Abstract
Chronic graft versus host disease (cGVHD) is a major complication that can develop after bone marrow transplantation. It involves an immune-mediated attack by transplanted donor lymphocytes, and often results in inflammatory damage of host target organs. Immune hyporesponsiveness induced by oral antigen administration has been recently shown to prevent the development of cGVHD in a murine model. The aim of this study was to evaluate whether tolerance induction in bone marrow transplant (BMT) recipients after transplantation, toward their pretransplant antigens, can alleviate preexisting cGVHD in a mouse model. cGVHD was generated by infusing 2.5 × 107splenocytes from B10.D2 donor mice, to sublethally irradiated (6 Gy) BALB/c recipient mice, which differ by minor histocompatibility antigens. Transplantation resulted in cGVHD, with characteristic scleroderma-like cutaneous fibrosis, increased skin collagen content, decreased body weight, and hepatic and small bowel inflammation. Oral tolerance was induced by feeding recipient BALB/c mice with proteins extracted from BALB/c splenocytes for 11 days after B10.D2 splenocyte transplantation. Tolerance induction was evidenced by a significant reduction in mixed lymphocyte response of effector splenocytes from tolerant BALB/c mice transplanted with B10.D2 splenocytes against BALB/c target splenocytes. Oral tolerance decreased skin collagen deposits. Reduction of collagen 1(I) gene expression and skin collagen were shown by in situ hybridization and histochemistry, respectively. Liver and bowel biopsy specimens revealed less inflammation. Serum IL-10 levels were higher in tolerant mice than in controls, whereas IFNγ was significantly reduced. Oral tolerance of BMT recipients toward their pretransplant antigens after splenocyte transplantation down-regulated the immune attack by transplanted cells, thus ameliorating cGVHD.
Chronic graft versus host disease (cGVHD) is a major complication that can develop after bone marrow transplantation.1 It is a multiorgan disorder, with skin manifestations resembling scleroderma. It also brings about salivary gland dysfunction, small bowel inflammation, and hepatic pathology, including chronic aggressive hepatitis, bridging necrosis, cirrhosis, and bile duct destruction.2-4 The pathogenesis involves activation of immunologic pathways similar to those involved in several autoimmune disorders.5,6 Dysregulation of the cytokine network has been shown to play a role in induction and maintenance of cGVHD in experimental models and humans.7-11
Oral administration of antigens is an effective means of inducing antigen-specific immunologic hyporesponsiveness.12,13Feeding small doses of the antigen has been reported to induce tolerance by the generation of negative immunoregulatory cells, whereas administering higher doses tends to bring about clonal inactivation or deletion.13 Oral tolerance has been shown effective in various experimental models of autoimmune disorders, including experimental autoimmune encephalitis, experimental arthritis, experimental colitis, and in down-regulating an antiviral immune response.14-17 Moreover, in the antiviral immunity models, it was found superior to other modes of peripheral tolerance induction.18-21 Oral tolerance has shown promising results when applied in the treatment of multiple sclerosis, rheumatoid arthritis, and diabetes in humans.22,23 Moreover, oral tolerance was also found to be effective in several models of animals, in which there was a preexisting immunity toward the target antigen.24 It was also possible to induce oral tolerance to major histocompatibility complex (MHC) molecules and to reduce host rejection of donor cells.25-27
We have previously shown that the induction of oral tolerance in bone marrow transplant (BMT) donors toward transplant recipient splenocytes before bone marrow transplantation can prevent the development of graft versus host inflammatory immune response.28 We used the mouse model of cGVHD, which involved transplantation of B10.D2 splenocytes into sublethally irradiated BALB/c mice. These mice strains are identical at MHC gene (H2-D) loci, but differ by their multiple minor histocompatibility antigens. Intravenous infusion of splenocytes from B10.D2 into BALB/c mice induces cGVHD manifested by scleroderma-like skin lesions, an increase in skin collagen, thickening of the dermis, and a loss of subdermal fat. Liver and gastrointestinal tract injuries in this model include inflammatory destruction of the intrahepatic bile ducts and the bowel mucosa.29-31 Feeding donor mice homogenates of recipient splenocytes before transplantation prevented the clinical and microscopic manifestations of cGVHD.28 However, clinical application of this method will require tolerance induction in BMT recipients after transplantation. The aim of this study was to evaluate the possibility of inducing tolerance in splenocyte recipients toward their pretransplant antigens, after transplantation. The results show that oral administration of BMT recipient antigens to splenocyte recipients after transplantation induced tolerance and ameliorated cGVHD in the mouse model.
