Abstract
Both in utero and postnatal hematopoietic stem cell (HSC) transplantation would benefit from the development of approaches that produce increased levels of engraftment or a reduction in the period of time required for reconstitution. We used the in utero model of human–sheep HSC transplantation to investigate ways of improving engraftment and differentiation of donor cells after transplantation. We hypothesized that providing a more suitable microenvironment in the form of human stromal cell progenitors simultaneously with the transplanted human HSC would result in higher rates of engraftment or differentiation of the human cells in this xenogeneic model. The results presented here demonstrate that the cotransplantation of both autologous and allogeneic human bone marrow-derived stromal cell progenitors resulted in an enhancement of long-term engraftment of human cells in the bone marrow of the chimeric animals and in earlier and higher levels of donor cells in circulation both during gestation and after birth. By using marked stromal cells, we have also demonstrated that injected stromal cells alone engraft and remain functional within the sheep hematopoietic microenvironment. Application of this method to clinical HSC transplantation could potentially lead to increased levels of long-term engraftment, a reduction in the time for hematopoietic reconstitution, and a means of delivery of foreign genes to the hematopoietic system.
Bone marrow transplantation (BMT) has been used successfully in the treatment of a wide variety of disorders including the storage diseases and other diseases of metabolism, diseases of immune function, and many hematologic and solid tumor malignancies. More recently, in utero hematopoietic stem cell (HSC) transplantation has emerged as an alternative for a limited number of diseases.1-4 However, although the successful application of this procedure could circumvent many of the limitations and risks currently associated with postnatal BMT in these diseases, in utero HSC transplantation, like postnatal BMT, has not yet achieved its full therapeutic potential. Thus, both in utero and postnatal BMT would benefit from the development of approaches that produce increased levels of engraftment or a reduction in the period of time required for reconstitution. It has been shown that the radiation exposure and standard- and high-dose therapies currently used in postnatal BMT induce important sequelae within the hematopoietic and stromal progenitor cell compartments.5-7 Likewise, the fetal microenvironment may not be fully conducive for the development of donor cells in the case of in utero HSC transplantation.8,9In the fetus, as in pediatric and adult recipients, donor HSC primarily engraft the bone marrow (BM).10 However, despite the presence of significant numbers of donor stem/progenitor cells in host marrow following in utero HSC transplantation, little or no mature donor-derived cells or their products are detected in the circulation until late in gestation, near birth.11,12 The mechanism(s) underlying this delay in the differentiation and appearance of donor cells into the peripheral blood (PB) is not known. It is possible that the onset of marrow hematopoiesis is associated with the development of a more permissive hematopoietic environment. In this regard, we have recently reported that cotransplantation of adult sheep stroma resulted in increased engraftment and early differentiation of donor cells in sheep fetuses.13 Based on this we investigated the possibility that the presence of a human adult microenvironment in the human–sheep xenograft model of human hematopoiesis may render the model more biologically relevant.14 The feasibility of engrafting marrow-derived progenitor stromal cells/mesenchymal cells after both in utero and postnatal transplantation has been reported by a number of authors.14-20 Pereira et al15reported the successful long-term engraftment of normal marrow stromal cells in several organs following intravenous infusion into mice exhibiting osteogenesis imperfecta. Nilsson et al16demonstrated that whole BM contains cells capable of engrafting nonablated mice and giving rise to competent osteoblasts and Horwitz et al20 demonstrated the transplantability and therapeutic effects of BM-derived marrow stromal cells (MSCs) in children with osteogenesis imperfecta. Also, the simultaneous injection of human stroma and human HSCs into bnx mice resulted in increased engraftment, but only when the stroma was transduced before transplant with a construct containing the human interleukin-3 (IL-3) complementary DNA (cDNA).17 A phase one trial has also been conducted in humans demonstrating that mesenchymal progenitor cells can be collected from BM aspirates, subjected to ex vivo expansion, and subsequently reinfused into patients with no adverse reactions.18 We have shown in vitro that although sheep stroma is able to support human hematopoiesis and induce multilineage differentiation of human progenitor cells, it does so at levels significantly lower than that of human stroma.21 For this reason, the human-sheep xenograft model of in utero HSC transplantation is ideal for testing the effects of the transplantation of stromal progenitor cells and the creation of a suitable microenvironment to which the human HSC can home, engraft, and proliferate/differentiate. In support of the suitability of this model to questions of microenvironmental influence on transplanted HSC, the in vivo administration of human cytokines such as IL-3, granulocyte/macrophage colony-stimulating factor (GM-CSF), or stem cell factor (SCF) to chimeric sheep resulted in an increased level of donor cell activity in chimeric animals.11 22 In the present studies we used the human–sheep xenograft model to determine the effect of transplanted human stromal progenitor cells on the engraftment and differentiation of human cells, in the hope of providing a more suitable microenvironment for the transplanted cells. To this end, we transplanted preimmune 55- to 60-day-old fetal sheep with purified adult human stem cells with or without autologous or allogeneic marrow stromal progenitor cells and examined donor HSC engraftment and differentiation from 43 days after transplantation until 3 years after birth.
The results presented here demonstrate that human stromal cells are able to engraft in preimmune fetal sheep and that cotransplantation of stroma results in not only a significant increase of donor hematopoietic activity in fetal circulation starting early in gestation but also in higher levels of human donor cells in BM at later time points after transplantation.
Materials and methods
Human donor cell preparation
Heparinized human BM was obtained from healthy donors after informed consent on experimental day 0 and 9. Low-density BM mononuclear cells were separated by a Ficoll-Hypaque density gradient (1.077g/mL) (Sigma, St Louis, MO) and washed twice in Iscove's modified Dulbecco's media (IMDM) (Gibco Laboratories, Grand Island, NY). BM mononuclear cells harvested at day 0 were used for preparation of the autologous and allogeneic stroma, whereas BM mononuclear cells collected at day 9 were sorted into CD34+, HLA-DR− or CD34+, Lin−, Thy+ cells.
