ FL-derived cells from BALB/c cultures, but on only a low percentage (7%) of cells from C57BL/6 cultures. In mice administered FL for 9 days at 10 μg per mouse per day, we found that splenic-derived CD11c+ DC from C57BL/6 and BALB/c mice were, respectively, 14% and 25% positive for Gr-1 (data not shown). Thus, cells generated from FL-supplemented BM cultures from both C57BL/6 and BALB/c mice generate populations of cells that are phenotypically similar to DC subsets previously identified in lymphoid tissues of normal and FL-treated mice.3 15
Flow cytometric analysis of cells generated in FL-supplemented BM.
BM cells from cultures from C57BL/6 or BALB/c mice were cultured at 1 × 106 cells per milliliter in FL for 9 days (see “Materials and methods”). Gates were determined with the use of isotype controls. Analyses were performed a minimum of 3 times for each marker.
Flow cytometric analysis of cells generated in FL-supplemented BM.
BM cells from cultures from C57BL/6 or BALB/c mice were cultured at 1 × 106 cells per milliliter in FL for 9 days (see “Materials and methods”). Gates were determined with the use of isotype controls. Analyses were performed a minimum of 3 times for each marker.
FL, but not SCF, supports in vitro DC development
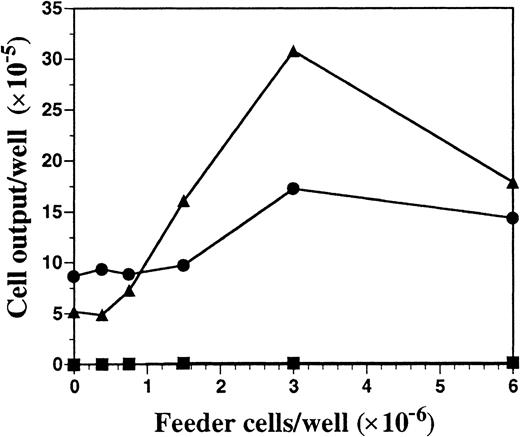
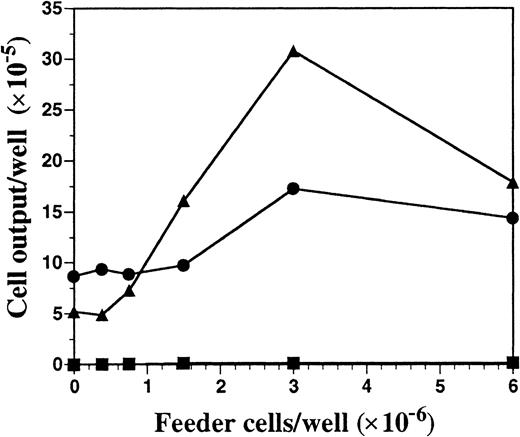
Unfractionated BM cells generated DCs in cultures only when the seeding density exceeded 5 × 105/mL (data not shown). Therefore, we investigated the role of endogenously produced soluble factor(s) in the generation of DCs by using a transwell system that allows for the selective diffusion of soluble factors. BM cells at low cell density (2 × 105 cells per milliliter in lower chamber) were cultured with increasing numbers of BM cells (upper chamber) in the presence or absence of FL or SCF, the ligand for c-kit that shares activities and structural homology to FL.22 Regardless of the BM feeder-cell density in the upper chamber, cells cultured at low cell density in the lower chamber did not survive in the absence of exogenous cytokines (Figure2). Inclusion of SCF in the cultures resulted in a 2-fold expansion of cells in the lower chamber, which was further increased when feeder cells were included in the upper chamber. However, fewer than 4% of the cells from the lower chamber, generated in the SCF-supplemented cultures, expressed CD11c or MHC class II, whereas 87% of the cells expressed high levels of Gr-1 and exhibited the morphology of immature granulocytes (data not shown). BM cells cultured at low density in FL-containing medium generated few cells when the BM feeder cells were excluded (25% of the input number). However, there was a linear dose response in the generation of cells in the lower chamber when increasing numbers of BM feeder cells were included in the top chamber, demonstrating the importance of cell density for the production of soluble factors (Figure 2). Cells harvested from the lower chambers under these conditions expressed CD11c (85%) and MHC class II (70%) similarly to what was shown in Figure 1. Thus, feeder cell-derived soluble factor(s) can support DC development in vitro when FL is the sole growth factor added exogenously.
Cell output from FL versus SCF-supplemented low–cell-density BM cultures in the presence of titrated BM cells with the use of transwell plates.
BM cells at low density (2 × 105 cells per milliliter) were cultured in the lower chambers of transwell plates, separated by a 0.4-μm filter from the titrated feeder cells in the upper chambers. Cells in the upper and lower chambers were cultured in either culture medium alone (▪), 100 ng/mL of FL (▴), or 100 ng/mL SCF (●) for 10 days and then harvested from the lower chamber, counted, and analyzed for expression of cell-surface antigens by flow cytometry. Results shown are representative of 3 experiments.
Cell output from FL versus SCF-supplemented low–cell-density BM cultures in the presence of titrated BM cells with the use of transwell plates.
BM cells at low density (2 × 105 cells per milliliter) were cultured in the lower chambers of transwell plates, separated by a 0.4-μm filter from the titrated feeder cells in the upper chambers. Cells in the upper and lower chambers were cultured in either culture medium alone (▪), 100 ng/mL of FL (▴), or 100 ng/mL SCF (●) for 10 days and then harvested from the lower chamber, counted, and analyzed for expression of cell-surface antigens by flow cytometry. Results shown are representative of 3 experiments.
