Abstract
NF-κB is required for prevention of apoptosis. We examined the importance of human T-cell leukemia virus–I (HTLV-I) Tax protein to stimulate NF-κB nuclear translocation, thus preventing apoptosis. Jurkat cells and JPX-9 cells in which the inducible Tax expression plasmid vector was stably transfected were used in the present study. Both Jurkat and Tax− JPX-9 cells had small amounts of basal nuclear NF-κB activity. The addition of NF-κB inhibitors suppressed NF-κB nuclear translocation of the cells, thus inducing apoptosis. Sequential activation of caspases from caspase-8 to caspase-3 was shown during this process. NF-κB nuclear translocation in JPX-9 cells was stimulated through Tax expression, and both the activation of caspases and apoptosis induced by NF-κB inhibitors were significantly suppressed in the Tax+ JPX-9 cells. The expression of Bcl-2, Bax, and Bcl-x was not changed among Jurkat, Tax− JPX-9, and Tax+ JPX-9 cells in the presence or absence of NF-κB inhibitors. X-chromosome–linked inhibitor of apoptosis (XIAP) protein expression in Tax−JPX-9 cells was significantly suppressed by NF-κB inhibitors, however, its expression in Tax+ JPX-9 cells was maintained even by the addition of NF-κB inhibitors. Our results suggest that the activation of NF-κB via Tax protein in HTLV-I infected cells renders the cells resistant to apoptosis. The expression of anti-apoptotic gene products such as XIAP to suppress caspase cascade, results in an increase of cytokine production and cell proliferation; one of the proposed mechanisms that promotes autoimmune disorders such as Sjögren’s syndrome and rheumatoid arthritis found in HTLV-I seropositive subjects.
INFECTION OF HUMAN host cells with human T-cell leukemia virus–I (HTLV-I) results in activation of these cells and induction of various cellular events, such as increased production of various types of cytokines1-3 and augmentation of cell proliferation.4 The central role of these events is believed to be initiated by transactivation of expression of host genes through HTLV-I Tax protein.5 The factors in host cells to induce gene expression by Tax is thought to be a set of enhancer-binding proteins.5 One such factor is NF-κB, which is implicated in Tax-mediated transactivation of various genes. We previously showed that interleukin-6 (IL-6) gene is transactivated by Tax protein via NF-κB binding site using transient cotransfection by Tax expression plasmid pMax-Neo with IL-6 promotor in COS1 cells, and JPX-9, in which pMAX-Neo is stably transfected in Jurkat cells.6 Previous studies suggested that increased production of cytokines and cell proliferation mediated by activation of NF-κB through Tax protein may have a major pathogenic role in the induction of autoimmune disorders described in humans such as HTLV-I associated myelopathy (HAM), Sjögren syndrome (SS), and rheumatoid arthritis (RA).7-11
A newly described function of NF-κB is its inhibitory action against apoptotic signals.12-19 A variety of stimuli such as radiation, depletion of growth factors, and signaling through Fas and tumor necrosis factor receptor (TNFR) induce apoptosis of the cells, whereas the common death pathways of the above stimuli appear to be mediated through activation of caspases.12-17 Accumulating evidence suggests that the apoptotic process is clearly inhibited by activation of NF-κB, which appears to be mediated through induction of anti-apoptotic gene products.12-19 It is possible to speculate that NF-κB is highly activated by Tax in HTLV-I infected cells, which increases the resistance against several apoptotic stimuli, resulting in increased production of cytokines and cell proliferation. These phenomena may increase the risk for onset of autoimmune disorders in HTLV-I seropositive subjects.
In the present study, we initially examined whether inhibition of activation of NF-κB induces apoptosis of cells through activation of the caspase cascade. In the next step, we also investigated whether HTLV-I Tax protein activates NF-κB, which in turn inhibits the activation of caspases and results in inhibition of apoptosis.
MATERIALS AND METHODS
Cell culture.
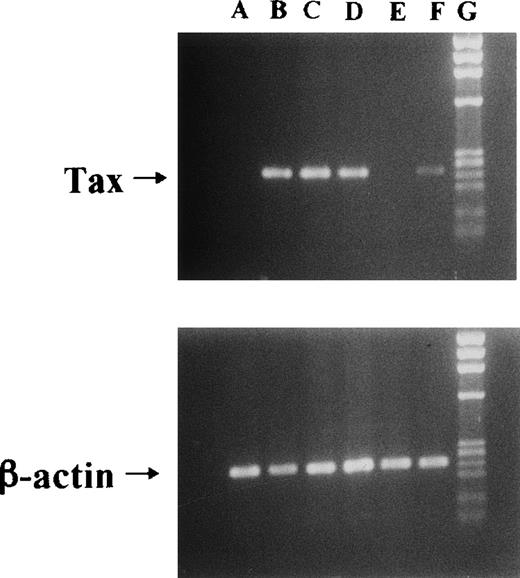
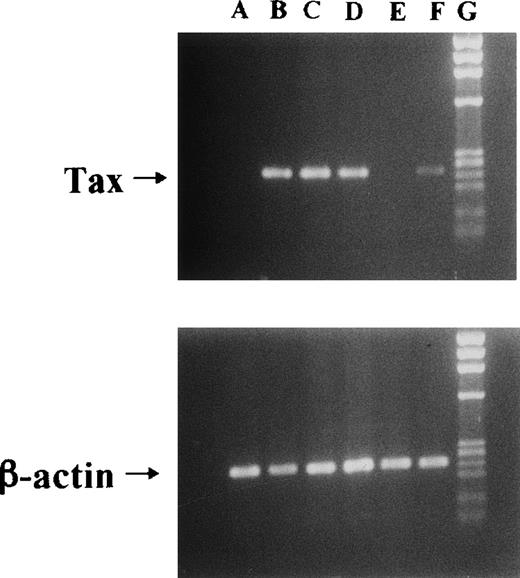
We used Jurkat (HTLV-I–uninfective T-cell line) and JPX-9 cells in the following experiments. JPX-9 is a Jurkat subclone generated by the stable introduction of the Tax expression plasmid vector, pMax-Neo (kindly provided by Prof Sugamura, Tohoku University, Sendai, Japan).6,20 Because metallothionein promotor is included in the plasmid vector, the addition of CdCl2(30 μmol/L) was necessary to induce Tax expression in JPX-9 cells.6,20 As previously described,6 20 Tax expression in JPX-9 cells induced by CdCl2 was detected within 3 hours as determined by reverse transcription-polymerase chain reaction (RT-PCR) and Western blotting (results of RT-PCR in Fig1).