Materials and methods
Animals
Donor mice were 12-week-old B10.D2 females (H2-D, mls-b), obtained from Jackson Laboratories (Anne Harbor, ME). Recipients were BALB/c mice (also H2-D, mls-b). The mice were kept in 12-hour light/dark cycles in the Animal Core of the Hadassah-Hebrew University Medical School. All animals were fed regular laboratory chow and drank water ad libitum. All animal experiments were approved by the Hebrew-University-Hadassah Institutional Committee for Care and Use of Laboratory Animals. After irradiation and splenocyte transplantation, the mice were maintained in laminar flow isolators.
Splenocyte transplantation
Three groups of B10.D2. mice, consisting of 10 animals each, were used as splenocyte donors. To induce cGVHD, 2.5 × 107 spleen cells from B10.D2 mice were injected intravenously into BALB/c recipient mice. Mice were given60Co whole-body irradiation (6 Gy) before transplantation.28-30
Preparation and administration of recipient splenocytes to donors
Spleens were excised from BALB/c and B10.D2 mice, and splenocytes were mechanically homogenized. After filtration through a 40-μm nylon cell strainer, remaining intact cells were spun down and removed. Proteins were measured using the Biorad Protein assay reagent (Biorad, Munich, Germany). Recipient mice were fed homogenates containing 50 μg of proteins, by atraumatic cannulae every other day, (for a total of 5 doses), 7 days after splenocyte transplantation. The dose of splenocytes used was determined by initial dosing experiments performed by others as well as ourselves.17 The 50 μg dose was found to be in the low-dose oral tolerance range.
Experimental groups
Three groups of recipient BALB/c mice, consisting of 10 animals each, were studied. Mice in all groups were transplanted with B10.D2 splenocytes as described above. Recipient mice in experimental group A were fed homogenates of splenocytes derived from recipient strain BALB/c mice. BALB/c recipient mice in control groups B and C were fed homogenates prepared from syngeneic B10.D2 mouse splenocytes or bovine serum albumin (BSA), respectively. Mice in all groups were fed 5 doses every other day, beginning 7 days after splenocyte transplantation.
Evaluation of tolerance induction in donor mice and assessment of a recipient's response toward donor cells by 1-way mixed lymphocyte reaction assay
Tolerance induction in donor B10.D2 mice toward the minor histocompatibility antigens of recipient mice was evaluated by 1-way mixed lymphocyte reaction (MLR) tests.32,33 The MLR test was performed in both directions in 5 animals from each group 52 days after transplantation: B10.D2 splenocytes versus BALB/c splenocytes, and vice versa. Effector BALB/c or B10.D2 splenocytes (1 × 106), were cultured in flat-bottom microwell plates (Sterilin catalogue no. M29ARTL; Sterilin, UK), with irradiated (30 Gy) B10.D2 or BALB/c spleen cells (1 × 106), respectively, in a total volume of 0.2 mL RPMI 1640 culture medium, supplemented with 100 μ/mL penicillin, 100 μg/mL streptomycin, 2 m mmol/L L-glutamine, with 5 × 10−5 2M-ME and 10% FCS, adsorbed into mice splenocytes. After 72 hours in a 37°C humidified 5% CO2 incubator, (1 μCi) 3H TdR (1.85 × 1011 Bq/nmol, Nuclear Research Center, Negev, Israel) was added to each well. Cells were collected 16 to 18 hours later on paper filters, using a multiple sample harvester (Titertek Cell Harvester 530; Flow Laboratories, McLean, VA). Radioactivity was measured with a liquid scintillation counter. In both tests, background results from tolerated and nontolerated B10.D2 splenocytes against themselves were subtracted.
Assessment of chronic graft versus host disease
cGVHD assessment was performed 52 days after transplantation using the following parameters: Total body and spleen weights, skin collagen content, collagen type α I (I) gene expression, and liver and small bowel inflammatory response.
Body and spleen weights
Body weights were recorded every week for all animals in all groups throughout the study. Spleens were weighed at the end of the study.