Stromal cell preparation
Stromal layers were established from human BM mononuclear cells plated at a density of 20 × 106 cells per T75 flask (Costar, Cambridge, MA) in 10% fetal bovine serum (FBS) (Gibco), 10% horse serum (HS) (Gibco), and 10−6 mol/L hydrocortisone (Sigma) in IMDM. On day 9 of culture stromal layers were trypsinized, washed, and resuspended at the desired concentration for injection in a volume of 0.5 mL. Day 9 was chosen because at this time, stromal layers were found to contain the highest percentage of STRO-1+ cells, a finding in agreement with previous studies.23
Immunofluorescence staining for flow cytometric analysis and selection of cells by cell sorting
Sorting of the CD34+, HLA-DR− or CD34+, Lin−, Thy+ population was performed on a FACS Vantage after labeling with CD34 (8G12 FITC), HLA-DR (phycoerythrin [PE]) (Becton Dickinson Immunocytometry Systems [BDIS], San Jose, CA), or with CD34, CDw90 (5E10 fluorescein isothiocyanate [FITC]) (Pharmingen, San Diego, CA) and CD2, CD14, CD15, CD16, CD19 all FITC, as well as glycophorin A (AMAC, Inc, Westbrook, ME) to exclude Lin+ cells as previously described.24,25 Flow cytometric analysis of the cell populations was performed on a FACScan (Becton Dickinson). Monoclonal antibodies to various cluster designations directly conjugated with FITC or PE were used according to the manufacturer's recommendation. These included: CD45, CD14, CD34, CD20, CD33, CD3, CD7, CD56, CD44, CD10, CD4, CD8 (BDIS), glycophorin A (AMAC), and STRO-1.23
Creation of the human–sheep chimeras and assessment of engraftment
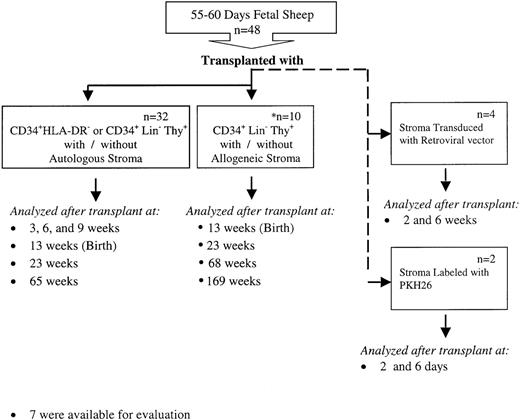
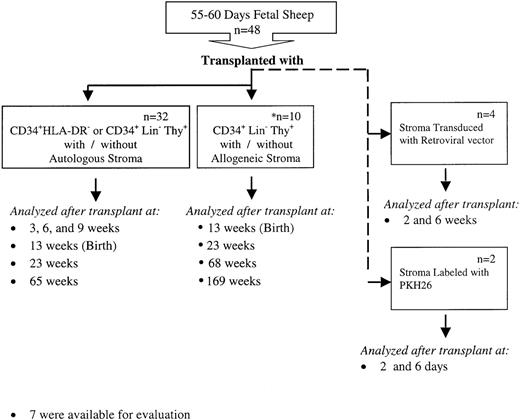
An overview of the experimental design is shown in Figure1. In autologous cotransplantation studies 32 fetal sheep were transplanted at 55 to 60 days of gestation following the procedure that has been described in detail previously.10-12 CD34+, Lin−, Thy+ (0.7-6 × 104 cells/fetus) or CD34+, HLA-DR−(6.5 × 104cells/fetus) with or without autologous stromal cells (5 × 104-7.5 × 105) were injected in l mL volume by intraperitoneal injection into fetal sheep 55 to 60 days old. The transplanted sheep were analyzed for donor (human) cell engraftment in BM, thymus, liver, spleen, and PB at 3, 6, and 9 weeks after transplantation (91, 112, and 133 days of gestation), and after birth at 1 week, 3 months, and 1 year of age (13, 23, and 65 weeks after transplantation). Of these 32 sheep, 15 were injected with human stroma and autologous human HSC with 1 of 2 phenotypes: CD34+, Lin−, Thy+ or CD34+, HLA-DR−. These populations were used because we have demonstrated in previous studies that both are equally capable of similar levels of engraftment and differentiation in our human–sheep xenograft model.24 25 Of the remaining 17 sheep, 12 were injected with human HSC alone with 1 of the 2 phenotypes described above. As a control, the remaining 5 sheep were injected with human stromal cells alone. Ten additional fetuses were transplanted with 105 CD34+, Lin−, Thy+ cells with or without 108 allogeneic stromal progenitor cells. In 7 animals analysis of human cell engraftment was performed at intervals after birth up to 3 years posttransplant (Figure 1).
Experimental design.
This figure depicts the overall design of our experiments to evaluate whether autologous or allogeneic stromal cells could enhance the levels of engraftment and donor cell differentiation of HSC transplanted in utero. Various hematopoietic tissues from these animals were then evaluated for the engraftment and differentiation of the transplanted HSC or stromal cells as detailed in the text.
Experimental design.
This figure depicts the overall design of our experiments to evaluate whether autologous or allogeneic stromal cells could enhance the levels of engraftment and donor cell differentiation of HSC transplanted in utero. Various hematopoietic tissues from these animals were then evaluated for the engraftment and differentiation of the transplanted HSC or stromal cells as detailed in the text.
Tracking of human stromal cells
For these experiments, 6 fetal sheep at 55 to 60 days gestational age were used. Four fetuses were injected with 107 human stromal cells that were genetically marked by in vitro retroviral transduction before transplantation and analyzed at 2 and 6 weeks after transplant. The 2 remaining fetuses were injected with an identical number of stromal cells that had been labeled with PKH26 and were analyzed at day 2 and 6 after transplant.
Transduction of human stroma with G1BgSvNa retroviral vector
Subconfluent stromal layers were transduced for 48 hours with rapidly thawed amphotropic retroviral supernatant diluted in an equal volume of Dulbecco's modified Eagle's medium with high glucose and 10% heat-inactivated FBS in the presence of 4 μg/mL protamine sulfate (Lyphomed, Deerfield, IL), with the vector preparation being replaced every 12 hours, for a total of 4 cycles of transduction. Passage 4 stromal cells were then selected in medium containing 1.5 mg/mL of G418 to ensure that the transplanted stromal cells were transduced.
Labeling of stromal cells with PKH26
Stromal layers 80% confluent, grown for 9 days as described above, were trypsinized and collected by centrifugation. Cells were then aliquoted at 2 × 107 cells per tube and labeled with PKH26 (Sigma) according to manufacturer's instructions.