Induction of Tax mRNA expression in JPX-9 cells by CdCl2. JPX-9 cells were treated with 30 μmol/L of CdCl2 for indicated hours. After incubation, the expression of Tax or β-actin mRNA in the cells was examined by RT-PCR analysis on 1% agarose gel electrophoresis. PCR products for Tax mRNA contain 246-bp fragments, whereas that for β-actin mRNA contains 236-bp fragments. Primer pairs used are 5′-AAACAGCCCTGCAGATACAAAGT-3′ (upper primer) and 5′-ACTGTAGAGCTGAGCCGATAACG-3′ (lower primer) for Tax, and 5′-GACGAGGCCCAGAGCAAGAGAG-3′ (upper primer) and 5′-ACGTACATGGCTGGGGTGTTG-3′ (lower primer) for β-actin. Lane A; CdCl2-untreated JPX-9 cells. Lane B; JPX-9 cells treated with CdCl2 for 3 hours. Lane C; JPX-9 cells treated with CdCl2 for 6 hours. Lane D; JPX-9 cells treated with CdCl2 for 12 hours. Lane E; Jurkat cells (negative control). Lane F; MT-2 cells (positive control). Lane G; DNA size marker (Hind III digests of λ DNA).
Induction of Tax mRNA expression in JPX-9 cells by CdCl2. JPX-9 cells were treated with 30 μmol/L of CdCl2 for indicated hours. After incubation, the expression of Tax or β-actin mRNA in the cells was examined by RT-PCR analysis on 1% agarose gel electrophoresis. PCR products for Tax mRNA contain 246-bp fragments, whereas that for β-actin mRNA contains 236-bp fragments. Primer pairs used are 5′-AAACAGCCCTGCAGATACAAAGT-3′ (upper primer) and 5′-ACTGTAGAGCTGAGCCGATAACG-3′ (lower primer) for Tax, and 5′-GACGAGGCCCAGAGCAAGAGAG-3′ (upper primer) and 5′-ACGTACATGGCTGGGGTGTTG-3′ (lower primer) for β-actin. Lane A; CdCl2-untreated JPX-9 cells. Lane B; JPX-9 cells treated with CdCl2 for 3 hours. Lane C; JPX-9 cells treated with CdCl2 for 6 hours. Lane D; JPX-9 cells treated with CdCl2 for 12 hours. Lane E; Jurkat cells (negative control). Lane F; MT-2 cells (positive control). Lane G; DNA size marker (Hind III digests of λ DNA).
Induction of apoptosis of Jurkat and JPX-9 cells by NF-κB inhibitors.
Apoptosis of Jurkat and JPX-9 cells was induced by the addition of pyrrolidine dithiocarbamate (PDTC; Sigma Chemical Co, St Louis, MO), N-acetylcysteine (NAC, Sigma), or Z-Leu-Leu-Leu-aldehyde (LLL-CHO, Peptide Institute, Inc, Osaka, Japan), a potent NF-κB inhibitor, as previously described.5,21,22 PDTC and NAC are antioxidants, which inhibit the action of reactive oxygen intermediates, and are thought to inhibit nuclear translocation of NF-κB in the cells.5 LLL-CHO is reported to inhibit IκBα degradation by inhibiting proteasome function. Therefore, dissociation of NF-κB from IκB is prevented by LLL-CHO, and nuclear NF-κB translocation is suppressed.22 In brief, Jurkat or JPX-9 cells, preincubated with or without CdCl2, were further cultured with various concentrations of NF-κB inhibitors for 24 hours in RPMI 1640 supplemented with 10% fetal bovine serum. After incubation, apoptotic cell death was quantified by the percentage of cells with hypodiploid DNA or by the detection of apoptotic nuclei using Hoechst 33258 dye staining (Wako Pure Chemical Industries, Osaka, Japan), as described previously.23 Briefly, the cells were fixed with 70% ethanol and treated with RNAase (100 μg/mL; Sigma) and then stained with propidium iodide (100 μg/mL; Sigma) for 30 minutes on ice. The stained cells were analyzed using a flow cytometer (Epics Profile-II; Coulter Immunology, Hialeah, FL) to detect the presence of hypodiploid DNA. We also used Hoechst 33258 dye staining to detect apoptotic cells. For this purpose, the cells were treated with or without CdCl2 or NF-κB inhibitors, then fixed with 2% glutaraldehyde solution (Wako) for 10 minutes and stained with 0.2 mmol/L Hoechst 33258 to visualize DNA. Cells were examined under a fluorescence microscope (AHB-LB; Olympus, Tokyo, Japan) to determine the number of cells with chromatin condensation and/or nuclear fragmentation.
In some experiments, we added a caspase-1 specific inhibitor, Ac-Try-Val-Ala-Asp-aldehyde (YVAD-CHO; Peptide Institute), caspase-3 specific inhibitor, Ac-Asp-Glu-Val-Asp-aldehyde (DEVD-CHO; Peptide Institute, Osaka, Japan), or caspase-8 specific inhibitor, Z-Ile-Glu(OMe)-Thr-Asp(OMe)-FMK (IETD-FMK; Enzyme Systems Products, Livermore, CA) to the cell culture 3 hours before adding NF-κB inhibitors, and examined their effects on apoptotic cell death.
Determination of nuclear NF-κB activity by electrophoretic mobility shift assay (EMSA).
Determination of nuclear NF-κB activity of Jurkat or JPX-9 cells in EMSA was examined by the use of Promega Gel Shift Assay System (Promega Co, Madison, WI). In brief, binding reactions of 20 μL total volume contained 7.5 μg of nuclear proteins isolated from Jurkat or JPX-9 cells, 32P-radiolabeled double-stranded oligonucleotide containing NF-κB binding site (5′-AGTTGAGGGGACTTTCCCAGGC-3′), and 3 μg of poly(dI-dC) in 10 mmol/L Tris (pH = 7.5), 50 mmol/L NaCl, 0.5 mmol/L EDTA, 1 mmol/L MgCl2, 0.5 mmol/L DTT, and 4% glycerol. Reactions were incubated for 30 minutes at room temperature and analyzed by 5% polyacrylamide gel electrophoresis. We used nuclear proteins from the HTLV-I–infected T-cell line MT-2 as a positive control for nuclear NF-κB activity in EMSA.
Enzyme activity assay and Western blot analysis of caspase-3 and caspase-8.