In situ hybridization of collagen messenger RNA and histochemical assessment of skin content
All mice from all experimental and control groups were killed 52 days after splenocyte transplantation. Skin biopsies were obtained from the ears and collected in phosphate-buffered saline (PBS). Biopsy specimens were fixed overnight in 4% paraformaldehyde in PBS at 4°C. Serial 5-μm sections were prepared after the samples had been dehydrated in graded ethanol, cleared in chloroform. For hybridization, sections were deparaffinized in xylene, rehydrated in graded ethanol, rinsed in distilled water for 5 minutes, and incubated in 2 × SSC at 70°C for 30 minutes. Sections were again rinsed in distilled water and treated with pronase (0.125 mg/mL in 50 mmol/L Tris-HCL, 5 mmol/L ethylenediaminetetraacetic acid, pH 7.5) for 10 minutes. After digestion, sections were blocked in 0.2% glycine, rinsed in distilled water, rapidly dehydrated in graded ethanol, air-dried for several hours, and postfixed in 10% formalin in PBS. The skin sections were hybridized with a digoxigenin-labeled collagen α1 (I) probe generated by excising a 1600-base pair (bp) insert from the plasmid (pUC18) and inserting into pSafyre (a generous gift of B. E. Kream, University of Connecticut. CT).34-36
Grading of histologic changes of chronic graft versus host disease
All mice from all groups were killed at 52 days after splenocyte transplantation. For evaluation of the degree of hepatic and intestinal inflammation, tissue was removed from all mice in both groups and kept in 10% formaldehyde. Five tissue sections from each mouse were embedded in paraffin, sectioned, and stained with hematoxylineosin by standard procedure. The degree of inflammation of coded microscopic sections of liver and small bowel were graded semiquantitatively from 0 to 4, as previously described, by 2 experienced pathologists who were unaware of the experimental conditions. Liver sections were graded according to standard scoring criteria as described.29,30,36 In brief: Grade 0, normal or less than 30% portal infiltration (based on percentage of portal tracts expanded by inflammatory cells) and normal bile duct epithelium; grade 1, 30% to 40% portal inflammation, and normal bile duct epithelium; grade 2, 40% to 60% portal inflammation, and abnormal bile duct epithelial morphology; grade 3, 60% to 80% portal inflammation and lymphocyte infiltration of bile ducts; and grade 4, 80% to 100% portal inflammation, and death of bile duct cells or disruption of bile ducts. Small bowel specimens were graded by the following scale: Grade 0, normal intestinal mucosa; grade 1, mild distortion of villous architecture with a normal amount of mucous cells, an occasional apoptotic cell in base of crypts with evidence of cryptic hyperplasia; grade 2, partial effacement or blunting of the villous architecture, mucous cell depletion, sloughing of epithelial cells within the lumen, marked apoptosis and crypt hyperplasia, and mononuclear cell infiltration within lamina propria and cryptitis; and grade 3, as in grade 2, plus patchy or total mucosal necrosis or ulceration.3,36 37
Serum cytokine levels
For evaluation of the effect of oral tolerance on the balance of proinflammatory and anti-inflammatory cytokines IFNα and IL10, serum levels were measured as previously described,10 11 by a highly sensitive RIA or enzyme immunoassay (R&D Systems, Minneapolis, MN), according to the manufacturers' instructions. These kits use an amplification system in which an alkaline phosphatase reaction product serves as a cofactor for the formation of a colored reporter system. The secondary enzyme system consists of alcohol dehydrogenase and diaphorase (amplifier). Serum cytokine levels were measured in 5 mice from each group, 28 days after splenocyte transplantation.
Statistical analysis
Results were analyzed by Student t test for the cytokine assays and by the Mann-Whitney rank sum test for histologic grading.
Results
Effect of oral toleration of splenocyte recipients on mixed lymphocyte reaction response of transplanted splenocytes toward pretransplant recipient splenocytes
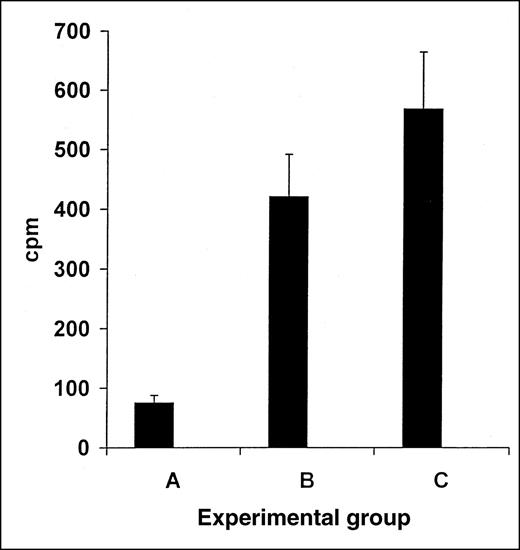
The induction of tolerance in splenocyte recipient BALB/c mice transplanted with B10.D2 splenocytes and fed with BALB/c splenocyte homogenate after transplantation, toward recipient BALB/c splenocyte antigens, was measured with the MLR test. Effector splenocytes from tolerant BALB/c mice showed a marked reduction in MLR response against BALB/c target splenocytes (Figure 1, group A, 75 cpm, n = 5), compared with the effector splenocytes from nontolerant BALB/c mice fed with B10.D2 splenocytes or BSA (control groups B and C, 420 or 568 cpm, respectively, n = 5,P < .005, Figure 1).