Quantiblot of human DNA
DNA samples were prepared by using a salting-out protocol.26 For detection and quantitation of human DNA in the several organs, Quantiblot (Perkin Elmer, Foster City, CA) was used according to manufacturer's instructions.
Detection of NeoR sequences by polymerase chain reaction (PCR)
Five hundred nanograms of total genomic DNA isolated from different sheep organs was subjected to analysis by PCR as follows: PCR primers 5′CTGAATGAACTGCAGGACGA3′ and 5′AGCCAACGCTATGTCCTGAT3′, which amplify a 504 base pair (bp) fragment of the bacterial neomycin phosphotransferase (NeoR) gene, were used at 0.5 μmol/L. Standard dNTP and MgCl2 (2.5 mmol/L) concentrations were used, and 1 unit of Amplitaq DNA polymerase (Perkin Elmer) was added to each 50-μL reaction. The reactions were run for 40 cycles consisting of denaturation at 95°C for 1 minute, annealing at 57°C for 1 minute, and extension at 72°C for 1.5 minutes. The positive control consisted of the plasmid pUC18Neo diluted in normal sheep DNA to a concentration of 1%.
Reverse transcriptase PCR (RT-PCR)
To examine the expression of human growth factors in the chimeric sheep, total cellular RNA was extracted by the guanidinium thiocyanate-acidic phenol-chloroform method. For the RT-PCR 1 μg of RNA was reverse transcribed in a 20-μL reaction mixture using a commercially available kit according to the manufacturer's recommendations (Perkin Elmer). For the PCR reaction, MgC12, Amplitaq DNA polymerase, PCR buffer, and 40 pmol of each primer were added to the total first strand cDNA mixture, giving a final volume of 100 μL, and PCR for SCF, GM-CSF, and granulocyte colony-stimulating factor (G-CSF) was then performed.
Southern blotting of PCR products
Southern blotting was performed according to standard procedures27 using Gene Screen Plus (DuPont) nylon membrane. Oligonucleotide probes specific for the Neo, SCF, GM-CSF, or G-CSF products were labeled with [32P]dATP by an end-labeling reaction. Following transfer, the DNA was cross-linked to the membrane by short-wave UV irradiation and the membrane was prehybridized at 65°C for 1 hour in 6× standard sodium citrate (SSC), 0.5% sodium dodecyl sulfate (SDS), and l00 μg/mL salmon sperm DNA; 2 × 106 cpm of labeled probe was added per milliliter of prehybridization solution, and dextran sulfate was added to a final concentration of 10%. The filter was then hybridized overnight at 65°C. The filter was washed 4 times at 65°C for 15 minutes each under conditions of increasing stringency. The filter was then autoradiographed for 1 to 12 hours (depending on the signal intensity) at −80°C with 2 intensifying screens (DuPont Cronex Lightning Plus).
β-Galactosidase staining of stromal layers
Stromal layers grown from the several hematopoietic organs were stained for β-galactosidase activity as follows. Cells were washed once with phosphate-buffered saline (PBS) and were then fixed for 5 minutes at room temperature in a solution of 0.5% glutaraldehyde in PBS. Following fixation, cells were rinsed once with PBS and were then washed twice for 5 minutes each in PBS. β-Galactosidase activity was then detected by incubating the cells overnight at 37°C in β-galactosidase staining solution, which consisted of PBS containing 5 mmol/L K4Fe(CN)6*3H2O, 5 mmol/L K3Fe(CN)6, 2 mmol/L MgCl2, and l mg/mL X-galactosidase. After overnight staining, the cells were washed once with PBS and were then counterstained with nuclear fast red. The slides were rinsed with H2O and visualized on an Olympus light microscope.
Statistical analysis
Results are expressed as mean ± SEM. Because of small group sizes, comparisons between experimental results were determined by a nonparametric rank sum test. A P value of less than .05 was considered statistically significant.
Results
Cotransplantation of autologous human stroma yields increased levels of human cells in circulation before birth: results from PB and BM at 3, 6, and 9 weeks after transplant
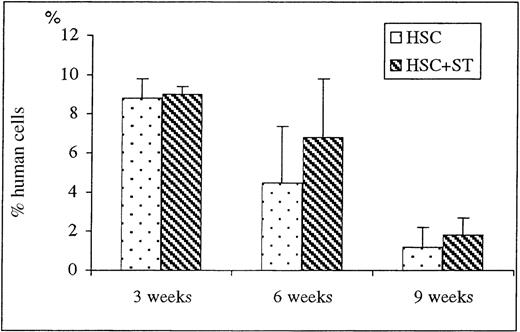
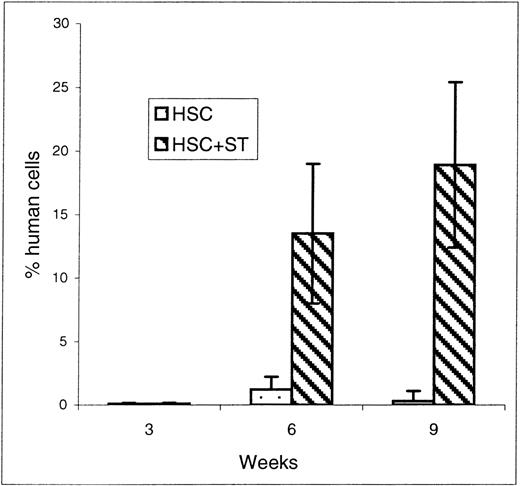
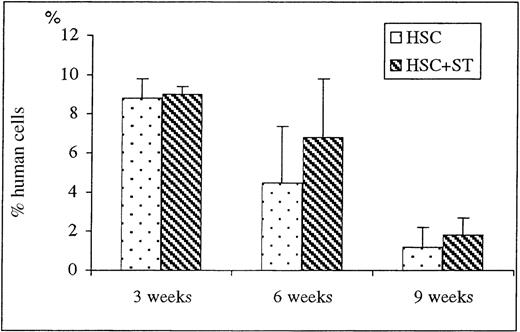
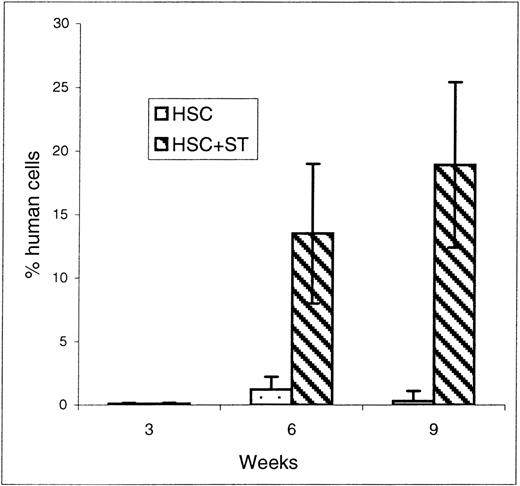