We measured the enzyme activity of caspase-3 in Jurkat and JPX-9 cells as described previously.24 In brief, treated Jurkat and JPX-9 cells were washed 3 times with phosphate-buffered saline (PBS), and cytosolic extracts were lysed with 100 μL extraction buffer (50 mmol/L HEPES, 50 mmol/L KCl, 5 mmol/L EGTA, 2 mmol/L MgCl2, 1 mmol/L DTT, 20 μmol/L cytochalasin B, 2 mmol/L PMSF, 1 μg/mL leupeptin, 1 μg/mL pepstatin, 10 μg/mL antipain, 3 μg/mL chymostatin, 1% NP-40, 1 μg/mL DNAase) and sonicated. Cell lysates were then diluted with 0.4 mL caspase-3 standard buffer (100 mmol/L HEPES, 10% Sucrose, 0.1% CHAPS, 10 mmol/L DTT, and 0.1 mg/mL ovalbumin) and incubated at 30°C for 1 hour with 100 μmol/L fluorescent substrate, Ac-DEVD-MCA (Peptide Institute). The specific inhibitor for caspase-1 (Ac-YVAD-CHO, Peptide Institute) was added to the reaction mixture at a concentration of 100 μmol/L to eliminate a possible cleavage of the substrate by caspase-1. Specific caspase-3 activity was determined by subtracting the values obtained in the presence of inhibitor. The fluorescence of the cleaved substrates was determined using a spectrofluorometer set at excitation/emission wavelengths of 380/460 nm. One unit corresponds to the activity that cleaves 1 pmol/min of the respective fluorescent substrate at 30°C.
We also examined the expression of pro-caspase-8 and pro-caspase-3 by Western blotting as previously described.25,26 These forms are rapidly converted into the active subunits on the course of activation.25,26 Thus, the decrease of pro-caspase-8/3 on Western blotting means the activation of pro-caspase-8/3.25 26 In brief, the cells were washed 3 times with PBS, lysed by the addition of extraction buffer, and sonicated. The protein concentration in cell extracts was determined by the Bio-Rad (Melville, NY) protein assay kit. An identical amount of the protein for each lysate (20 μg/well) was subjected to 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). Proteins were transferred to a nitrocellulose filter, and the filter was blocked for 1 hour using 5% nonfat dried milk in PBS containing 0.1% Tween 20 (PBS-T), washed with PBS-T, and incubated at room temperature for 1 hour in 1:1,000 dilution of mouse anti-human caspase-3 monoclonal antibody (MoAb; Transduction Laboratories, Lexington, KY) or mouse anti-human caspase-8 MoAb (MBL; Nagoya, Japan). These antibodies preferentially recognize 32 kD of pro-caspase-3 or 55/54 kD of pro-caspase-8, respectively. The filter was washed with PBS-T and incubated with 1:1,000 dilution of sheep anti-mouse immunoglobulin-G (IgG), coupled with horseradish peroxidase. The enhanced chemiluminescence (ECL) system (Amersham) was used for detection. In some experiments, we added a caspase-8 inhibitor, IETD-FMK, to the culture, and also examined the expression of pro-caspase-3. We used MoAb against β-actin (Sigma) for an internal control protein in Western blot analysis.
Expression of Bcl-2 related proteins and inhibitor of apoptosis protein (IAP) family proteins in Jurkat and JPX-9 cells.
The expression of Bcl-2 related proteins and IAP family of gene products is important in regulating certain apoptotic stimuli.17-19,27 28 Thus, we examined the expression of Bcl-2, Bax, Bcl-x, XIAP, cIAP1, and cIAP2 in Jurkat and JPX-9 cells by Western blotting. In brief, cells were collected and lysed by the addition of lysis buffer (50 mmol/L Tris buffer, pH 8, 150 mmol/L NaCl, 0.02% sodium azaide, 0.1% SDS, 100 μg/mL PMSF, 1 μg/mL of aprotinin, 1% NP-40, 0.5% sodium deoxycholate) for 20 minutes at 4°C, and insoluble material was removed by centrifugation at 13,000 rpm for 30 minutes at 4°C. The supernatant was collected and the protein concentration was determined by the Bio-Rad (Melville, NY) protein assay kit. An identical amount of protein for each lysate (20 μg/mL) was subjected to SDS-PAGE. Proteins were transferred to a nitrocellulose filter and the filter was blocked for 1.5 hours using 5% nonfat dried milk in PBS containing 0.1% Tween 20 (PBS-T), washed with PBS-T and incubated at room temperature for 1 hour in the presence of each antibody (Bcl-2: mouse monoclonal, DAKO Japan, Kyoto; Bax: rabbit polyclonal, Santa Cruz Biotechnology, Santa Cruz, CA; Bcl-x: rabbit polyclonal, Transduction Laboratories, Lexington, KY; XIAP: mouse monoclonal, MBL; cIAP1: rabbit polyclonal, Santa Cruz Biotechnology; cIAP2: rabbit polyclonal, Santa Cruz Biotechnology). The filter was washed with PBS-T and incubated with 1:1000 dilution of sheep anti-mouse IgG or donkey anti-rabbit IgG, coupled with horseradish peroxidase. ECL system (Amersham) was used for detection.
Statistical analysis.
Data were expressed as mean ± SD. Differences between groups were tested for statistical significance using the Student’st-test. A P value less than .05 was considered significant.
RESULTS
Induction of apoptosis of Jurkat and JPX-9 cells by NF-κB inhibitors.
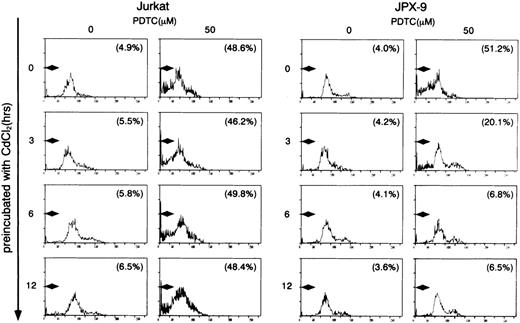
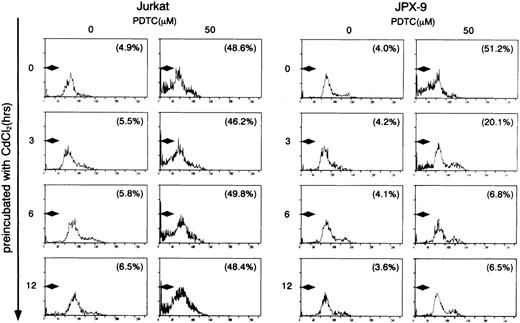
We initially examined the apoptotic cell death of Jurkat and JPX-9 cells induced by PDTC. As shown in Table 1, PDTC induced apoptosis, determined by flow cytometry, of Jurkat and JPX-9 cells in the absence of CdCl2. The percentage of apoptotic cells was similar in Jurkat and JPX-9 cells. The maximal effect was noted at a PDTC concentration of 50 μmol/L. Similar results were obtained when apoptosis was quantified by the Hoechst 33258 staining method (data not shown). Based on these initial results, we used 50 μmol/L of PDTC in the remaining experiments. In addition to PDTC, NAC also clearly induced apoptosis of Jurkat and JPX-9 cells (Table 2). Furthermore, the addition of 10 μmol/L of LLL-CHO for 24 hours induced apoptosis in both Jurkat and JPX-9 cells in the absence of CdCl2 (55.9% ± 3.9% in Jurkat cells and 56.9% ± 4.3% in JPX-9 cells from 4 experiments determined by flow cytometry). In contrast, when JPX-9 cells were preincubated with 30 μmol/L of CdCl2 to induce Tax expression, a significant suppression of apoptosis of the cells induced by PDTC was observed. This effect was not detected in Jurkat cells preincubated with or without CdCl2 or JPX-9 cells without CdCl2 (Fig 2). Inhibition of apoptosis occurred after preincubation with CdCl2 after 3 hours, and reached a peak level when preincubated for 6 hours (Fig 2). Similar results were obtained in CdCl2-pretreated JPX-9 cells when NAC or LLL-CHO was used as a NF-κB inhibitor. In the presence of NAC (50 mmol/L), CdCl2 preincubation decreased hypodiploid DNA+ cells from 44.5% ± 3.2% to 4.9% ± 0.3%, while in the presence of LLL-CHO (10 μmol/L), hypodiploid DNA+ cells decreased from 56.5% ± 3.9% to 5.9% ± 0.4% with CdCl2 preincubation (results from 4 experiments). As previously described,6 20 Tax expression in JPX-9 cells induced by preincubation with CdCl2 for 6 hours was determined by RT-PCR (Fig 1) and Western blot analysis.