Role of endogenous IL-6 in DC development from FL-supplemented BM cultures
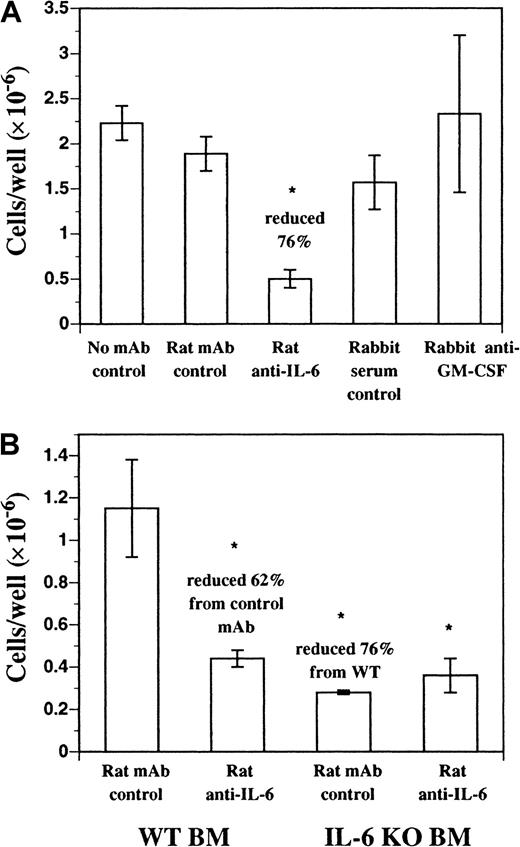
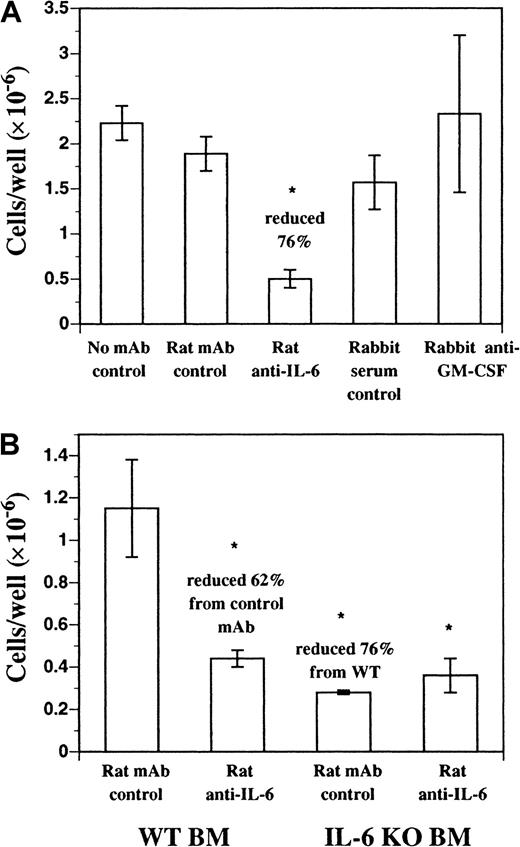
We investigated the role of endogenous soluble factors in DC development in FL-supplemented cultures using neutralizing mAbs. Neutralizing mAbs against IL-2, IL-3, IL-4, IL-7, IL-11, IL-15, granulocyte colony-stimulating factor (G-CSF), CSF-1, and transforming growth factor (TGF)–β1-3 failed to inhibit DC development in vitro from FL-supplemented BM cultures (data not shown). Neutralizing mAbs against murine IL-6 inhibited approximately 70% to 80% of the DC development during 9 days of culture (Figure3A). A similar effect was seen with neutralizing mAbs against the murine IL-6 receptor (IL-6R) and gp130, both components of the IL-6 receptor complex (data not shown). In addition, FL-supplemented cultures of BM cells from IL-6 gene KO mice produced fewer cells, compared with normal BM, and as expected were not affected by the inclusion of the neutralizing anti–IL-6 mAb (Figure3B). Residual cells harvested from FL-supplemented cultures of BM from IL-6 gene KO mice and control cultures where anti–IL-6 mAb was included still expressed CD11b, CD11c, CD86, and MHC class II similarly to control cultures (data not shown). BM cells cultured in recombinant murine IL-6 alone did not generate DCs (data not shown). Finally, polyclonal antibody against murine GM-CSF did not affect DC development in this culture system (Figure 3A). Thus, IL-6, but not GM-CSF, is an important growth factor for DC development from FL-supplemented BM cultures.
DC development from BM cells cultured in FL.
DC development from BM cells cultured in FL requires endogenous IL-6, but not GM-CSF. (A) C57BL/6 BM cells were cultured in FL plus anti–mIL-6 (murine IL-6) mAb, anti–mGM-CSF mAb, or control antibodies for 9 days as described in “Materials and methods.” Replicate wells (n = 3) were harvested and scored for cellularity. (B) C57BL/6 or IL-6 gene KO BM cells were cultured in FL plus anti–mIL-6 or control mAb for 9 days as described in “Materials and methods.” Replicate wells (n = 3) were harvested and scored for cellularity and results are presented as mean ± SD. Each experiment was performed a minimum of 3 times. *Significantly different from normal BM cells cultured in FL plus rat mAb isotype control (P < .05).
DC development from BM cells cultured in FL.
DC development from BM cells cultured in FL requires endogenous IL-6, but not GM-CSF. (A) C57BL/6 BM cells were cultured in FL plus anti–mIL-6 (murine IL-6) mAb, anti–mGM-CSF mAb, or control antibodies for 9 days as described in “Materials and methods.” Replicate wells (n = 3) were harvested and scored for cellularity. (B) C57BL/6 or IL-6 gene KO BM cells were cultured in FL plus anti–mIL-6 or control mAb for 9 days as described in “Materials and methods.” Replicate wells (n = 3) were harvested and scored for cellularity and results are presented as mean ± SD. Each experiment was performed a minimum of 3 times. *Significantly different from normal BM cells cultured in FL plus rat mAb isotype control (P < .05).
Kinetics of DC development in FL-supplemented BM cultures
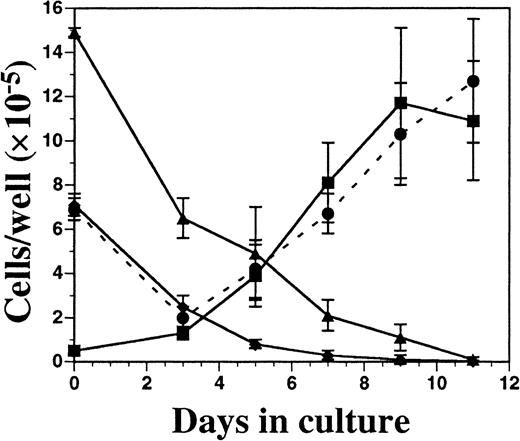
Cells from FL-supplemented BM cultures were harvested periodically over an 11-day period, counted, and analyzed for cell-surface phenotype. Although the total cellularity was fairly constant over time (1 to 3 × 106 cells per well), there was a considerable change in the relative proportion of monocytic-myeloid and B-lymphoid cells, as assessed by flow cytometry (Figure4). The number of cells expressing CD19 (B cells) and Gr-1 (primarily granulocytes) declined over time, while there was a concomitant increase in cells expressing CD11c and MHC class II. After 9 days of culture, there were no detectable erythroid cells (TER-119+), T cells (CD3ε+), or NK cells (NK1.1+) present (data not shown). Significant numbers of CD11c+ cells were not present until 5 to 7 days of culture, and CD11c expression closely correlated with development of MHC class II expression.