To determine the effect of cotransplanting stroma on early donor HSC engraftment and appearance of donor cells within the PB, recipients of HSC and HSC plus stroma were killed at 3, 6, and 9 weeks after transplant and their BM and PB were analyzed by flow cytometry for the engraftment/differentiation of human cells. The results of flow cytometric analyses of the BM and PB of the sheep killed before birth are shown in Figures 2 and3, respectively. At the time point of 3 weeks after transplant, there were no significant differences in the levels of donor cell engraftment in the BM (Figure 2) and appearance in PB (Figure 3) between the groups that received HSC alone (n = 2) or with stroma (n = 2). In contrast, as early as 6 weeks after transplant, there were marked increases in the levels of human cells observed in the PB (Figure 3) of the animals that had received HSC with stroma (CD45: 13.5 ± 5.5%; n = 4) when compared to the group that had received HSC alone (CD45: 1.2 ± 0.6%; n = 3) (P < .03). Although the presence of human cells in PB was multilineage in both groups, the pronounced difference in the levels of human cells between these 2 groups can be attributed primarily to the higher levels of lymphoid progenitors (CD7) and erythroid (glycophorin A-positive) cell precursors in sheep that received human HSC plus stroma. The sheep that received HSC plus stroma had an average of 10% CD7+ cells versus only 3.4% in the sheep that received HSC alone. Likewise, sheep receiving HSC plus stroma had an average of 2.25% glycophorin A-positive cells, whereas those that received HSC alone had 0.6%. Even more pronounced differences were observed in the PB at 9 weeks after transplant, with human CD45 levels as high as 18.9 ± 6.5% in the sheep receiving stroma plus HSC (n = 4) compared to only 0.3 ± 0.1% in those injected with HSC alone (n = 3) (P < .03) (Figure 3). At this time, however, pronounced increases in donor cells were seen not only in the lymphoid and erythroid precursor cell populations, but also in the myeloid and monocytic lineages (Table 1).
FACS analysis of BM at early time points after transplantation.
Sheep that had been transplanted in utero with human HSC alone or in combination with autologous stromal cells were killed at early time points of 3, 6, and 9 weeks after transplant and their long bones collected. Single cell suspension were then obtained as detailed in “Materials and methods,” and FACS analysis for human-specific cell markers was performed to evaluate whether stromal cells could enhance the levels of engraftment of donor human cells within the animals transplanted in utero. Values represent the mean ± 1 SEM.
FACS analysis of BM at early time points after transplantation.
Sheep that had been transplanted in utero with human HSC alone or in combination with autologous stromal cells were killed at early time points of 3, 6, and 9 weeks after transplant and their long bones collected. Single cell suspension were then obtained as detailed in “Materials and methods,” and FACS analysis for human-specific cell markers was performed to evaluate whether stromal cells could enhance the levels of engraftment of donor human cells within the animals transplanted in utero. Values represent the mean ± 1 SEM.
FACS analysis of PB at early time points after transplantation.
Sheep that had been transplanted in utero with human HSC alone or in combination with autologous stromal cells were killed at early time points of 3, 6, and 9 weeks after transplant and their PB was collected. FACS analysis for the presence of the human-specific marker CD45 was then performed to evaluate whether stromal cells could enable the early entry of donor human cells within the periphery of animals transplanted in utero. Values represent the mean ± 1 SEM.
FACS analysis of PB at early time points after transplantation.
Sheep that had been transplanted in utero with human HSC alone or in combination with autologous stromal cells were killed at early time points of 3, 6, and 9 weeks after transplant and their PB was collected. FACS analysis for the presence of the human-specific marker CD45 was then performed to evaluate whether stromal cells could enable the early entry of donor human cells within the periphery of animals transplanted in utero. Values represent the mean ± 1 SEM.
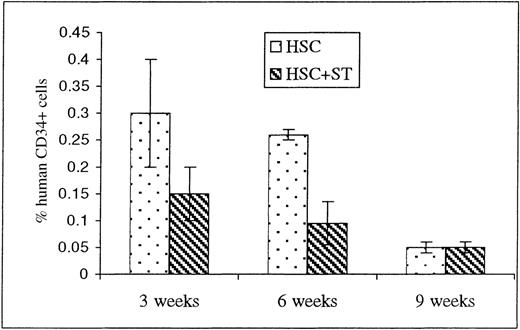
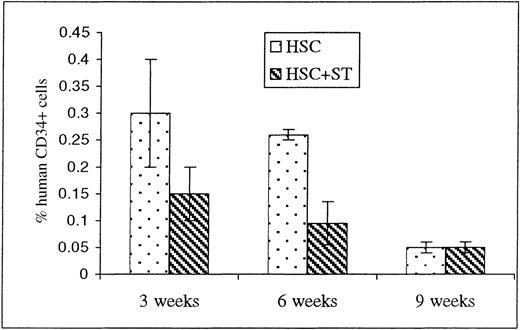
Multilineage engraftment was also seen at all 3 time points in the BM of animals that received HSC either alone or with stroma, but no significant differences were seen between the 2 groups (Figure 2). Of note, however, is the fact that the levels of CD34+ cells at both 3 and 6 weeks after transplant appear to be slightly higher in the animals that were injected with HSC alone when compared to those receiving stroma concomitantly (Figure 4).
Engraftment of CD34+ cells within the sheep bone marrow (BM) following in utero HSC transplantation.
To determine whether cotransplanting stromal cells with the HSC graft produced early donor cell differentiation within the periphery by inducing all of the grafted HSC to undergo terminal differentiation, BM was obtained from animals killed at 3, 6, and 9 weeks after transplant and analyzed by flow cytometry for the presence of CD34. Values shown represent the mean ± 1 SEM.
Engraftment of CD34+ cells within the sheep bone marrow (BM) following in utero HSC transplantation.