Induction of apoptosis in Jurkat and JPX-9 cells by PDTC determined by flow cytometry. Jurkat and JPX-9 cells were preincubated with 30 μmol/L of CdCl2 for indicated time intervals, washed with PBS, and further cultured with 50 μmol/L of PDTC for 24 hours. After cultivation, apoptotic cell death was determined by flow cytometry as described in the text. Numbers in parentheses are percentages of cells with hypodiploid DNA. The results are representative examples of 6 similar experiments.
Induction of apoptosis in Jurkat and JPX-9 cells by PDTC determined by flow cytometry. Jurkat and JPX-9 cells were preincubated with 30 μmol/L of CdCl2 for indicated time intervals, washed with PBS, and further cultured with 50 μmol/L of PDTC for 24 hours. After cultivation, apoptotic cell death was determined by flow cytometry as described in the text. Numbers in parentheses are percentages of cells with hypodiploid DNA. The results are representative examples of 6 similar experiments.
Activation of caspase-3 during the apoptotic process induced by NF-κB inhibitors.
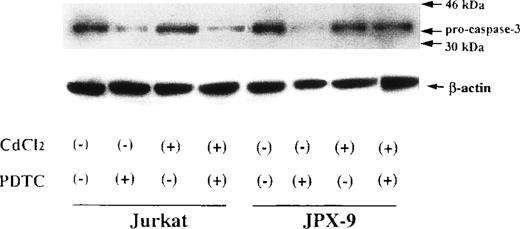
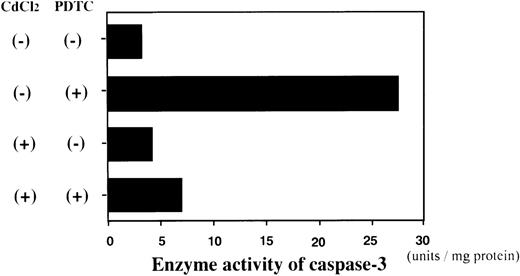
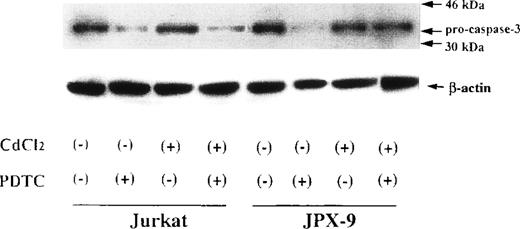
We next examined whether activation of caspase-3 is involved in the apoptotic process induced by NF-κB inhibitors. During the process of caspase-3 activation, the expression of pro-caspase-3 is decreased, and enzyme activity is increased.25 As shown in Fig3, the expression of pro-caspase-3 was significantly decreased during PDTC-induced apoptosis of Jurkat cells with or without preincubation with CdCl2. In contrast, preincubation of JPX-9 cells with CdCl2 inhibited the decrease in expression of pro-caspase-3 (Fig 3). Similar results were obtained in JPX-9 cells preincubated with CdCl2 when NAC or LLL-CHO was used (Fig 4). The enzyme activity of caspase-3 was markedly increased in apoptotic JPX-9 cells incubated with PDTC in the absence of CdCl2. This increase was inhibited in JPX-9 cells preincubated with CdCl2 (Fig5). Similar results were obtained when NAC or LLL-CHO was used in the experiments instead of PDTC (data not shown). In addition, the peptide DEVD-CHO inhibited the apoptosis of Jurkat and JPX-9 cells induced by PDTC (Table3). The inhibitory effect of DEVO-CHO was also evident in apoptotis induced by NAC or LLL-CHO. (Apoptosis of JPX-9 cells by NAC, 45.5% ± 3.4%, decreased to 5.8% ± 0.4%, and that by LLL-CHO, 56.9% ± 4.1%, decreased to 8.7% ± 0.7% in the presence of DEVD-CHO. The data are mean ± SD from 4 experiments of hypodiploid DNA+ cells). These data suggest that while caspase-3 is involved in the apoptotic process induced by NF-κB inactivation, the HTLV-I Tax protein inhibits the activation of caspase-3.
Expression of pro-caspase-3 in Jurkat and JPX-9 cells determined by Western blotting. Jurkat and JPX-9 cells were preincubated with or without CdCl2 for 6 hours. After washing with PBS, the cells were further cultured with or without 50 μmol/L of PDTC for 24 hours. After cultivation, we examined the expression of pro-caspase-3 as described in the text. Results are representative examples of 6 similar experiments. Note that the expression of pro-caspase-3 in Jurkat cells is significantly decreased by PDTC irrespective of CdCl2 pretreatment, whereas that of JPX-9 cells in the presence of PDTC is almost suppressed by preincubation of the cells with CdCl2.
Expression of pro-caspase-3 in Jurkat and JPX-9 cells determined by Western blotting. Jurkat and JPX-9 cells were preincubated with or without CdCl2 for 6 hours. After washing with PBS, the cells were further cultured with or without 50 μmol/L of PDTC for 24 hours. After cultivation, we examined the expression of pro-caspase-3 as described in the text. Results are representative examples of 6 similar experiments. Note that the expression of pro-caspase-3 in Jurkat cells is significantly decreased by PDTC irrespective of CdCl2 pretreatment, whereas that of JPX-9 cells in the presence of PDTC is almost suppressed by preincubation of the cells with CdCl2.