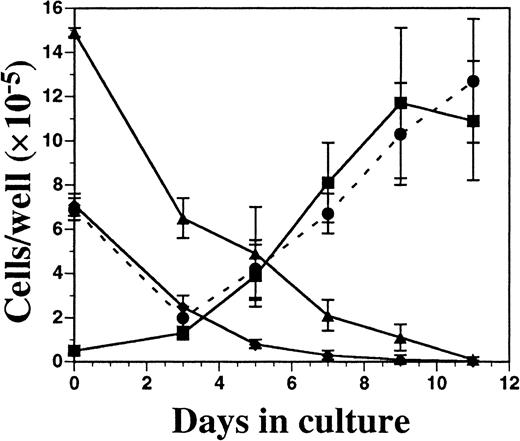
Kinetics of DC development from FL-supplemented C57BL/6 BM cultures.
BM cells were cultured in FL at 1 × 106 cells per milliliter and, on days 3, 5, 7, 9, and 11 of culture, were harvested for enumeration of cells and analyzed for the expression of Gr-1 (▴), CD19 (♦), CD11c (▪), and MHC class II (●). Results are presented as the mean ± SD from 3 separate experiments. Day 0 data were generated from freshly isolated BM cells. Gates for flow cytometric analysis were determined with the use of isotype controls as described in “Materials and methods.” Analysis was performed a minimum of 3 times for each cell-surface marker.
Kinetics of DC development from FL-supplemented C57BL/6 BM cultures.
BM cells were cultured in FL at 1 × 106 cells per milliliter and, on days 3, 5, 7, 9, and 11 of culture, were harvested for enumeration of cells and analyzed for the expression of Gr-1 (▴), CD19 (♦), CD11c (▪), and MHC class II (●). Results are presented as the mean ± SD from 3 separate experiments. Day 0 data were generated from freshly isolated BM cells. Gates for flow cytometric analysis were determined with the use of isotype controls as described in “Materials and methods.” Analysis was performed a minimum of 3 times for each cell-surface marker.
Activation of FL-derived DCs
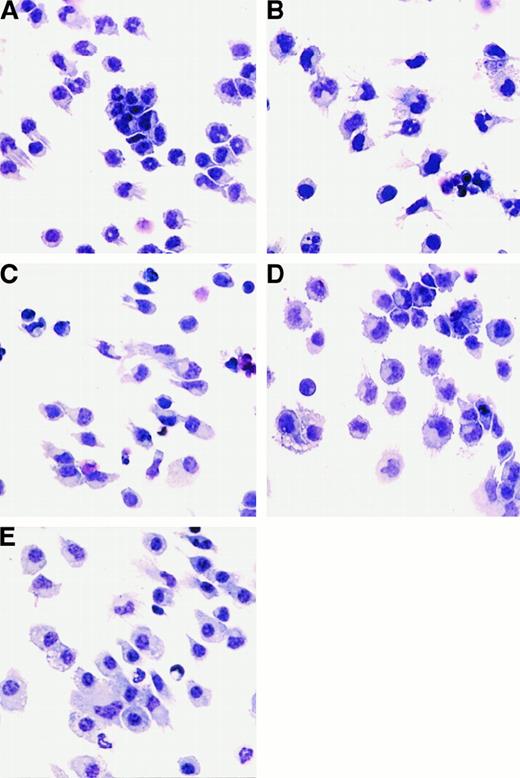
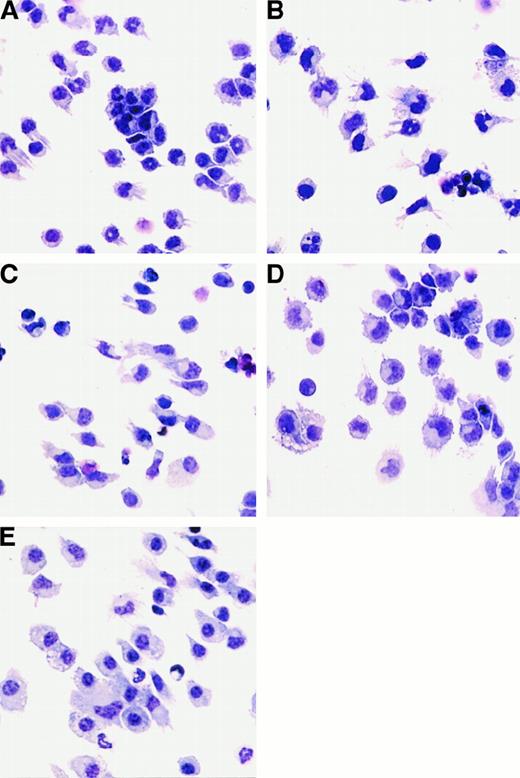
Since the DCs generated from FL-supplemented cultures expressed low levels of DC-associated molecules (Figure 1), we concluded that DCs with an immature phenotype were being generated. We investigated whether these cells could be activated in vitro with cytokines or LPS. DCs generated from FL-supplemented BM cultures were treated with 1 μg/mL E coli–derived LPS, 1000 U/mL IFN-α, or 10 ng/mL GM-CSF to assess morphological and phenotypic changes associated with activation. LPS, IFN-α, or GM-CSF was added to FL-supplemented DC cultures during the final 24 hours of culture (day 8). There was no statistically significant change in cellularity in the cultures after 24 hours of stimulation with LPS, IFN-α, or GM-CSF (data not shown). Cultures supplemented with FL alone contained small, lymphoid-sized cells, growing as single-cell suspensions, although some cells grew in small clusters (fewer than 20 cells per cluster). Some of the cells also possessed short dendrites (Figure5A). Cells from LPS-treated cultures were larger; formed clusters (more than 50 cells per cluster) that adhered to the plastic culture dishes; and when separated by gentle pipetting, exhibited long dendrites (Figure 5B). Cells from IFN-α–treated cultures also increased in size and had extended dendrites, but did not form large adherent clusters (Figure 5C). FL-derived DCs treated with GM-CSF for 24 hours formed large clusters and increased in size, but few cells possessed long dendrites (Figure 5D). In addition, for comparative purposes, we generated DCs in cultures supplemented with GM-CSF and IL-4 as described.16 BM cells cultured for 7 days in medium supplemented with 20 ng/mL GM-CSF and 20 ng/mL IL-4 consisted primarily of adherent giant-multinucleated cells and macrophagelike cells, as well as nonadherent cells consisting of monocytes and cells with dendriform bodies. Only those cells in the nonadherent fraction were used to compare with FL-derived DCs (Figure 5E).
Morphology of cells generated from FL-supplemented BM cultures.