To determine whether cotransplanting stromal cells with the HSC graft produced early donor cell differentiation within the periphery by inducing all of the grafted HSC to undergo terminal differentiation, BM was obtained from animals killed at 3, 6, and 9 weeks after transplant and analyzed by flow cytometry for the presence of CD34. Values shown represent the mean ± 1 SEM.
Levels of engraftment of human cells in PB and BM at 3 days, 3 months, and 12 months after birth in sheep transplanted with human HSC with or without human autologous stroma
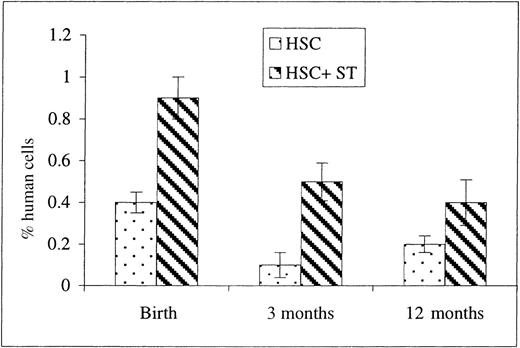
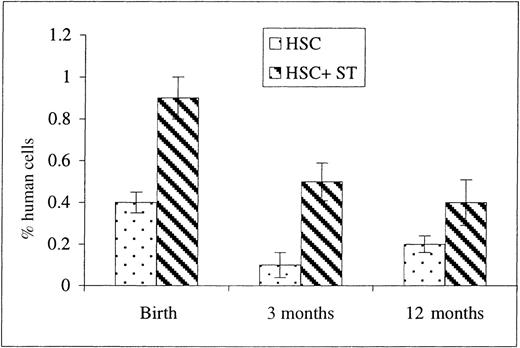
Once we had demonstrated that the cotransplantation of stroma with HSC resulted in an enhancement in early donor cell appearance within the PB, we next examined whether this enhancement was long-lasting. To address this issue, flow cytometric analyses of PB and BM were also performed on the transplanted sheep at 3 days, 3 months, and 1 year of age (13, 23, and 65 weeks after transplantation, respectively). As can be seen in Figure 5, the presence of human donor cells in PB was observed in both groups at all time points after birth, albeit at lower levels than those seen at earlier time points after transplantation. As in the earlier time points, however, the levels were significantly higher in the group that had received stromal cells compared to HSC alone. When BM from these same animals was analyzed, we found that higher levels of human cells were present in the animals that had been cotransplanted with autologous stroma (2.2 ± 0.3% at birth, n = 5; 1.63 ± 0.5% at 3 months of age, n = 5; and 0.17 ± 0.06% at 1 year of age, n = 4) when compared with sheep receiving HSC alone in which the levels of human cells found in the BM did not exceed 0.2% at any time. This is in contrast to the results obtained in the sheep analyzed before birth, in which no differences in the levels of engraftment in the BM were observed between the 2 experimental groups. It is also of note that we were never able, at any time point, to detect human CD45+cells by flow cytometry in any of the sheep that were transplanted with human stroma alone.
Donor human cell presence within PB after birth (13, 23, and 65 weeks after transplantation).
To determine whether cotransplanting stroma altered the long-term engraftment/differentiation of donor human cells, sheep that had been transplanted in utero with either HSC alone or in conjunction with autologous stromal cells were allowed to complete gestation and were subsequently analyzed by flow cytometry at birth, 3 months, and 12 months for the presence of human CD45+ cells in their PB. Values represent the mean ± 1 SEM.
Donor human cell presence within PB after birth (13, 23, and 65 weeks after transplantation).
To determine whether cotransplanting stroma altered the long-term engraftment/differentiation of donor human cells, sheep that had been transplanted in utero with either HSC alone or in conjunction with autologous stromal cells were allowed to complete gestation and were subsequently analyzed by flow cytometry at birth, 3 months, and 12 months for the presence of human CD45+ cells in their PB. Values represent the mean ± 1 SEM.
Cotransplantation of human allogeneic stroma with human HSC
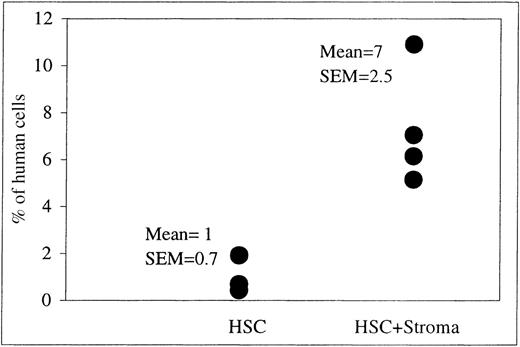
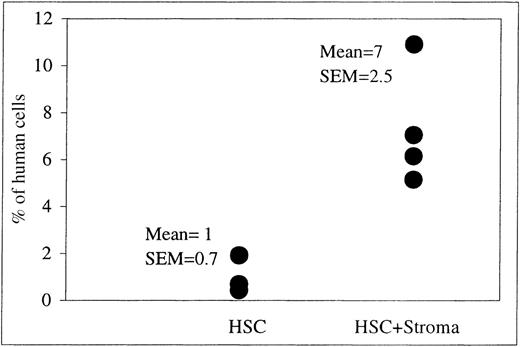
We next examined whether allogeneic stroma was also capable of increasing the levels of engraftment and differentiation of human donor cells in our sheep model. To address this issue, sheep that had received allogeneic stroma in combination with human HSC were analyzed at 13, 23, 68, and 169 weeks after transplant and compared to the results obtained with sheep receiving either HSC alone or HSC with autologous stroma. Analysis of these animals revealed that the allogeneic stroma was as effective as the autologous stroma at enhancing the appearance of donor human cells within the PB at all times after transplant resulting in levels of human cells in the PB similar to those described above (data not shown). In addition, by including the 169-week time point, we were able to demonstrate that the pronounced enhancement in the levels of donor cells within the periphery achieved by cotransplanting allogeneic stroma with HSC persisted for over 3 years after transplant. As can be seen in Figure6, the levels of human cells in the PB of sheep receiving HSC plus allogeneic stroma (n = 4) varied from 5.15% to 10.9% (mean: 7% ± 2.5%), whereas sheep receiving HSC alone (n = 3) had levels ranging from 0.43 to 1.91 (mean: 1% ± 0.7%) (P < .03). Furthermore, as with autologous stroma, allogeneic stroma also produced an increase in the levels of marrow long-term engraftment at all time points after birth (percent human cells in BM of HSC plus stroma, 0.5 ± 0.08, n = 4; HSC alone, 0.2 ± 0.1, n = 3) (P < .03).