Activation of caspase-3 in JPX-9 cells by NAC or LLL-CHO, which is inhibited by preincubation of the cells with CdCl2. JPX-9 cells were preincubated with or without CdCl2 for 6 hours. After washing with PBS, the cells were further cultured with or without 50 mmol/L of NAC or 10 μmol/L of LLL-CHO for 24 hours. After cultivation, the expression of pro-caspase-3 was determined as described in the text. Results are representative examples of 4 similar experiments. Note that the expression of pro-caspase-3 in CdCl2-untreated JPX-9 cells is significantly decreased by 2 kinds of NF-κB inhibitors, whereas that is almost inhibited by preincubation of the cells with CdCl2.
Activation of caspase-3 in JPX-9 cells by NAC or LLL-CHO, which is inhibited by preincubation of the cells with CdCl2. JPX-9 cells were preincubated with or without CdCl2 for 6 hours. After washing with PBS, the cells were further cultured with or without 50 mmol/L of NAC or 10 μmol/L of LLL-CHO for 24 hours. After cultivation, the expression of pro-caspase-3 was determined as described in the text. Results are representative examples of 4 similar experiments. Note that the expression of pro-caspase-3 in CdCl2-untreated JPX-9 cells is significantly decreased by 2 kinds of NF-κB inhibitors, whereas that is almost inhibited by preincubation of the cells with CdCl2.
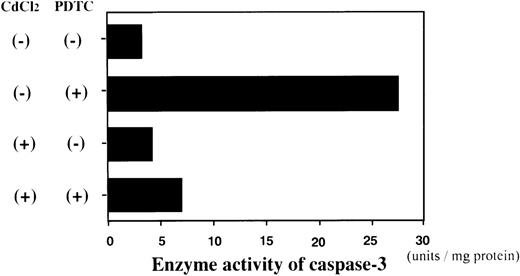
Enzyme activity assay of caspase-3 in JPX-9 cells. JPX-9 cells were preincubated with or without CdCl2 for 6 hours, and further cultured in the presence or absence of 50 μmol/L of PDTC for 24 hours. After incubation, the enzyme activity of caspase-3 was determined as described in the text. Results are representative examples of 5 similar experiments. Note that caspase-3 enzyme activity is markedly increased in CdCl2-untreated JPX-9 cells by PDTC, whereas it is significantly suppressed by preincubation of cells with CdCl2.
Enzyme activity assay of caspase-3 in JPX-9 cells. JPX-9 cells were preincubated with or without CdCl2 for 6 hours, and further cultured in the presence or absence of 50 μmol/L of PDTC for 24 hours. After incubation, the enzyme activity of caspase-3 was determined as described in the text. Results are representative examples of 5 similar experiments. Note that caspase-3 enzyme activity is markedly increased in CdCl2-untreated JPX-9 cells by PDTC, whereas it is significantly suppressed by preincubation of cells with CdCl2.
Sequential activation of caspase-8 to caspase-3 is an essential signal to induce apoptosis during inhibition of NF-κB.
Recent reports have suggested that sequential activation of caspases, initiating from caspase-8, is essential for the activation of caspase-3 during certain apoptotic stimuli.17,26,29 Thus, we next examined the involvement of caspase-8 and caspase-1 in the apoptotic process of Jurkat and JPX-9 cells induced by NF-κB inactivation. Blocking experiments using peptide inhibitors indicated the importance of caspase-8 in PDTC-induced apoptosis because the caspase-8 inhibitor, IETD-FMK, significantly abrogated PDTC-induced apoptosis, whereas the caspase-1 inhibitor, YVAD-CHO, did not affect this process (Table4). Similar results were obtained in NAC- or LLL-CHO–induced apoptosis (CdCl2-untreated, and NAC-treated JPX-9 cells without IETD-FMK contained 42.2% ± 2.2% of hypodiploid DNA+ cells, which was decreased to 6.1% ± 0.4% by the addition of IETD-FMK. CdCl2-untreated, and LLL-CHO-treated JPX-9 cells without IETD-FMK contained 56.9 ± 4.4% of hypodiploid DNA+cells, which was decreased to 8.9 ± 0.5% by IETD-FMK. The results are mean ± SD from four experiments). When we added the caspase-8 inhibitor IETD-FMK to cultures, in which the cells did not undergo apoptosis, the expression of pro-caspase-3 was not decreased (Fig6), indicating that the activation of caspase-3 was clearly suppressed by the caspase-8 inhibitor. These results suggest that caspase-8 is upstream of caspase-3 during the apoptotic signal induced by inactivation of NF-κB. In addition, 2 isoforms of pro-caspase-8 determined by Western blotting26were significantly decreased in CdCl2-untreated JPX-9 cells after the addition of PDTC. This phenomenon was significantly suppressed by CdCl2 pretreatment (Fig7). These data also suggest that the inhibitory effect of Tax protein on inactivation of caspase-3 is mediated through inactivation of caspase-8.
Suppression of the activation of caspase-3 by caspase-8 inhibitor in JPX-9 cells during apoptotic process induced by NF-κB inhibitors. CdCl2-untreated JPX-9 cells were cultured with or without NF-κB inhibitors (PDTC, NAC, or LLL-CHO) in the presence or absence of caspase-8 inhibitor Z-IETD-FMK for 24 hours. After incubation, the expression of pro-caspase-3 was determined as described in the text. Note that the decrease of pro-caspase-3 expression, which indicates caspase-3 activation, in JPX-9 cells induced by NF-κB inhibitors is almost recovered by the addition of Z-IETD-FMK. Results are representative examples of 4 similar experiments.
Suppression of the activation of caspase-3 by caspase-8 inhibitor in JPX-9 cells during apoptotic process induced by NF-κB inhibitors. CdCl2-untreated JPX-9 cells were cultured with or without NF-κB inhibitors (PDTC, NAC, or LLL-CHO) in the presence or absence of caspase-8 inhibitor Z-IETD-FMK for 24 hours. After incubation, the expression of pro-caspase-3 was determined as described in the text. Note that the decrease of pro-caspase-3 expression, which indicates caspase-3 activation, in JPX-9 cells induced by NF-κB inhibitors is almost recovered by the addition of Z-IETD-FMK. Results are representative examples of 4 similar experiments.
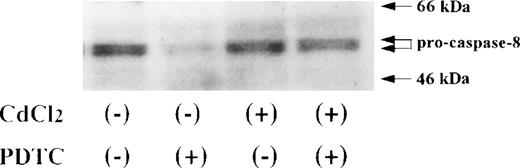
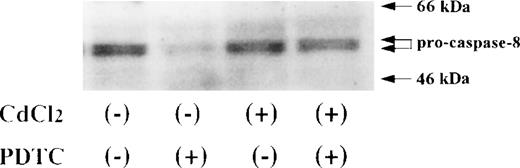
Activation of caspase-8 in JPX-9 cells during PDTC-induced apoptosis. JPX-9 cells were preincubated with or without CdCl2 for 6 hours, washed with PBS, and further incubated with or without PDTC (50 μmol/L) for 24 hours. After incubation, the expression of procaspase-8 was examined as described in the text. Note that the decrease of pro-caspase-8 expression, which indicates caspase-8 activation, was almost recovered even in the presence of PDTC by preincubation of the cells with CdCl2. Results are representative examples of 5 similar experiments.