Cells were harvested and cytospins prepared. Cytospun cells were photographed at 400 × magnification. BM cells from C57BL/6 mice were cultured in FL for 9 days alone (A) or stimulated for the final 24 hours of culture with the addition of LPS (B), IFN-α (C), or GM-CSF (D). (E) “Myeloid” DCs cultured in GM-CSF and IL-4 for 7 days.
Morphology of cells generated from FL-supplemented BM cultures.
Cells were harvested and cytospins prepared. Cytospun cells were photographed at 400 × magnification. BM cells from C57BL/6 mice were cultured in FL for 9 days alone (A) or stimulated for the final 24 hours of culture with the addition of LPS (B), IFN-α (C), or GM-CSF (D). (E) “Myeloid” DCs cultured in GM-CSF and IL-4 for 7 days.
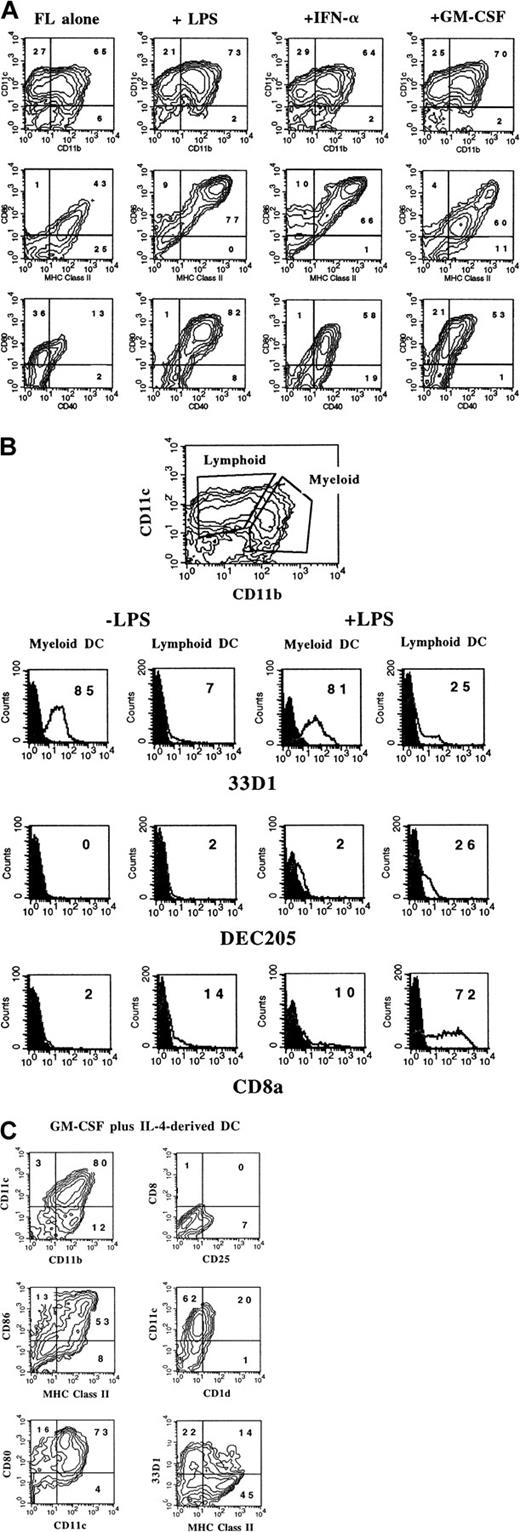
The cell-surface phenotype of activated DCs from FL-supplemented cultures was assessed by flow cytometry. There was little change in expression of CD11b or CD11c after 24-hour treatment of cultures with LPS, IFN-α, or GM-CSF; however, there were notable differences in the expression of other cell-surface antigens. DCs generated in FL alone did not express appreciable levels of CD25 (IL-2Rα), DEC205, CD1d, or CD8α and expressed relatively low levels of CD40, MHC class II, CD86, and CD80 (Table 1; Figure6A-B). LPS treatment resulted in the up-regulation of CD25, CD40, DEC205, MHC class II, CD86, CD80, CD1d, and CD8α (Table 1; Figure 6A-B). CD8α and DEC205 were expressed primarily by the CD11bdull population (Figure 6B). In contrast, expression of c-fms (CD115), the receptor for CSF-1, decreased after LPS treatment (Table 1). Like LPS, IFN-α increased the expression of CD25, CD40, DEC205, MHC class II, CD86, CD80, CD1d, and CD8α and decreased expression of c-fms. Surprisingly, although overnight treatment with GM-CSF resulted in up-regulation of CD40 and CD80, there was only a moderate increase in DEC205, MHC class II, CD86, and CD1d (Table 1; Figure 6A). In addition, unlike LPS or IFN-α, GM-CSF induced no increase of CD8α cell-surface expression, yet c-fmsexpression increased (Table 1). Expression of 33D1 was restricted to the myeloid DC subset, and there was little change in expression after stimulation with LPS, IFN-α, and GM-CSF (Figure 6B and data not shown). Interestingly, many DCs from unactivated cultures (FL alone) constitutively coexpressed c-kit, as well as Sca-1 (stem cell antigen/Ly6A/E), and the expression of both cell-surface antigens increased upon activation with LPS or IFN-α (Table 1). Both c-kit and Sca-1 are cell-surface antigens typically used in murine stem-cell purification protocols.23-25
Flow cytometric analysis of activated BM-derived DCs.
(A) FL-supplemented BM cells from C57BL/6 mice were cultured for 9 days and stimulated with LPS, IFN-α, or GM-CSF during the last 24 hours of culture. (B) Three-color analysis of FL-derived (± LPS) DCs with CD11b and CD11c used to define myeloid- and lymphoid-type DCs. (C) DCs generated in BM cultures supplemented with GM-CSF and IL-4 for 7 days. Gates were determined with the use of isotype controls as described in “Materials and methods.” Results shown are representative of more than 5 experiments.
Flow cytometric analysis of activated BM-derived DCs.
(A) FL-supplemented BM cells from C57BL/6 mice were cultured for 9 days and stimulated with LPS, IFN-α, or GM-CSF during the last 24 hours of culture. (B) Three-color analysis of FL-derived (± LPS) DCs with CD11b and CD11c used to define myeloid- and lymphoid-type DCs. (C) DCs generated in BM cultures supplemented with GM-CSF and IL-4 for 7 days. Gates were determined with the use of isotype controls as described in “Materials and methods.” Results shown are representative of more than 5 experiments.