FACS analysis on PB at 169 weeks after transplantation.
The PB was obtained at intervals from sheep transplanted in utero with HSC alone (n = 3) or in combination with allogeneic human stromal cells (n = 4). This scatter plot shows the levels of human CD45+ cells in the PB of each of these sheep at 169 weeks after transplantation as well as the mean percentage of human cells present ± 1 SEM for each experimental group as a whole.
FACS analysis on PB at 169 weeks after transplantation.
The PB was obtained at intervals from sheep transplanted in utero with HSC alone (n = 3) or in combination with allogeneic human stromal cells (n = 4). This scatter plot shows the levels of human CD45+ cells in the PB of each of these sheep at 169 weeks after transplantation as well as the mean percentage of human cells present ± 1 SEM for each experimental group as a whole.
Detection of human DNA in human–sheep chimeras
DNA analysis was used to further demonstrate engraftment of human cells in transplanted sheep and to investigate the possibility that human stromal cells were able to engraft following in utero transplantation. To this end, DNA was isolated from both whole organs and cultured BM stromal layers of the transplanted sheep and analyzed using the Quantiblot assay to detect the presence of human DNA. Table2 summarizes the results obtained from a Quantiblot assay performed on 8 randomly selected transplanted sheep at 9 weeks after transplantation. Human DNA was detected in all of the freshly collected BM samples and in all but 1 of the cultured marrow stromal layers (sheep injected with CD34+HLA−DR− human BM cells). Of note is the presence of human DNA in the BM and spleen of sheep that had been injected with stroma alone, indicating that injected stromal cells are capable of engraftment in the human-sheep xenograft model.
Tracking of human stromal cells in sheep fetuses
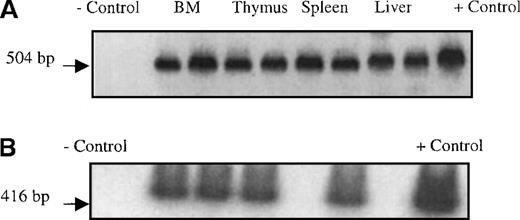
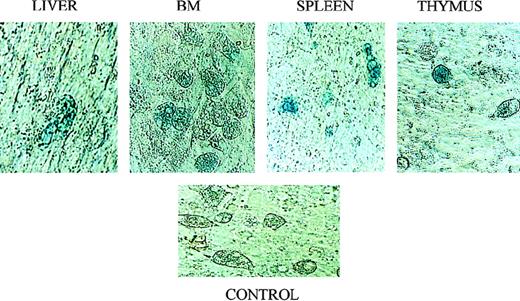
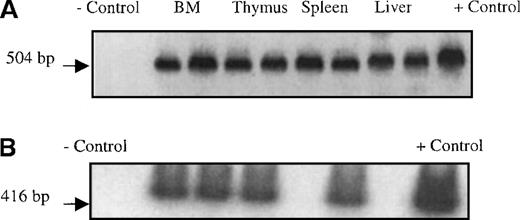
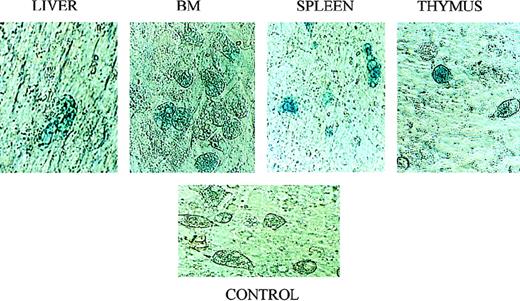
Although the Quantiblot system enabled us to demonstrate that human stromal cells had the ability to engraft in sheep hematopoietic organs following in utero transplantation, we wished to find another method of tracking transplanted stromal cells that would allow us to identify engrafted stromal cells in situ. Four additional fetal sheep were transplanted in utero with passage 4 stromal cells that had been transduced with a retroviral vector containing theNeoR and the LacZ genes. We then used the presence/expression of these genes to track where the human stromal cells had engrafted. The retroviral vector used contained a nuclear targeting signal at the start of the LacZ gene, thus eliminating any ambiguity caused by the presence of lysosomal enzymes with β-galactosidase–like activity. Transplanted sheep were killed at either 2 weeks or 6 weeks after transplant, and hematopoietic tissues collected. Stromal layers were then grown from these organs, and histochemical staining for β-galactosidase was performed. PCR for the NeoR gene was also performed on DNA obtained from the freshly harvested organs. Figure7A shows a NeoR-specific PCR performed on freshly isolated tissues from sheep transplanted with the retroviral-marked stromal cells, showing the ability of these transplanted human stromal cells to engraft in several hematopoietic organs. Figure 8 shows that stromal layers grown from bone marrow, thymus, spleen, and liver all contained cells that expressed β-galactosidase, demonstrating that marked human stromal cells are present within these organs. Although we did not use specific markers or culture conditions that would allow us to distinguish between the various types of cells into which human stromal progenitors are known to be able to differentiate,28-30 by morphologic examination we were able to identify transduced adipocytes and fibroblast-like cells (Figure 8). Importantly, flow cytometric analysis demonstrated that CD45+ cells were not present in these stromal layers, thus excluding the presence of leukocytes as the source of transduced cells in the stromal layers.
PCR on isolated tissues.
(A) Presence of NeoR gene in hematopoietic organs from sheep transplanted in utero with retrovirally marked stromal cells. Following sacrifice, single cell suspensions of liver, spleen, thymus, and BM were prepared as detailed in “Materials and methods,” and the DNA obtained from these cells was then subjected to PCR analysis with primers specific for the vector-encodedNeoR gene. The reagent control consisted of all of the constituents of the PCR reaction mixture except template DNA (lane 1 from the left). The negative control (−) DNA was isolated from the PB mononuclear cells from a normal control ram (lane 2). The positive control consisted of the plasmid pUC18Neo diluted in normal sheep DNA to a concentration of 1% (lane 11). The remainder of the samples consisted of DNA extracted from the organs (as labeled in the figure) of 2 different transplanted sheep. For each organ there are 2 samples; the sample on the left is from the time point of 2 weeks after transplant, and the sample on the right from 6 weeks after transplant. (B) SCF RT-PCR on marrow stromal cells. To evaluate whether the transplanted stromal cells were expressing mRNA for hematopoietic growth factors BM stromas grown from 5 sheep transplanted with human stroma cells alone were harvested and RNA was isolated. This RNA was then reverse transcribed into cDNA and used as a template for SCF-specific PCR. The reagent control consisted of all of the reaction constituents except template DNA, and the negative control consisted of RNA isolated from the BM mononuclear cells of a normal control sheep (lane 1). The positive control was RNA isolated from human BM mononuclear cells (lane 8). Lanes 2 to 6 consisted of RNA extracted from stromal layers cultured from BM of 5 different sheep injected with human stromal cells alone. In all but 1 animal (lane 5) we were able to detect mRNA for SCF. We were able, however, to amplify a fragment of β-actin message from this sample demonstrating the presence of intact RNA.
PCR on isolated tissues.
(A) Presence of NeoR gene in hematopoietic organs from sheep transplanted in utero with retrovirally marked stromal cells. Following sacrifice, single cell suspensions of liver, spleen, thymus, and BM were prepared as detailed in “Materials and methods,” and the DNA obtained from these cells was then subjected to PCR analysis with primers specific for the vector-encodedNeoR gene. The reagent control consisted of all of the constituents of the PCR reaction mixture except template DNA (lane 1 from the left). The negative control (−) DNA was isolated from the PB mononuclear cells from a normal control ram (lane 2). The positive control consisted of the plasmid pUC18Neo diluted in normal sheep DNA to a concentration of 1% (lane 11). The remainder of the samples consisted of DNA extracted from the organs (as labeled in the figure) of 2 different transplanted sheep. For each organ there are 2 samples; the sample on the left is from the time point of 2 weeks after transplant, and the sample on the right from 6 weeks after transplant. (B) SCF RT-PCR on marrow stromal cells. To evaluate whether the transplanted stromal cells were expressing mRNA for hematopoietic growth factors BM stromas grown from 5 sheep transplanted with human stroma cells alone were harvested and RNA was isolated. This RNA was then reverse transcribed into cDNA and used as a template for SCF-specific PCR. The reagent control consisted of all of the reaction constituents except template DNA, and the negative control consisted of RNA isolated from the BM mononuclear cells of a normal control sheep (lane 1). The positive control was RNA isolated from human BM mononuclear cells (lane 8). Lanes 2 to 6 consisted of RNA extracted from stromal layers cultured from BM of 5 different sheep injected with human stromal cells alone. In all but 1 animal (lane 5) we were able to detect mRNA for SCF. We were able, however, to amplify a fragment of β-actin message from this sample demonstrating the presence of intact RNA.
X-galactosidase staining confirms engraftment of transplanted stromal cells in multiple hematopoietic organs.
Following transplantation in utero with retrovirally marked stromal cells, sheep were killed and stromal layers established in vitro from their liver, spleen, thymus, and BM. These layers were then evaluated for expression of the vector-encoded LacZ gene by histochemical X-galactosidase staining according to standard procedures. See text for details. The control consisted of a stromal layer derived from the BM of a normal control sheep that was processed and stained identically to the experimental samples.
X-galactosidase staining confirms engraftment of transplanted stromal cells in multiple hematopoietic organs.
Following transplantation in utero with retrovirally marked stromal cells, sheep were killed and stromal layers established in vitro from their liver, spleen, thymus, and BM. These layers were then evaluated for expression of the vector-encoded LacZ gene by histochemical X-galactosidase staining according to standard procedures. See text for details. The control consisted of a stromal layer derived from the BM of a normal control sheep that was processed and stained identically to the experimental samples.
Because the stromal layers used for retroviral transduction were passaged several times and constituted a much more homogeneous population of stromal cells than the ones cotransplanted with the HSC, we wished to track stromal cells that had been grown under exactly the same culture conditions that were used in the cotransplantation experiments. Stromal layers were grown for 9 days and then marked with PKH26 as described in “Materials and methods.” Flow cytometric analysis of these stromal layers before transplant demonstrated that 37% to 42% of the cells were STRO-1+, whereas 8% to 10% were CD8+, 10% to 14% were CD14+, 12% to 15% were positive for CD19, and 1% to 2% were CD34+. Two sheep fetuses were transplanted with PKH26-labeled stromal cells and analyzed at 2 and 6 days after transplant for PKH26+, CD45− cells. As a control, the peritoneal lavage was also analyzed for the presence of these cells to ensure the accuracy of the site of injection. As can be seen in Table3, stromal cells were found in PB, BM, liver, and spleen, corroborating the data obtained with the retroviral-transduced stroma. Of note is that at least a percentage of these cells use the bloodstream to migrate to their homing site as can be seen by the presence of 1.7% PKH26+CD45− cells in the PB at day 2 after transplant.
RT-PCR detects human SCF production
Based on our finding of human cells within the stromal layers cultured from the chimeric sheep, we wished to determine if these human cells were providing support to the transplanted human HSC. We performed RT-PCR to evaluate whether human SCF was being produced within the BM stromas of the sheep transplanted with stromal cells alone. As Figure 7B shows, we were able to detect messenger RNA (mRNA) for human SCF in the marrow stromal layers from several of these sheep. These results demonstrate that the injected stromal cells were not only capable of engrafting within the sheep marrow, but also maintained their ability to produce human-specific hematopoietic factors at least at the mRNA level. In addition, we performed RT-PCR analysis to examine the production of human G-CSF and GM-CSF on these same sheep, but we were not able to detect the mRNAs for either of these factors (data not shown).