Reduction in MLR response in transplant recipients via induction of oral tolerance toward recipient's splenocytes.
Induction of tolerance in recipient BALB/c mice toward the recipient splenocytes was measured using the MLR test. Responding tolerated BALB/c mice transplanted with B10.D2 splenocytes and fed with BALB/c splenocyte homogenates after transplantation, were tested versus BALB/c, showed marked reduction in the MLR response (A). In contrast, nontolerant control BALB/c mice fed with B10.D2 splenocyte homogenates, tested versus similar BALB/c targets showed marked MLR response (B,P < .005). Similarly, nontolerant control BALB/c mice splenocytes fed with BSA, tested versus similar BALB/c targets showed marked MLR response (C, P < .005).
Reduction in MLR response in transplant recipients via induction of oral tolerance toward recipient's splenocytes.
Induction of tolerance in recipient BALB/c mice toward the recipient splenocytes was measured using the MLR test. Responding tolerated BALB/c mice transplanted with B10.D2 splenocytes and fed with BALB/c splenocyte homogenates after transplantation, were tested versus BALB/c, showed marked reduction in the MLR response (A). In contrast, nontolerant control BALB/c mice fed with B10.D2 splenocyte homogenates, tested versus similar BALB/c targets showed marked MLR response (B,P < .005). Similarly, nontolerant control BALB/c mice splenocytes fed with BSA, tested versus similar BALB/c targets showed marked MLR response (C, P < .005).
Effect of oral toleration of bone marrow transplant recipients on chronic graft versus host disease manifestations
Body and spleen weights.
Whole body weights were followed on all mice from all groups and were not significantly different among the 3 experimental groups during and at the end of the study (52 days). Mean body weights at the end of study measured 19.6 ± 0.55 g, 18.8 ± 1.64 g, and 19.5 ± 0.55 g, for mice in groups A, B, and C, respectively (P = .12, A vs B; P = .3, A vs C). Similarly, no significant change was observed in spleen weights at the end of the study for the 3 groups (0.139 ± 0.019 g vs 0.117 ± 0.025 g vs 0.142 ± 0.046 g, respectively, P = .3, A vs B;P = .2, A vs C).
Skin collagen 1(I) gene expression in splenocyte recipients.
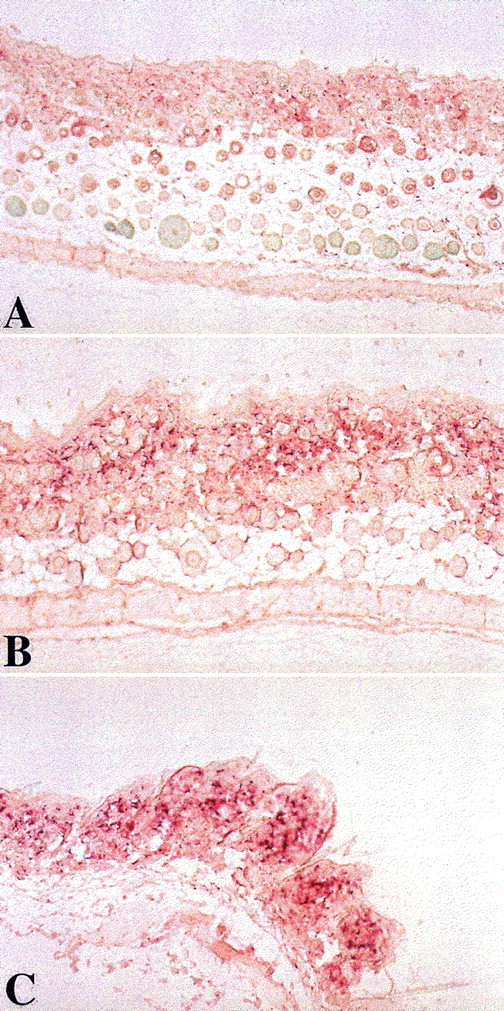
Skin biopsies were performed on all splenocyte recipients from all groups 52 days after transplantation. Skin collagen α1 messenger RNA (mRNA) content was determined by in situ hybridization of a skin section with a mouse collagen α1 (I) probe. Mice in experimental group A, fed with homogenates of splenocytes derived from recipient strain BALB/c mice, 7 days after transplantation, showed much less collagen α1 (I) gene expression (Figure2A). In contrast, biopsy specimens from control nontolerant recipients in groups B and C, fed after transplantation with homogenates prepared from B10.D2 mouse splenocytes or BSA, respectively, showed significantly higher levels of collagen αI gene expression (Figure 2B, 2C). With the use of the standardized quantitative measurements, group A recipients achieved a sum score of 634.5, compared with 2240.5 and 2491.5 in control groups B and C (n = 6, P < .005).