For comparative purposes, we generated DCs using BM cultures supplemented with GM-CSF plus IL-4 as previously described.16 Flow cytometric analysis showed that most of the cells constitutively expressed high levels of CD11b, CD11c, CD80, CD86, and MHC class II (Figure 6C). Some of the cells from these cultures also expressed CD1d, 33D1, CD25, CD40, DEC205, and Sca-1, but not c-kit or CD8α (Figure 6C; Table 1). The phenotype of DCs derived from GM-CSF plus IL-4 did not change significantly after a 24-hour stimulation with LPS, IFN-α, or TNF-α as previously reported (Labeur et al13 and data not shown). Thus, unlike DCs generated in GM-CSF plus IL-4, cells generated in FL-supplemented BM cultures, when activated with LPS or IFN-α overnight, express a phenotype similar to both lymphoid (CD11bdull/CD8α+) DCs and myeloid (CD11bbright/CD8α−) DCs found in lymphoid tissues.
DCs can be generated from flt3+, lineage-depleted BM cell progenitors
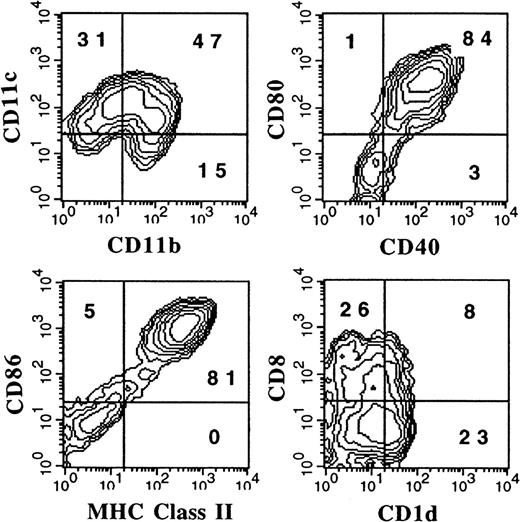
To address the question of whether DCs generated from FL-supplemented BM cultures arise from pre-existing DCs or progenitor cells, the following experiment was performed. Cells from the BM expressing flt3, a receptor found on lymphohematopoietic progenitor cells,22 and lacking a number of lineage-specific markers (CD3ε, CD11b, CD11c, CD122, B220, Gr-1, NK1.1, TER119, F4-80, and Ly6c) were purified. This flt3+, lineage-depleted population represented fewer than 0.3% of whole BM. Sorted cells were cultured in FL plus conditioned medium from cultured spleen cells, which has previously been described to contain an IL-6–like activity and could promote murine DC development in vitro.18 After 8 days of culture, the cells were stimulated with LPS and harvested 24 hours later. Addition of LPS was used to up-regulate DC-associated markers as shown in Figures 6A and 6B. Flow cytometric analysis demonstrated 2 discrete populations as determined by levels of CD11b and CD11c (Figure 7). Similarly to DCs generated from whole BM cultured in FL and stimulated with LPS, most cells expressed high levels of MHC class II, CD80, CD86, and CD40 (Figures 6A and 7). CD8α and 33D1 were expressed primarily by the CD11bdull and CD11bbright populations, respectively (data not shown). Thus, both myeloid- and lymphoid-type DCs, as assessed by phenotype, could be generated from hematopoietic progenitors by means of FL and the conditioned medium from spleen cells.
Flow cytometric analysis of DCs derived from flt3+/lineage− cells.
Flt3+/lineage− cells were sorted from BM and cultured for 9 days in 200 ng/mL FL plus conditioned media from spleen cells. LPS at 1 μg/mL was added during the last 24 hours to induce maturation. Gates were determined with the use of isotype controls as described in “Materials and methods.” Results shown are representative of 5 experiments.
Flow cytometric analysis of DCs derived from flt3+/lineage− cells.
Flt3+/lineage− cells were sorted from BM and cultured for 9 days in 200 ng/mL FL plus conditioned media from spleen cells. LPS at 1 μg/mL was added during the last 24 hours to induce maturation. Gates were determined with the use of isotype controls as described in “Materials and methods.” Results shown are representative of 5 experiments.
FL-derived DCs stimulate allogeneic T-cell proliferation
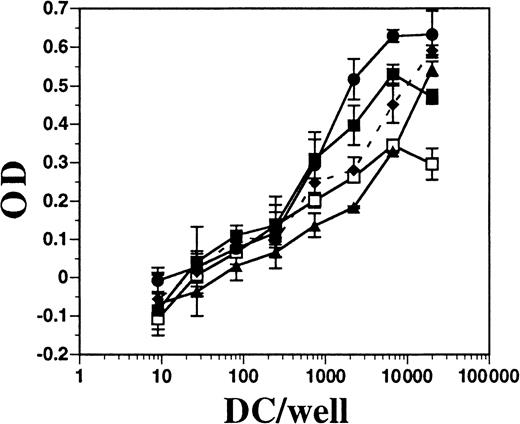
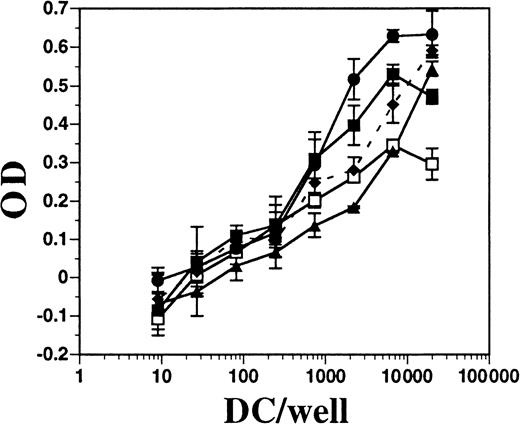
DCs from BM cultures supplemented with FL or with GM-CSF plus IL-4, as well as DCs derived from the spleens of mice treated with FL for 10 days, were assayed for allo-stimulatory activity in an MLR assay. Each population of DCs was approximately 75% to 80% positive for CD11c and contained fewer than 1% contaminating T cells (data not shown). All populations of DCs had potent allo-stimulatory activity, although those generated in vitro with FL alone had the least (Figure8). BM cells cultured in FL and stimulated for 24 hours with IFN-α or LPS had enhanced allo-stimulatory capacity over those in FL alone (approximately 10-fold, at 1000 DCs per well). DCs generated in BM cultures supplemented with GM-CSF plus IL-4 were comparable in activity to DCs derived from cultures supplemented with FL and activated with LPS. Splenic-derived DCs consistently exhibited less activity than in vitro–derived DCs. Thus, FL-derived DCs activated with IFN-α or LPS, which induced up-regulation of MHC class II and costimulatory molecules, resulted in potent allo-stimulatory activity to induce T-cell proliferation in an MLR assay.
Comparison of allo-stimulatory activity of FL-derived and GM-CSF plus IL-4–derived DCs.