Activation of caspase-8 in JPX-9 cells during PDTC-induced apoptosis. JPX-9 cells were preincubated with or without CdCl2 for 6 hours, washed with PBS, and further incubated with or without PDTC (50 μmol/L) for 24 hours. After incubation, the expression of procaspase-8 was examined as described in the text. Note that the decrease of pro-caspase-8 expression, which indicates caspase-8 activation, was almost recovered even in the presence of PDTC by preincubation of the cells with CdCl2. Results are representative examples of 5 similar experiments.
We confirmed the relationship between NF-κB inactivation and activation of caspase cascade by the use of EMSA and kinetic studies of caspase activation. EMSA showed that small amount of nuclear NF-κB activity was present in CdCl2-untreated JPX-9 cells. The treatment of these cells with NF-κB inhibitors for 3 hours mostly abrogated NF-κB nuclear translocation (Fig8A). Both flow cytometric analysis and Hoechst 33258 dye staining showed that these cells (treated with NF-κB inhibitors for 3 hours) did not undergo apoptosis (data not shown). However, CdCl2-treated JPX-9 cells contained a significant amount of nuclear NF-κB, and substantial NF-κB nuclear activity still remained after the addition of NF-κB inhibitors in these cells (Fig 8B). Nuclear NF-κB activity of Jurkat cells was not increased by CdCl2 (Fig 8C). Kinetic studies of the expression of pro-caspase-8 or -3 suggested that both activation of the caspase cascade and induction of apoptosis was operated after the inhibition of NF-κB nuclear translocation because the decrease of pro-caspase protein expression was not seen until 24 hours after incubation with PDTC (Fig 9).
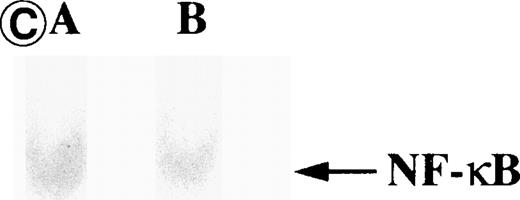
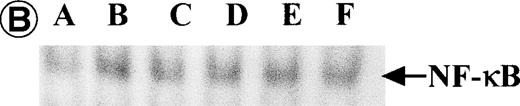
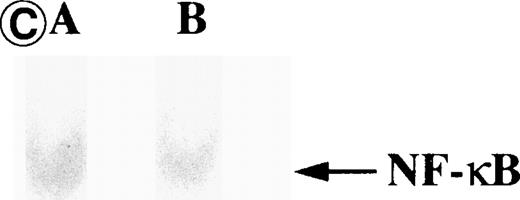
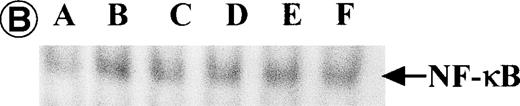
Nuclear NF-κB activity determined by EMSA. Nuclear proteins of JPX-9 cells treated with (B; CdCl2 treatment for 6 hours) or without CdCl2 (A) in the presence or absence of NF-κB inhibitors, was incubated with radiolabeled oligonucleotide containing NF-κB binding site, and nuclear NF-κB activity was examined by EMSA as described in the text. (A) Nuclear NF-κB activity in CdCl2-untreated JPX-9 cells. Lane A; positive control (MT-2 cells). Lane B; CdCl2-untreated JPX-9 cells without NF-κB inhibitors. Lane C; CdCl2-untreated JPX-9 cells, and then cultured in 50 μmol/L of PDTC for 3 hours. Lane D; CdCl2-untreated JPX-9 cells, and then cultured in 50 mmol/L of NAC for 3 hours. Lane E; CdCl2-untreated JPX-9 cells, and then cultured in 10 μmol/L of LLL-CHO for 3 hours. Note that basal nuclear NF-κB activity in CdCl2-untreated JPX-9 cells was clearly suppressed by 3 kinds of NF-κB inhibitors. (B) Nuclear NF-κB activity in CdCl2-treated JPX-9 cells. Lane A; control (CdCl2-untreated JPX-9 cells without NF-κB inhibitors). Lane B; CdCl2-treated JPX-9 cells (without NF-κB inhibitors). Lane C; CdCl2-treated JPX-9 cells, and then cultured in 50 μmol/L of PDTC for 3 hours. Lane D; CdCl2-treated JPX-9 cells, and then cultured in 50 mmol/L of NAC for 3 hours. Lane E; CdCl2-treated JPX-9 cells, and then cultured in 10 μmol/L of LLL-CHO for 3 hours. Lane F; CdCl2-treated JPX-9 cells, and then cultured for 3 hours without NF-κB inhibitors. Note that nuclear NF-κB in JPX-9 cells was clearly augmented by CdCl2 treatment, and its activity is still high in the presence of 3 kinds of NF-κB inhibitors. (C) Nuclear NF-κB activity of Jurkat cells was not augmented by incubation of the cells with CdCl2. Lane A; Jurkat cells without CdCl2 treatment. Lane B; Jurkat cells treated with CdCl2 for 6 hours. Results (Fig 8A through C) are representative of 5 experiments.
Nuclear NF-κB activity determined by EMSA. Nuclear proteins of JPX-9 cells treated with (B; CdCl2 treatment for 6 hours) or without CdCl2 (A) in the presence or absence of NF-κB inhibitors, was incubated with radiolabeled oligonucleotide containing NF-κB binding site, and nuclear NF-κB activity was examined by EMSA as described in the text. (A) Nuclear NF-κB activity in CdCl2-untreated JPX-9 cells. Lane A; positive control (MT-2 cells). Lane B; CdCl2-untreated JPX-9 cells without NF-κB inhibitors. Lane C; CdCl2-untreated JPX-9 cells, and then cultured in 50 μmol/L of PDTC for 3 hours. Lane D; CdCl2-untreated JPX-9 cells, and then cultured in 50 mmol/L of NAC for 3 hours. Lane E; CdCl2-untreated JPX-9 cells, and then cultured in 10 μmol/L of LLL-CHO for 3 hours. Note that basal nuclear NF-κB activity in CdCl2-untreated JPX-9 cells was clearly suppressed by 3 kinds of NF-κB inhibitors. (B) Nuclear NF-κB activity in CdCl2-treated JPX-9 cells. Lane A; control (CdCl2-untreated JPX-9 cells without NF-κB inhibitors). Lane B; CdCl2-treated JPX-9 cells (without NF-κB inhibitors). Lane C; CdCl2-treated JPX-9 cells, and then cultured in 50 μmol/L of PDTC for 3 hours. Lane D; CdCl2-treated JPX-9 cells, and then cultured in 50 mmol/L of NAC for 3 hours. Lane E; CdCl2-treated JPX-9 cells, and then cultured in 10 μmol/L of LLL-CHO for 3 hours. Lane F; CdCl2-treated JPX-9 cells, and then cultured for 3 hours without NF-κB inhibitors. Note that nuclear NF-κB in JPX-9 cells was clearly augmented by CdCl2 treatment, and its activity is still high in the presence of 3 kinds of NF-κB inhibitors. (C) Nuclear NF-κB activity of Jurkat cells was not augmented by incubation of the cells with CdCl2. Lane A; Jurkat cells without CdCl2 treatment. Lane B; Jurkat cells treated with CdCl2 for 6 hours. Results (Fig 8A through C) are representative of 5 experiments.