Discussion
In the present studies, the human-sheep in utero transplantation model was used to investigate whether providing a source of exogenous stromal progenitor cells could enhance the engraftment and differentiation of donor human HSC following transplantation. The long-term engraftment and multilineage differentiation of human HSC in this model have been well documented.10-12 However, despite the presence of donor (human) cells in the BM, their appearance in blood does not occur until late in gestation. Because in the human-sheep model, no irradiation is necessary, this enables us to transplant cells into a functionally intact microenvironment that is naturally “primed” to receive donor HSC. The requirement for functional stromal support for definitive hematopoiesis has been well established and evidence of qualitative differences in hematopoiesis supported by stroma derived from different ontological environments has been reported.8,9 In an in utero sheep-sheep transplantation study we reported that by providing an adult BM environment we could achieve higher and earlier levels of circulating donor cells in the PB, demonstrating that the transplantation of a mature stromal environment improved engraftment and qualitatively changed hematopoiesis.13
In the human-sheep model we have also demonstrated the formation of human stromal elements after transplantation of pure populations of human HSC but at relatively low levels,31 probably not enough to provide the human transplanted stem cells with a more suitable human microenvironment. We therefore hypothesized that cotransplanting human stromal progenitor cells would enhance the levels of engraftment and differentiation of the donor human cells in this xenogeneic model of in utero human HSC transplantation.
The results presented here demonstrate that the cotransplantation of human stromal elements with human HSC results in increased levels of circulating human donor cells within the PB starting at early time points after transplant and that this effect was maintained for at least 3 years after birth. In the BM, enhancement of human hematopoiesis by human stroma was only seen at later time points but seemed to be long-lasting as well. As early as 6 weeks after transplantation the analysis of chimeric sheep PB transplanted with the combined cell populations showed mean levels of human CD45+cells as high as 13.5% ± 5.5%, levels that continued to increase to reach a mean maximum of 18.9% ± 6.5% at 9 weeks. Although at 6 weeks the difference in levels of human cells between the 2 transplanted groups, with or without stroma, were mainly explained by a higher number of lymphoid cell and erythroid cell precursors in circulation, at 9 weeks an increase of cells of all lineages was seen in the PB. After birth the levels of human cells in blood decreased gradually in both groups, remained higher in the group cotransplanted with stroma, and were still present in detectable levels at 1 year of age. The reason for this decrease is not clear, but it could be a function of the exponential expansion in the sheep hematopoietic compartment as the animal's age and size increase without a synchronous proliferation of the human stem cell compartment. We also observed, at 1 year after transplant, identical levels of CD34+ cells in the bone marrow of both groups of animals suggesting that the differentiation of HSC induced by stromal cells was not responsible for the subsequent (after birth) decreased numbers of human cells in circulation by possible exhaustion of the stem cell compartment. Our results also demonstrate that autologous and allogeneic stromal cells were equally effective in producing an enhancement in the levels of human donor cells in the PB within the fetal sheep recipients. As we reported previously, sheep stroma is able to maintain human hematopoiesis and support multilineage differentiation, but less efficiently than its human counterpart.21 Thus we believe that the presence of an adult humanized microenvironment, even if allogeneic, in our model, is beneficial for the transplanted human HSC. Also of note is that at 3 years after transplant the mean levels of human cells in PB increased to higher levels than seen during the first year of life. Longer time course analysis will determine if the higher levels of human cells will be maintained in these animals or if a cycling phenomenon resulting in periods during which human hematopoiesis is stimulated in these animals is responsible.
Our results are of particular interest given the fact that the transplantability of stromal cells has thus far been a source of controversy.18-20,32-36 Although several investigators have demonstrated in preclinical animal models and in 1 human study that stromal cells are able to be transplanted and can subsequently generate donor-derived osteoblasts, other studies performed in BMT recipients suggested that all stromal elements were host in origin with the exception of stromal macrophages.34-36 Likewise, other studies performed in immunodeficient mice have demonstrated that transplanted stromal cells engraft within the lung, liver, and the spleen, but not in the BM.17 The results presented here confirm the presence of human donor-derived stromal cells within the BM of the recipients, providing evidence that, in our model following in utero transplantation, stromal cells are capable of engrafting the marrow, and retain the ability to produce SCF at least at the mRNA level. In addition to the BM, we were also able to demonstrate the presence of human stromal cells within the spleen, liver, and thymus of the recipients, showing that adult human stromal cells can engraft in multiple hematopoietic organs within the fetal sheep recipients. Although the transplanted stromal cells were not a homogeneous population, and did contain monocytes/macrophages, CD8+cells, and trace numbers of CD34+ cells, roughly 40% of the injected cells were STRO-1+, confirming that they were stromal progenitor cells. The use of the PKH dye in conjunction with an antibody to CD45 enabled us to confirm that the cells that engrafted within the various hematopoietic organs were indeed stromal cells and not simply contaminating hematopoietic cells. In addition, by injecting stromal cells that had been transduced with a retroviral vector containing the LacZ gene, we were able to demonstrate that the injected stromal cells gave rise to both adipocytes and fibroblast-like cells following their engraftment within the marrow. It is possible that the transplanted cells possessed a wider differentiative potential, but were restricted to these 2 lineages by the culture conditions we used to grow the stromal layers. Because we did not inject pure populations of stromal cell progenitors, the possibility that either the trace amounts of CD34+ HSC or CD8+ facilitator cells present within our stromal cells were responsible for the enhancement of engraftment/differentiation of human cells in our model cannot be ruled out. After comparing large numbers of sheep that have been transplanted with widely variant numbers of human HSC, we can conclude that the trace quantities of CD34+ cells within our stromal cell populations could not account for the degree of enhancement we observed in the present studies. Although it is possible that so-called facilitator cells may be in part responsible for this enhanced engraftment37-39we do not feel that this is likely, because injecting autologous PB mononuclear cells with the human HSC did not result in enhancement of donor cell engraftment or differentiation (unpublished observations). However the role of CD8+ cells in establishing functional stromal cell layers37 within the fetal sheep microenvironment cannot be ruled out. Studies cotransplanting pure populations of human stromal cell progenitors with HSC are in progress and will allow us to answer that question.
In conclusion, these studies demonstrate that cotransplanting an appropriate functional hematopoietic microenvironment with HSC may represent a means of achieving higher and earlier levels of circulating donor cells, enhance long-term engraftment within the BM, and suggest that stromal cells may be a useful vehicle for the delivery of secreted exogenous gene products to the hematopoietic microenvironment, making cotransplantation of stroma a promising tool as an adjunct to a variety of novel therapeutic applications.
Supported by grants HL49042, HL52955, and DK51427 from the National Institutes of Health and the Department of Veterans Affairs.
Reprints:Graça Almeida-Porada, VA Medical Center (151B), 1000 Locust St, Reno, NV 89520; e-mail: almei_g@med.unr.edu.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.