Induction of oral tolerance in splenocyte recipients toward pretransplant recipient lymphocytes decreased skin collagen production.
Skin biopsies were performed on splenocyte recipients 52 days after transplantation. Skin collagen α1 mRNA content was determined by in situ hybridization of skin section with a mouse collagen α1(I) probe. Mice in experimental group A, fed with homogenates of splenocytes derived from recipient strain BALB/c mice, 7 days after transplantation, showed much less collagen α1 (I) gene expression (panel A). In contrast, control nontolerant recipients in groups B and C, fed after transplantation, with homogenates prepared from B10.D2 mouse splenocytes or BSA, respectively, showed significantly higher levels of collagen αI gene expression (panels B and C). (Original magnification ×10.)
Induction of oral tolerance in splenocyte recipients toward pretransplant recipient lymphocytes decreased skin collagen production.
Skin biopsies were performed on splenocyte recipients 52 days after transplantation. Skin collagen α1 mRNA content was determined by in situ hybridization of skin section with a mouse collagen α1(I) probe. Mice in experimental group A, fed with homogenates of splenocytes derived from recipient strain BALB/c mice, 7 days after transplantation, showed much less collagen α1 (I) gene expression (panel A). In contrast, control nontolerant recipients in groups B and C, fed after transplantation, with homogenates prepared from B10.D2 mouse splenocytes or BSA, respectively, showed significantly higher levels of collagen αI gene expression (panels B and C). (Original magnification ×10.)
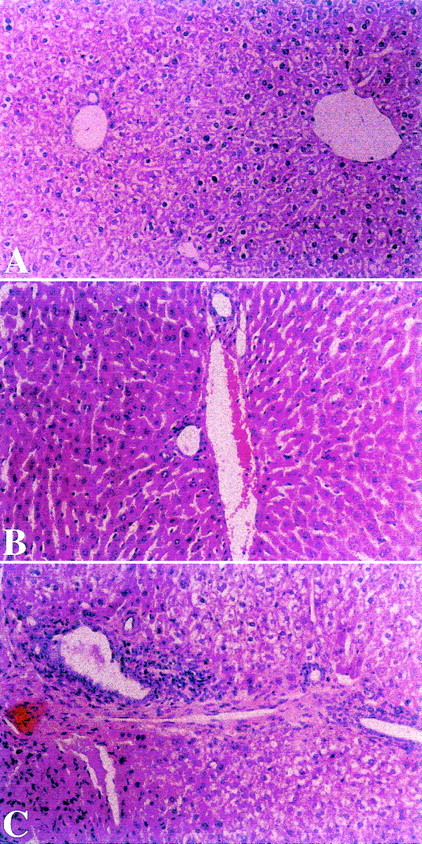
Amelioration of cGVHD associated-liver disease by oral tolerance.
Liver biopsies were performed on all splenocyte recipients from all groups 52 days after transplantation. Mice in experimental group A, fed after transplantation with homogenates of splenocytes derived from recipient strain BALB/c mice, showed mild degrees of portal inflammation, lymphocyte infiltration, and/or disruption of intrahepatic bile ducts (Figure 3A). In contrast, biopsy specimens from control nontolerant recipients in groups B and C, fed after transplantation with homogenates prepared from B10.D2 mouse splenocytes or BSA, respectively, showed portal inflammation and bile duct destruction (Figure 3B and C). With the use of the standardized score grading for liver and bile duct involvement in cGVHD, group A recipients achieved a sum score of 1.90 ± 0.42, compared with 2.3 ± 0.41 and 2.58 ± 0.38 in control groups B and C (n = 6, group A vs B, P < .005; group A vs C,P < .004).
Effect of toleration on histologic evaluation of liver in transplants recipients.
Liver biopsies were performed on splenocyte recipients 52 days after transplantation. Mice in experimental group A, fed after transplantation with homogenates of splenocytes derived from recipient strain BALB/c mice showed mild degree of portal inflammation (Panel A). In contrast, biopsies from control nontolerant recipients in groups B and C, fed after transplantation with homogenates prepared from B10.D2 mouse splenocytes or BSA, respectively, showed portal and bile duct inflammation (Panels B and C). (HαE, original magnification ×40.)