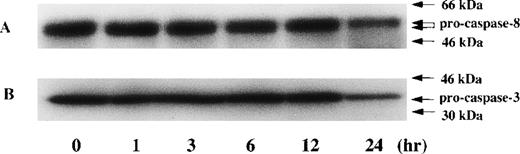
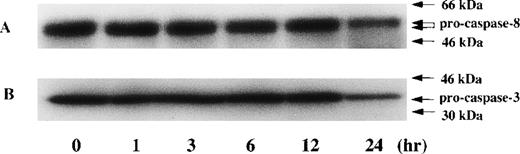
Time kinetic study of activation in caspase-8 and caspase-3 during PDTC-induced apoptosis. CdCl2-untreated JPX-9 cells were cultured for indicated hours in the presence of 50 μmol/L of PDTC. After incubation, the expression of pro-caspase-8 (A) and procaspase-3 (B) was examined by Western blotting. Note that the decrease of pro-caspase protein expression was found at 24 hours incubation of JPX-9 cells with PDTC, whereas that was not determined at 3 hours incubation of the cells with PDTC in which nuclear NF-κB activity had already been suppressed. Results are representative of 4 experiments.
Time kinetic study of activation in caspase-8 and caspase-3 during PDTC-induced apoptosis. CdCl2-untreated JPX-9 cells were cultured for indicated hours in the presence of 50 μmol/L of PDTC. After incubation, the expression of pro-caspase-8 (A) and procaspase-3 (B) was examined by Western blotting. Note that the decrease of pro-caspase protein expression was found at 24 hours incubation of JPX-9 cells with PDTC, whereas that was not determined at 3 hours incubation of the cells with PDTC in which nuclear NF-κB activity had already been suppressed. Results are representative of 4 experiments.
Expression of Bcl-2 related proteins and IAP family proteins in Jurkat and JPX-9 cells.
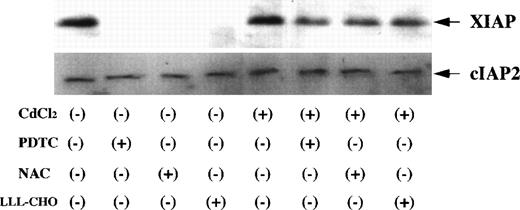
Bcl-2 related proteins, such as Bcl-2, Bax, and Bcl-x, are involved in the regulation of apoptotic process.17 27 Therefore, we investigated changes in the expression of Bcl-2, Bax, and Bcl-x on Jurkat and JPX-9 cells by Western blot analysis. There was no significant change of Bcl-2 expression in both Jurkat and JPX-9 cells with or without of CdCl2 or NF-κB inhibitors (Fig10) although the amount of Bcl-2 in Jurkat cells was higher than that in JPX-9 cells. In addition, the expression of Bax and Bcl-x was not different in both Jurkat and JPX-9 cells under these culture conditions (data not shown). Expression of IAP family proteins was also examined. As shown in Fig11, XIAP expression in CdCl2-untreated JPX-9 cells almost disappeared after the addition of NF-κB inhibitors. However, XIAP expression in CdCl2-treated JPX-9 cells was maintained even after addition of NF-κB inhibitors. cIAP2 expression in JPX-9 cells was not changed in these culture conditions (Fig 11), and cIAP1 expression was not detected in Jurkat or JPX-9 cells (data not shown).
Bcl-2 expression in Jurkat and JPX-9 cells. (A) Jurkat and JPX-9 cells were preincubated with or without CdCl2 for 6 hours, washed with PBS, and further incubated with or without PDTC (50 μmol/L) for 24 hours. After incubation, the expression of Bcl-2 was examined as described in the text. (B) JPX-9 cells were preincubated with or without CdCl2 for 6 hours, washed with PBS, and further incubated with or without NAC (50 mmol/L) or LLL-CHO (10 μmol/L) for 24 hours. After incubation, the expression of Bcl-2 was examined as described in the text. Note that the expression of Bcl-2 in Jurkat or JPX-9 was not different in each culture condition. Results are representative examples of 5 similar experiments.
Bcl-2 expression in Jurkat and JPX-9 cells. (A) Jurkat and JPX-9 cells were preincubated with or without CdCl2 for 6 hours, washed with PBS, and further incubated with or without PDTC (50 μmol/L) for 24 hours. After incubation, the expression of Bcl-2 was examined as described in the text. (B) JPX-9 cells were preincubated with or without CdCl2 for 6 hours, washed with PBS, and further incubated with or without NAC (50 mmol/L) or LLL-CHO (10 μmol/L) for 24 hours. After incubation, the expression of Bcl-2 was examined as described in the text. Note that the expression of Bcl-2 in Jurkat or JPX-9 was not different in each culture condition. Results are representative examples of 5 similar experiments.
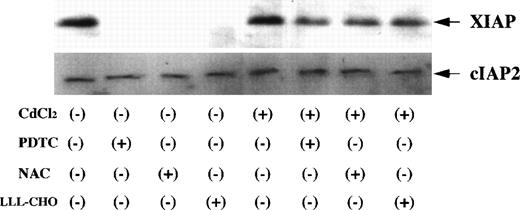
Expression of XIAP and cIAP2 in JPX-9 cells. JPX-9 cells were preincubated with or without CdCl2 for 6 hours, washed with PBS, and further incubated with or without PDTC (50 μmol/L), NAC (50 mmol/L), or LLL-CHO (10 μmol/L) for 24 hours. After incubation, the expression of XIAP and cIAP2 was examined as described in the text. Note that XIAP expression in CdCl2-untreated JPX-9 cells is almost suppressed by NF-κB inhibitors, however, its expression in CdCl2-treated JPX-9 cells is maintained even in the presence of NF-κB inhibitors. In contrast, cIAP2 expression in JPX-9 cells was not changed in each culture condition. Results are representative examples of 4 similar experiments.