DCs were enriched from spleens of mice treated with FL and generated from the BM of C57BL/6 mice cultured in GM-CSF plus IL-4 or in FL, as described in “Materials and methods.” Splenic-derived DCs from mice treated with FL for 10 days (■) and BM-derived DCs from cultures using GM-CSF and IL-4 (●), FL alone (▴), FL plus IFN-α (♦ and dashes), or FL plus LPS (▪) were cultured at various numbers in the presence of a constant number (1 × 105 per well) of T cells from DBA/2 mice for 4 days. Alamar blue was added for another 24 hours before measuring optical density (OD). DBA/2 T cells alone gave a mean OD of −0.044 ± 0.031. Background OD from DCs alone was subtracted from DCs with T cells.
Comparison of allo-stimulatory activity of FL-derived and GM-CSF plus IL-4–derived DCs.
DCs were enriched from spleens of mice treated with FL and generated from the BM of C57BL/6 mice cultured in GM-CSF plus IL-4 or in FL, as described in “Materials and methods.” Splenic-derived DCs from mice treated with FL for 10 days (■) and BM-derived DCs from cultures using GM-CSF and IL-4 (●), FL alone (▴), FL plus IFN-α (♦ and dashes), or FL plus LPS (▪) were cultured at various numbers in the presence of a constant number (1 × 105 per well) of T cells from DBA/2 mice for 4 days. Alamar blue was added for another 24 hours before measuring optical density (OD). DBA/2 T cells alone gave a mean OD of −0.044 ± 0.031. Background OD from DCs alone was subtracted from DCs with T cells.
Processing and presentation of OVA protein to OVA-specific CD4 T cells
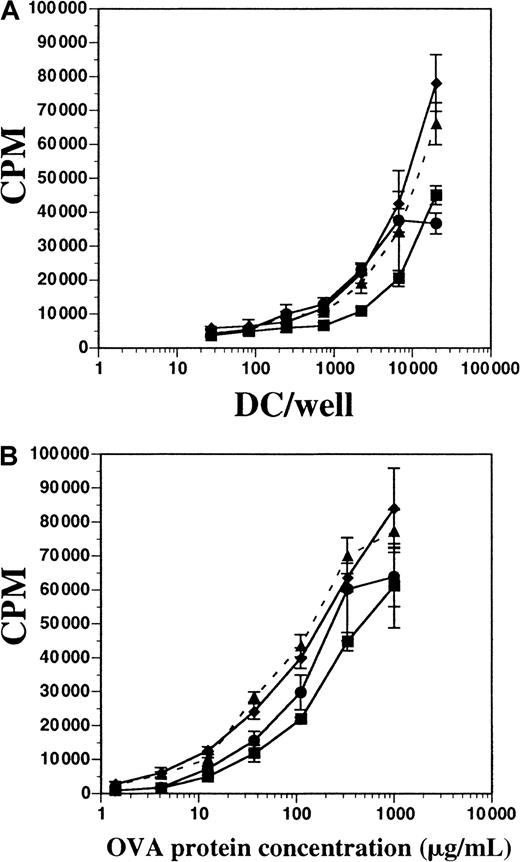
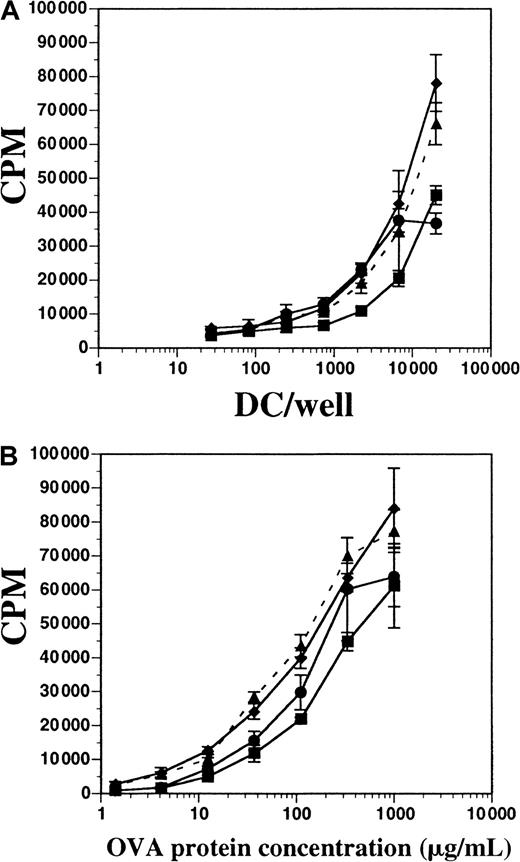
We tested the capacity of both FL-derived and GM-CSF plus IL-4–derived DCs to process OVA protein and present it to OVA-specific CD4+ T cells in vitro. T cells from D011.10 transgenic mice express a TCRαβ specific for the OVA peptide fragment 323-339 presented on I-Ad MHC class II molecules.20OVA-specific CD4+ T cells were enriched from spleens and LNs of D011.10 mice and then cultured either with varying numbers of DCs in a constant concentration of OVA protein or with constant numbers of DCs with titrated OVA protein; proliferation was measured after 5 days. DCs were derived from in vitro cultures containing FL (± LPS) or GM-CSF plus IL-4, as well as cultures enriched from the spleens of mice treated with FL for 10 days. All populations of DCs were approximately 72% to 76% positive for CD11c and contained fewer than 1% contaminating T cells (data not shown). In terms of inducing T-cell proliferation, DCs derived in vitro with FL and stimulated with LPS during the final 8 hours of culture demonstrated activity similar to DCs from cultures supplemented with FL alone; this similarity occurred both when DCs were titrated and OVA protein remained constant (300 μg/mL) and when OVA protein was titrated and APCs remained constant (Figure 9A-B). FL-derived DCs stimulated with IFN-α for 8 hours were also used and found to have activity similar to those stimulated with LPS (data not shown). When OVA protein was held constant, and APCs were titrated, DCs generated in vitro with the use of GM-CSF plus IL-4 demonstrated activity similar to FL-derived DCs. Additionally, those DCs generated in GM-CSF plus IL-4 were less stimulatory to T cells than FL-derived DCs when DCs were held constant and the OVA protein was titrated (Figure 9B). Splenic-derived DCs enriched from the spleens of mice treated with FL for 10 days were also used for comparison, but found to be consistently less stimulatory to T-cell proliferation when compared with the in vitro–derived DCs. These data demonstrate that DCs generated in vitro with FL or FL plus IFN-α are competent at processing and presenting OVA protein to OVA peptide–specific CD4+ T cells, and were comparable to those DCs generated in cultures supplemented with GM-CSF and IL-4.
Comparison of antigen processing and presentation by DCs generated from FL-supplemented or GM-CSF plus IL-4–supplemented BM cultures.