Effect of toleration on histologic evaluation of liver in transplants recipients.
Liver biopsies were performed on splenocyte recipients 52 days after transplantation. Mice in experimental group A, fed after transplantation with homogenates of splenocytes derived from recipient strain BALB/c mice showed mild degree of portal inflammation (Panel A). In contrast, biopsies from control nontolerant recipients in groups B and C, fed after transplantation with homogenates prepared from B10.D2 mouse splenocytes or BSA, respectively, showed portal and bile duct inflammation (Panels B and C). (HαE, original magnification ×40.)
Alleviation of cGVHD of small bowel by oral tolerance.
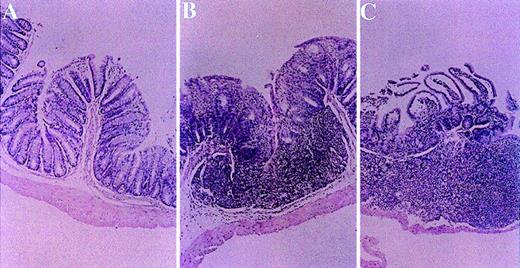
Small bowel biopsies were performed in all mice from all experimental and control groups 52 days after transplantation. Significant alleviation of all parameters was observed in mice from experimental group A fed with homogenates of splenocytes derived from recipient strain BALB/c mice (Figure 4A). In contrast, biopsy specimens from control nontolerant recipients in groups B and C, fed with homogenates prepared from syngeneic B10.D2 mouse splenocytes or BSA, respectively, manifested severe mucosal damage, distortion of villous architecture, reduction in the number of mucosal cells and crypt hyperplasia, mononuclear cell infiltration within the lamina propria, and cryptitis (Figure 4B and C). With the use of the standardized score grading for bowel involvement in cGVHD, the sum scores for small bowel involvement in groups A, B, and C measured 2.10 ± 0.74 versus 3.17 ± 0.52 and 2.67 ± 0.75, respectively (group A vs group B,P < .002; group A vs group C, P < .005).
Effect of toleration on histologic evaluation of small bowel in transplant recipients.
Small bowel biopsies were performed in all groups 52 days after transplantation. Alleviation of all histologic parameters of cGVHD was observed in mice from experimental group A fed with homogenates of splenocytes derived from recipient strain BALB/c mice (panel A). In contrast, biopsy specimens from control nontolerant recipients in group B and C, which were fed with homogenates prepared from syngeneic B10.D2 mouse splenocytes or BSA, respectively, manifested mucosal damage, distortion of villous architecture, reduction in the number of mucosal cells and crypt hyperplasia, mononuclear cell infiltration within the lamina propria, and cryptitis (Panels B and C). (HαE, original magnification ×10.)
Effect of toleration on histologic evaluation of small bowel in transplant recipients.
Small bowel biopsies were performed in all groups 52 days after transplantation. Alleviation of all histologic parameters of cGVHD was observed in mice from experimental group A fed with homogenates of splenocytes derived from recipient strain BALB/c mice (panel A). In contrast, biopsy specimens from control nontolerant recipients in group B and C, which were fed with homogenates prepared from syngeneic B10.D2 mouse splenocytes or BSA, respectively, manifested mucosal damage, distortion of villous architecture, reduction in the number of mucosal cells and crypt hyperplasia, mononuclear cell infiltration within the lamina propria, and cryptitis (Panels B and C). (HαE, original magnification ×10.)
Serum cytokine levels
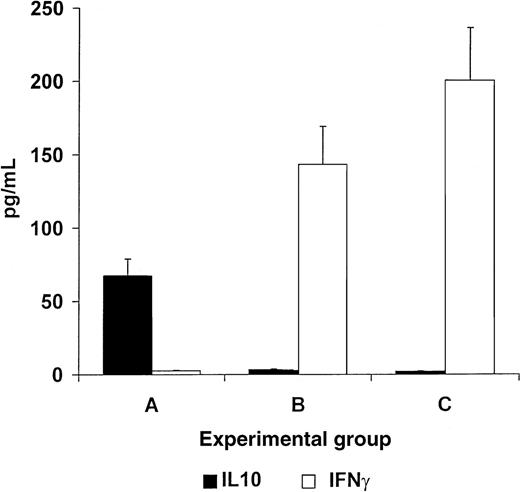
Mice in experimental group A, fed with homogenates of splenocytes derived from recipient strain BALB/c mice showed an increase in serum IL10 levels and a decrease in serum IFNγ levels (67.4 ± 9.64 vs 0.9 ± 0.11 pg/mL for IL10 and IFNγ, respectively,P < .005, Figure 5). In contrast, control nontolerant recipients in groups B and C, fed with homogenates prepared from B10.D2 mouse splenocytes or BSA, respectively, showed an increase in serum IFNγ levels and a decrease in serum IL10 levels (143.4 ± 14.64 vs 16 ± 1.88 pg/mL; and 200.6 ± 31.3 vs 11.1 ± 1.6 pg/mL for IFNγ and IL10, respectively, P < .005, Figure 5).