Expression of XIAP and cIAP2 in JPX-9 cells. JPX-9 cells were preincubated with or without CdCl2 for 6 hours, washed with PBS, and further incubated with or without PDTC (50 μmol/L), NAC (50 mmol/L), or LLL-CHO (10 μmol/L) for 24 hours. After incubation, the expression of XIAP and cIAP2 was examined as described in the text. Note that XIAP expression in CdCl2-untreated JPX-9 cells is almost suppressed by NF-κB inhibitors, however, its expression in CdCl2-treated JPX-9 cells is maintained even in the presence of NF-κB inhibitors. In contrast, cIAP2 expression in JPX-9 cells was not changed in each culture condition. Results are representative examples of 4 similar experiments.
DISCUSSION
The apoptotic process involves a complex machinery regulated by molecular interactions of various gene products.12-19,22,28One of the major gene products that induce apoptosis is the caspase family, which is conserved from nematodes to mammals.17 In humans, more than 10 such proteins are divided in 3 subfamilies.17 Among these, a sequential activation of caspase-8 to caspase-1, to caspase-3 is one of the major cascades involved in the induction of apoptotic cell death including signaling through Fas and TNFR.17,26,29 In contrast, a major molecule that inhibits apoptosis is the nuclear transcription factor NF-κB.12-19 Binding of TNF to TNFR stimulates both the caspase cascade and NF-κB nuclear translocation. In this model, TNF-induced cytotoxicity increases when NF-κB activation is inhibited,12-19 suggesting a molecular interaction between caspases and NF-κB. Thus, we initially examined whether inactivation of NF-κB induces sequential activation of the caspase cascade, resulting in the induction of apoptosis.
Our results showed that both Jurkat and JPX-9 cells underwent apoptosis in the presence of NF-κB inhibitors, without the addition of CdCl2. As suggested in the recent study that both the cleavage of poly(ADP-ribose) polymerase (PARP) and induction of apoptosis in HuT-78 cells was induced by NF-κB inactivation,30 a significant activation of caspase-3 in the cells was noted as determined by Western blotting and enzyme activity assay during this process. Furthermore, the addition of DEVD-CHO, a caspase-3 inhibitor, abrogated apoptotic cell death induced by NF-κB inhibitors, suggesting that caspase-3 is a major effector caspase to induce apoptosis when NF-κB nuclear translocation is inhibited. We next examined whether caspase-8 and caspase-1, caspases located upstream of caspase-3, are involved during an apoptotic process induced by NF-κB inhibitors. According to the results using peptide inhibitors, the involvement of not caspase-1 but caspase-8 is apparent because the caspase-1 inhibitor YVAD-CHO did not inhibit the apoptotic process. However, the caspase-8 inhibitor IETD-FMK significantly suppressed apoptosis induced by NF-κB inhibitors. The evidence that caspase-1 is not involved in apoptotic process has been reported in Fas-mediated apoptosis of synovial cells.31 Western blot analysis showed that pro-caspase-8 expression is markedly decreased when we added NF-κB inhibitors to the cells, indicating that activation of caspase-8 is an essential event in the induction of apoptosis by NF-κB inhibition. The caspase-8 inhibitor IETD-FMK inhibited the activation of caspase-3 in CdCl2-untreated JPX-9 cells induced by NF-κB inhibitors, while caspase-8 activation in JPX-9 cells by NF-κB inhibitors was clearly suppressed in the cells pretreated with CdCl2. These data strongly suggest that sequential activation from caspase-8 to caspase-3 is a central apoptotic pathway in the process of apoptosis induced by inactivation of NF-κB.
What are the molecular mechanisms of HTLV-I Tax protein that inhibit the activation of caspase cascade? EMSA showed that the treatment of CdCl2-untreated JPX-9 cells with NF-κB inhibitors clearly suppressed basal nuclear NF-κB activity of the cells, whereas nuclear NF-κB activity in JPX-9 cells was significantly augmented by CdCl2. Furthermore, residual NF-κB activity in CdCl2-treated JPX-9 cells was still high even with the addition of NF-κB inhibitors. These data indicate that nuclear NF-κB activity is a principal factor to suppress the activation of caspase cascade initiated from caspase-8, and Tax-mediated inhibition of caspase cascade is mediated through hyperactivation of NF-κB in the cells. However, a kinetic study (Fig 9) suggests that gene products regulated by NF-κB modulate the activation of caspase cascade because the activation of the caspase cascade was followed by inhibition of NF-κB nuclear translocation. Recent reports have suggested that the expression of IAP family proteins is regulated by NF-κB, and their expression in the cells is important to inhibit the activation of caspase-3.18,19 We showed in the present study that the expression of XIAP in CdCl2-untreated JPX-9 cells was clearly suppressed by NF-κB inhibitors. In contrast, its expression in CdCl2-treated JPX-9 cells was maintained even with the addition of NF-κB inhibitors, suggesting an importance of Tax-mediated NF-κB activation in XIAP expression in the cells. cIAP1 expression was not detected, and the expression of cIAP2 was not changed with or without CdCl2 or NF-κB inhibitors. Furthermore, the expression of Bcl-2, Bax, and Bcl-x was also not changed in Jurkat and JPX-9 cells with or without CdCl2 or NF-κB inhibitors. Therefore, we speculate that Tax-mediated anti-apoptotic effect is accomplished, in part, through XIAP expression, which is regulated by NF-κB. Recent experiments suggest that while XIAP directly inhibits the activation of caspase-3, it does not prevent the caspase-8–induced proteolytic activation of caspase-3.28 Because Tax-mediated anti-apoptotic effects appear to act on caspase-8, anti-apoptotic molecules other than XIAP such as FLICE-inhibitory protein32 may also be responsible for this process.
In conclusion, we showed the inhibitory effect of HTLV-I Tax in the activation of caspase cascade and suggest a role for Tax in the prevention of apoptosis. The lack of apoptotic cell death inlpr and in gld mice causes various autoimmune disorders by the inability to eliminate self-reactive T cells.33,34Various kinds of viruses encode anti-apoptotic proteins such as an E1B for an adenovirus35 and BHRF1 for an Epstein-Barr virus,36 and transgenic mice overexpressing Tax protein develop diseases quite similar to SS and RA.37 38 Although the precise molecular mechanisms in the process are not fully understood at present, our present data may imply new insight to why autoimmune disorders are developed in HTLV-I seropositive subjects. Perhaps, viral protein-mediated inhibition of apoptosis could also be involved in autoimmune disorders induced by viruses other than HTLV-I.
ACKNOWLEDGMENT
We thank Prof Sugamura for providing us Jurkat and JPX-9 cells. We also thank Y. Matsuo, E. Nogami, and N. Fukuda for their excellent technical assistance. We also thank Dr F.G. Issa, Sydney, Australia, for the careful reading and editing of the manuscript.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Atsushi Kawakami, MD, The First Department of Internal Medicine, Nagasaki University School of Medicine, 1-7-1 Sakamoto, Nagasaki 852-8501, Japan.