(A) Splenic-derived DCs from mice treated with FL for 10 days (▪) and BM-derived DCs from cultures using GM-CSF and IL-4 (●), FL alone (▴ and dashes), or FL plus LPS (♦) were cultured with constant OVA protein (300 μg/mL) and 1 × 105 cells per well OVA-specific D011.10 T cells. (B) DC and T cells were cultured at constant concentrations (2 × 104 DCs per well), and OVA protein was titrated. T-cell proliferation was measured on day 5 for both assays. Background counts from OVA-specific T cells cultured without DCs in OVA protein (300 μg/mL) were fewer than 4000 cpm, and T cells without OVA protein and with DCs (2 × 104 DCs per well) were fewer than 1000 cpm. Background counts from DCs alone were fewer than 1000 cpm. Data represented are the mean ± SD of triplicate wells, and the experiement was performed 5 times.
Comparison of antigen processing and presentation by DCs generated from FL-supplemented or GM-CSF plus IL-4–supplemented BM cultures.
(A) Splenic-derived DCs from mice treated with FL for 10 days (▪) and BM-derived DCs from cultures using GM-CSF and IL-4 (●), FL alone (▴ and dashes), or FL plus LPS (♦) were cultured with constant OVA protein (300 μg/mL) and 1 × 105 cells per well OVA-specific D011.10 T cells. (B) DC and T cells were cultured at constant concentrations (2 × 104 DCs per well), and OVA protein was titrated. T-cell proliferation was measured on day 5 for both assays. Background counts from OVA-specific T cells cultured without DCs in OVA protein (300 μg/mL) were fewer than 4000 cpm, and T cells without OVA protein and with DCs (2 × 104 DCs per well) were fewer than 1000 cpm. Background counts from DCs alone were fewer than 1000 cpm. Data represented are the mean ± SD of triplicate wells, and the experiement was performed 5 times.
IL-12 production is restricted to the lymphoid-type DCs
Among the most profound functional differences between myeloid- and lymphoid-type DCs is IL-12 production, which is limited to the lymphoid subset.26-28 We asked whether sorted DCs generated in vitro from FL-supplemented cultures could produce IL-12 p70 and, if so, whether it would be restricted to the CD11bdull lymphoid DC subset. DC subsets sorted with the use of the gates as shown in Figure 6B were cultured for 40 hours in the presence of SAC (Pansorbin), GM-CSF, and IFN-γ. Lymphoid-type DCs generated from FL-supplemented BM cultures made 10-fold more IL-12 p70 than their myeloid DC counterparts (Table2). No detectable IL-12 p70 was produced from unstimulated DCs (data not shown).
Discussion
Here we describe a novel culture system to generate murine DCs with cell-surface antigen expression and functional phenotype similar to those of DCs residing in lymphoid tissues. This culture system requires the addition of a single growth factor, FL, whereas a structurally and functionally related growth factor, SCF, promotes the generation of immature granulocytes. Maximal DC numbers are achieved after 9 to 10 days of culture, which is similar to the kinetics of FL-induced DC expansion in vivo.3 The cell-density dependence of this culture system suggests the involvement of endogenous factor(s) as well. Rasko et al29 have demonstrated the cell-density dependence of murine BM cells in forming colonies in response to FL alone, which suggests the presence of endogenous factors. Addition of neutralizing mAb to IL-2, IL-3, IL-4, IL-7, IL-11, IL-15, CSF-1, G-CSF, GM-CSF, or TGF-β1-3 to the FL-supplemented BM cultures does not inhibit DC development, whereas neutralizing anti–IL-6 mAb significantly decreases DC yields, and BM cultures from IL-6 gene KO mice generate fewer DCs than the wild-type cultures. Additionally, neutralizing mAbs to IL-6R and gp130 are inhibitory (data not shown). Interestingly, residual cells harvested from cultures where neutralizing anti–IL-6 or IL-6R mAb were included had similar cell-surface marker expression for CD11b, CD11c, CD86, and MHC class II (data not shown). This suggests that IL-6 acts as a DC-proliferative factor and is not necessary for DC differentiation in this in vitro culture system. These findings are consistent with results from previous studies demonstrating a role for IL-6 in DC development. Human CD34+ progenitors driven toward DC development with GM-CSF, SCF, and TNF-α produce IL-6 in vitro and require IL-6 for optimal DC expansion.30 In addition, a recently described culture system for the generation of murine DCs from cultured spleen cells also exhibited an IL-6–like activity.18 More recent data from our laboratory suggest that the IL-6 activity comes from the lineage-negative population in BM (data not shown). This would be consistent with a previous report demonstrating lineage-negative progenitor cells from murine BM cells as a source of IL-6.31 The mechanisms by which IL-6 and perhaps other gp130-stimulating growth factors, such as IL-11, LIF, and oncostatin-M, affect DC development in vivo remains to be determined.
GM-CSF has the capacity to induce both DC development from precursor cells and activation of DCs in vitro and in vivo.5,6,11,32-43 In the present study, GM-CSF was not required for DC generation from mouse BM in vitro, which is similar to a previous report that anti–GM-CSF mAb does not inhibit DC generation from murine thymic precursors cultured in a cytokine cocktail (TNF-α, IL-1β, IL-7, SCF, and IL-3).14 Lymphoid tissues from GM-CSF gene KO mice have normal DC numbers8; however, these mice exhibit a defect in antigen-specific T-cell and B-cell activation at the level of the APCs, which could be rectified by an injection of exogenous GM-CSF with the immunogen.9 Taken together, this suggests that the role of GM-CSF in acquired immunity may lie at the level of DC activation, survival, and/or migration to lymphoid tissues, rather than DC development.
We have demonstrated that as little as 24 hours' exposure to GM-CSF caused these immature DCs to cluster and up-regulate expression of CD40 and CD80, but there was surprisingly little effect on MHC class II, CD86, and other DC-associated antigens. Optimal stimulation of FL-derived DCs with GM-CSF may require longer incubation periods or the addition of other proinflammatory cytokines. LPS and IFN-α had more potent effects, including increased expression of cell-surface antigens expressed on mature DCs from lymphoid tissues and important for DC function (ie, MHC class II, CD40, CD80, CD86, DEC205, and CD1d).3,13,26,35,36 Similar effects can be elicited in mice within 6 hours of LPS injection, and this is accompanied by DC migration from the marginal zones to the T-cell areas of spleen.37 IFN-α has also been described as a potent DC activator.38,39 IFN-α induced maturation of human DCs generated in GM-CSF, TNF-α, and IL-4 under serum-free conditions.39 Other cell-surface antigens, such as CD8α, CD25, Sca-1, and c-kit, whose function in DC biology is unclear, were also up-regulated by LPS and IFN-α by means of the FL-based culture system.