Oral tolerance induction in splenocyte recipients toward pretransplant recipients lymphocytes induces a reversal of the cytokine profile, from a Th1 to a Th2 type of response.
Mice in experimental group A, fed with homogenates of splenocytes derived from recipient strain BALB/c mice manifested increased serum IL10 levels (black bars) and decreased serum IFNγ levels (white bars, A). In contrast, control nontolerant recipients in groups B and C, fed with homogenates prepared from B10.D2 mouse splenocytes or BSA, respectively, showed an increase in serum IFNγ levels and decreased serum IL10 levels (B and C).
Oral tolerance induction in splenocyte recipients toward pretransplant recipients lymphocytes induces a reversal of the cytokine profile, from a Th1 to a Th2 type of response.
Mice in experimental group A, fed with homogenates of splenocytes derived from recipient strain BALB/c mice manifested increased serum IL10 levels (black bars) and decreased serum IFNγ levels (white bars, A). In contrast, control nontolerant recipients in groups B and C, fed with homogenates prepared from B10.D2 mouse splenocytes or BSA, respectively, showed an increase in serum IFNγ levels and decreased serum IL10 levels (B and C).
Discussion
Chronic GVHD accounts for much of the morbidity and mortality associated with allogeneic bone marrow transplantation. The results of this study show that oral administration of low doses of splenocyte homogenates of the recipient strain, after transplantation of allogeneic splenocytes, induces tolerance toward a recipient's alloantigens and ameliorates cGVHD manifestations.
Oral tolerance is a recognized procedure for induction of antigen-specific peripheral immune hyporesponsiveness and has been shown to prevent or alleviate several autoimmune disorders in animals.14-17,22-24 We have shown previously that oral administration of low doses of splenocyte homogenates of the recipient strain to donors before splenocyte transplantation induces tolerance toward recipient alloantigens.28 Tolerance was documented by a significant reduction in MLR response of donor lymphocytes toward recipient splenocytes, and by the prevention of the inflammatory immune response, which in turn resulted in a prevention of skin, small bowel, and liver manifestations of cGVHD.28 However, clinical application of this method would require inducing tolerance in the presence of preexisting disease, as well as immune modulating the recipient rather than the donor.
The results of this study show that oral tolerance of splenocyte recipients toward their pretransplant antigens down-regulated the inflammatory response, thus ameliorating cGHVD. In parallel, tolerance was shown in vitro by a significant reduction in the MLR response of recipient splenocytes toward pretransplant recipient splenocytes, after transplantation of donor lymphocytes. In contrast, tolerance was not induced by feeding recipient mice with splenocyte homogenates from the donor strain or BSA. This may indicate that tolerance was antigen-specific, probably due to minor histocompatibility epitopes, which differ between donor and recipient strains.
The skin lesions of cGVHD in the murine model, manifested mainly by increased collagen synthesis, dermal fibrosis, mononuclear cell infiltration, and loss of dermal fat and appendages, are similar to those of human cGHVD and idiopathic scleroderma.4 The results of the current study showed that oral tolerance of the recipients toward pretransplant splenocyte homogenates partially ameliorated collagen 1α (I) gene expression and skin collagen content after splenocyte engraftment. Hepatic involvement in cGVHD is characterized by mononuclear cell inflammation of portal tracts and bile duct destruction inside the liver.30 Murine cGVHD across minor HLA antigens results in cell-mediated destruction of bile ducts inside the liver.30 31 In this study, we demonstrated that induction of oral tolerance toward recipient splenocytes partially alleviated these changes. Similarly, recipient toleration after splenocyte transplantation alleviated the small bowel inflammatory response. Tolerant recipients disclosed less small bowel mucosal inflammation and destruction. Tolerance induction using homogenates of recipient splenocytes was associated with the down-regulation of proinflammatory cytokines, IFNαb secreted by Th1 helper cells, along with increased serum levels of anti-inflammatory cytokine, IL10.