CD8α+ DCs are found in murine thymus, spleen, and peripheral LNs.15 We found that stimulation of immature DCs with LPS or IFN-α, but not GM-CSF, resulted in up-regulation of CD8α, and this is the first report of in vitro–derived murine DCs expressing this molecule. A recent report has described CD8α up-regulation on murine skin-derived Langerhan cells induced by culturing cells in GM-CSF plus CD40L.40 Surprisingly, though, these cells also expressed CD8β, which we did not find on the BM-derived DCs (data not shown). We also found that TNF-α induced potent DC maturation by up-regulating MHC class II, CD80, and CD86, but not CD8α (data not shown).
LPS and IFN-α down-regulated receptor c-fms expression. The down-regulation of this receptor for CSF-1 has been previously reported on human DCs.41,42 The results of Olweus et al42 suggest that CD34+/CD123bright human DC progenitors are derived from the granulomonocytic pathway since this population initially expresses c-fms and differentiates to CD1a+/c-fms− DCs after culture for 5 days in IL-3 plus GM-CSF.
The coexpression of both c-kit and Sca-1 following activation of DCs is a novel observation. Both of these cell-surface antigens have been previously described as being expressed on lymphoid tissue DC,15,32,43-47 but never together in the same report. Murine hematopoietic stem cells from C57BL/6 mice are described as lineage marker negative (such as B220, Gr-1, CD11b, CD3ε, and NK1.1) and positive for Sca-1 and c-kit.23-25We found that after stimulation using LPS or IFN-α, nearly all DCs expressed high levels of both Sca-1 and c-kit, and this included the CD11bdull lymphoid-type DC subset. Therefore, some activated DCs have the same phenotype as described for stem cells from C57BL/6 mice. As a result of this observation, we recommend that investigators purifying stem cells from C57BL/6 mice include DC-specific cell-surface antigens (CD11c and MHC class II) for complete lineage depletion.
We have also shown that, similarly to FL-treated mice, both lymphoid- and myeloid-type DCs, as defined by phenotype, are generated when BM is cultured in vitro with FL.3,26 Myeloid-type DCs are characterized as CD11bbright, CD11c+, 33D1+, and CD8α−, whereas lymphoid-type DCs are CD11bdull, CD11c+, 33D1−, and CD8α+. The 33D1 antigen, which was originally described as a marker of cells within the marginal zones,21 was expressed exclusively by the CD11bbright cells. Pulendran et al26 previously demonstrated that 33D1 was restricted to the myeloid-type DC subset that was increased in the spleens of mice after administration of FL. Conversely, we found that there was no expression of 33D1 on the CD11bdull subset, but some of this population did express CD8α and DEC205 after activation with LPS and IFN-α (Figure 6B, Table 1, and data not shown). In addition, both CD11bdull and CD11bbright subsets were generated from flt3+, lineage-depleted BM cells cultured in FL plus conditioned medium from cultured spleen cells.
One of the pressing issues in murine DC development is whether myeloid- and lymphoid-type DCs represent separate lineages. Numerous studies have addressed this issue by culturing progenitors in combinations of cytokines excluding GM-CSF, or by injecting defined progenitors directly into mice.14,43,45 However, more recent reports have indicated that thymic- and splenic-derived lymphoid-type DC development is not associated with T-cell development since Notch 1 KOs or mice deficient in both c-kit and IL-2Rγ chain have thymic DCs, but no T cells or detectable T-cell progenitors.48 49 We have not yet been able to demonstrate that only one type of DC could be generated in the above culture with the use of whole BM or highly purified progenitors, and in addition, we find both types are typically generated at ratio of 1:1. This suggests that lymphoid- and myeloid-type DC progenitors are at an equal frequency and require the same cytokines for development, or that development of a common progenitor into a lymphoid- or myeloid-type DC is a stochastic process.
In an MLR assay, DCs generated in vitro from BM supplemented with FL and stimulated with LPS had similar allo-stimulatory activity as those DCs generated in GM-CSF plus IL-4. In addition, DCs generated in FL plus IFN-α or LPS had activity comparable to DCs derived from GM-CSF plus IL-4 to stimulate OVA-specific T cells to proliferate. Surprisingly, DCs generated in vitro with FL alone were just as active as those DCs stimulated with IFN-α. This may be a result of the DCs becoming activated by the low levels of endotoxin that contaminate OVA protein (data not shown). However, until each DC subset from FL-supplemented BM cultures is separated, it is unclear as to the contribution of each to these function assays. Although we have found that FL-derived and GM-CSF–derived DCs demonstrate similar functional activity in the assays we employed, there were subtle phenotypic and morphological differences. Most notably, the DCs generated in GM-CSF plus IL-4 were larger, had a higher level of autoflouresence, were constitutively activated (surface expression of MHC class II, CD80, CD86, CD1d, and CD40), and lacked expression of c-kit and CD8α.
Finally, to address whether lymphoid-type DCs generated from FL-supplemented BM cultures represent those found in lymphoid tissues, we stimulated both DC subsets to produce IL-12, which has been described as being restricted to the lymphoid-type subset as defined by CD11b expression26 or CD8α expression.27,28Administration of LPS or soluble Toxoplasma gondiitachyzoite extract to mice results in production of IL-12 p40 by DCs found in T-cell areas of the spleen, and these cells coexpress CD11c, CD8α, and DEC205.27 We found that lymphoid-type DCs from BM cultures produced 10-fold more IL-12 p70 than their myeloid-type counterparts. In addition, these data suggest that the differences in IL-12 production by DCs is intrinsic to the subset and not a result of the microenvironment from which the DCs were derived. Surprisingly, the GM-CSF plus IL-4–derived DCs, which are considered to represent myeloid-type DCs, make IL-12 p70 (greater than 1000 pg/mL) in response to LPS alone (data not shown and Labeur et al13). However, it has not been demonstrated that these in vitro–derived myeloid-type DCs represent the myeloid-DC subset found in lymphoid tissues in vivo.
The culture system described in this report will be useful for our understanding of the development of DCs for their potential use as adjuvants both for infectious disease and in tumor biology.
Acknowledgments
We thank Gary Carlton, Daniel Hirschstein, and Steve Braddy for technical assistance and Anne Aumell and Drs Hilary McKenna, Stewart Lyman, and Douglas Williams for critical advice and review of this manuscript.
The authors are employees of Immunex Corporation.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Kenneth Brasel, Immunobiology Department, Immunex Corporation, 51 University St, Seattle, WA 98101; e-mail:brasel@immunex.com.