Oral tolerance was previously shown effective in preventing the secondary immune response in animals primed against target antigens.13 It was recently demonstrated that oral tolerance induction alleviates secondary immune response in the presence of preexisting antiviral immunity in animals.24,38,39 However, several studies have shown that oral tolerance induction in the setting of a previously primed animal is less effective in down-regulating immune-mediated inflammatory response, and in various animal models, conflicting results have been published.40,41 Induction of oral tolerance has shown promising results in various human immune-mediated diseases, including multiple sclerosis, rheumatoid arthritis, scleroderma, and diabetes.42-44 The results of this study showed that tolerance induction of splenocyte recipients after transplantation was effective in ameliorating the disease, however, somewhat less potently than the prevention achieved by tolerance induction in splenocyte donors before transplantation.28This phenomenon can be explained by the fact that tolerance was induced in the recipients before their immune system was fully mature.
An immune-bystander effect was previously suggested to be of importance in oral tolerance.45 This may also have taken place in this study in which tolerance was induced by feeding BALBc mice with splenocyte homogenates, leading to amelioration of the attack on the major target organs by transplanted lymphocytes. The bystander effect may be explained by secretion of immunomodulatory cytokines triggered by tolerizing antigens, which may down-regulate the response against other antigens.46,47 In addition, disease target proteins, eg, minor alloantigens of the recipient type, are common to splenocytes and to target organs. Several studies support our observation that tolerance can be directed toward specific lymphocyte-associated antigens. Intrathymic injection of recipient-type splenocytes into donors has been shown to prevent GVHD.48Oral administration of MHC allopeptide mixture before immunization down-regulated cell-mediated immunity and reduced delayed-type hypersensitivity responses to allogeneic splenocytes.25 26
Currently, there is no effective treatment for cGVHD. Efforts to control GVHD have been directed against autoreactive T cells. Gluccorticoids, azathioprine, FK506, or cyclosporine ameliorate cGVHD by immunosuppressive mechanisms.1 Depleting the graft of T cells by a variety of techniques reduces GVHD by limiting the number of alloreactive cells in the infused marrow. But pharmacologic prophylaxis and T-cell depletion are not enough to control GVHD. Moreover, these regimens expose patients to generalized immunosuppression with many undesirable side effects, significant drug toxicity and suppression of the graft versus leukemia (GVL) effect.1 The ultimate goal in GVHD prophylaxis is to reduce GHVD-related organ damage, while sparing the antitumor GVL effect. In contrast, induction of specific tolerance toward disease-target antigens could potentially allow long-term alleviation of the disease, leaving the general immunologic defense of the recipient intact, and keeping the GVL effect. As tumor antigens are presumably important for GVL, tolerance induction in the future may require induction of hyporesponsiveness toward specific recipient epitopes, while keeping the immunogenicity toward tumor epitopes.
Clinical and experimental data suggest the role of a cytokine dysbalance in the induction and maintenance of target organ damage in cGVHD.49-52 The cytokine cascade results in amplification of local tissue injury and further promotes the inflammatory response via induction of cytotoxic T cells (CTL) and natural killer (NK) cell responses. Recently, prevention of GVHD has been attempted through interruption of the proinflammatory amplification by inducing a shift from proinflammatory to anti-inflammatory cytokine profile in the donor T-cell population before bone marrow transplantation.9Monoclonal antibodies against TNFα, or IL10, alleviated the skin, intestine, and liver manifestations of cGVHD.49 However, administration of immunosuppressive cytokines were either ineffective or toxic, limiting their potential application in clinical situations. The novel method shown in this study of inducing a shift to an anti-inflammatory cytokine response in the GVHD setting may permit overcoming these difficulties.
In conclusion, using the murine cGVHD model, we have demonstrated that oral toleration of recipients toward their pretransplant proteins, after transplantation, has down-regulated the immune attack by donor effector cells on host target tissue, thus alleviating the skin, liver, and small intestine manifestations of cGVHD. This effect was associated with changes in the cytokine secretion profile of donor immune cells from a proinflammatory to an anti-inflammatory pattern. The method also has the potential of being further developed for application in clinical practice to tolerize BMT recipient toward their pretransplant antigens, after blood marrow transplantation, for alleviation of cGVDH, while sparing general immune defense of the host.
Y.I. and I.G. contributed equally to this article.
Supported in part by the following grants: Hadasit-Yissum; a grant from ENZO Biochem Inc, NY; and The Roaman-Epstein Liver Research Foundation (to Y.I.).
Reprints:Yaron Ilan, Liver Unit, Department of Medicine, Hadassah University Hospital, POB 12000, Jerusalem, Israel IL-91120.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal