Abstract
In this study, we test the hypothesis that prothrombin levels may modulate activated protein C (APC) anticoagulant activity. Prothrombin in purified systems or plasma dramatically inhibited the ability of APC to inactivate factor Va and to anticoagulate plasma. This was not due solely to competition for binding to the membrane surface, as prothrombin also inhibited factor Va inactivation by APC in the absence of a membrane surface. Compared with normal factor Va, inactivation of factor Va Leiden by APC was much less sensitive to prothrombin inhibition. This may account for the observation that the Leiden mutation has less of an effect on plasma-based clotting assays than would be predicted from the purified system. Reduction of protein C levels to 20% of normal constitutes a significant risk of thrombosis, yet these levels are observed in neonates and patients on oral anticoagulant therapy. In both situations, the correspondingly low prothrombin levels would result in an increased effectiveness of the remaining functional APC of ≈5-fold. Thus, while the protein C activation system is impaired by the reduction in protein C levels, the APC that is formed is a more effective anticoagulant, allowing protein C levels to be reduced without significant thrombotic risk. In situations where prothrombin is high and protein C levels are low, as in early stages of oral anticoagulant therapy, the reduction in protein C would result only in impaired function of the anticoagulant system, possibly explaining the tendency for warfarin-induced skin necrosis.
IN THE LAST DECADE, mutations of components within the protein C anticoagulant pathway and a prothrombin dimorphism in the 3′ untranslated region of the gene (G20210A) have emerged as dominant contributors to thrombotic risk.1-4 Why defects in the protein C system are associated with thrombotic risk is relatively well understood, as failure of this system to operate optimally results in more stable factor Va and prothrombin activation complexes, almost certainly resulting in the observed hypercoagulable state. The protein C concentration in plasma (≈65 nmol/L)5 is well below the concentration required for optimal activation by the physiological activation complex (≈0.7 μmol/L).6 In contrast, prothrombin circulates at the highest level of any of the vitamin K-dependent coagulation zymogens. Its circulating concentration (≈1.4 μmol/L) is about 5 times the Km for prothrombin activation by the physiological activation complex,7 leading to the prediction that changes in prothrombin level should have little effect on the hemostatic balance.8 In addition to the risk associated with the prothrombin dimorphism, another feature observed experimentally is that the decrease in prothrombin level is the best predictor of antithrombotic efficacy of oral anticoagulants.9Furthermore, optimal clinical anticoagulation seems to correlate best with prothrombin levels and specifically when these levels are between about 12% to 24% of normal.10 11 These observations seem at variance with the biochemistry.
Another unanswered question is why oral anticoagulants work so effectively given that these agents reduce not only the clotting factor levels, but also reduce the levels of protein C and protein S comparably.12,13 Patients with 10% to 20% protein C or protein S are at risk of thrombosis,3 and the combination would be expected to constitute a more severe risk.14 In contrast, reductions to 20% or so of the coagulation zymogens constitutes little risk of hemorrhage.
Of the defects in the protein C pathway associated with venous thrombosis, activated protein C (APC) resistance due to a polymorphism in factor Va (factor V Leiden) is by far the most common.3In purified systems, the inactivation of factor Va Leiden is much slower than normal factor Va (about 20 to 30 times), but in plasma, the presence of factor V Leiden has only about a 2-fold to 4-fold effect on the APC anticoagulant activity of APC when the coagulation cascade is triggered at the level of factor Xa.15 16 Based on the reduced rates of inhibition observed in the purified system, one would predict a more pronounced effect in plasma.
One possible explanation for these unanswered questions is that the prothrombin concentration potently impacts the ability of the protein C pathway to function. Indeed, prothrombin inhibition of APC function has been observed.17 In these studies, the investigators concluded that inhibition is mediated by blocking protein S function. An alternative mechanism could involve prothrombin protection of factor Va from APC proteolysis. Factor Va is known to bind to prothrombin,18 and the cleavage of factor Va by APC results in the loss of prothrombin binding capacity.18 19 Given these interactions of prothrombin with factor Va and the unanswered questions related to the mechanisms of prothrombin regulation of hemostasis, we examined the ability of prothrombin to modulate APC inactivation of factor Va in purified systems and to influence the ability of APC to anticoagulate plasma. We find that the prothrombin concentration is a very strong determinant of APC anticoagulant activity and inhibition of APC inactivation of factor Va can occur on both membrane surfaces and in solution and effectively retards factor Va inactivation by APC in the absence of protein S.
MATERIALS AND METHODS
Proteins and reagents.
Human prothrombin,20 APC,21 Gla domainless APC (GD-APC),22 protein S,23 factor Xa,24 and the factor X activator from Russell’s viper venom (X-CP)25 were prepared as described previously. Human factor Va was purified by immunoaffinity chromatography from normal plasma26 or Factor V Leiden plasma27 as described. Human factor IX was a kind gift of Centeon L.L.C. (King of Prussia, PA). An APC chimera in which the protein C Gla domain is replaced with that of prothrombin (APC/PTGla) was prepared as described.26 Bovine serum albumin (BSA), ovalbumin, gelatin, 3-(N-morpholino) propanesulfonic acid (MOPS), Tris-HCl, and salts were from Sigma (St Louis, MO). The chromogenic substrates Spectrozyme TH and Spectrozyme PCa were from American Diagnostica (Greenwich, CT). The phospholipids used in these studies were 1-palmitoyl-2-oleoyl-sn-glycero-3 phosphatidylserine (PS), 1-palmitoyl-2-oleoyl-sn-glycero-3 phosphatidylcholine (PC), and 1,2-dilinoleoyl-sn-glycero-3-phosphatidylethanolamine (PE) and were purchased from Avanti Polar Lipids, Inc (Alabaster, AL). 1-Palmitoyl-2-[1-14C-oleoyl]PC was from DuPont NEN (Boston, MA). Factor V–deficient human plasma was prepared by the method of Bloom et al.28
Measurement of APC activity in the purified system.
Factor Va inactivation was analyzed with a 3-stage assay essentially as described (see Smirnov and Esmon29 for experimental details). All reactions were run at room temperature in 96-well polyvinyl chloride plates (Costar, Cambridge, MA). All reagents were diluted in 150 mmol/L NaCl, 20 mmol/L Tris-HCl, pH 7.4, 0.02% azide (TBS) containing 1 mg/mL gelatin, and 5 mg/mL BSA. Briefly, factor Va (0.2 nmol/L unless noted) was incubated with APC or the chimera APC/PTGla in the first stage for 30 minutes with phospholipid in the presence or absence of protein S. The concentrations of reactants are indicated in the figure legends. In the second stage, after inhibition of APC with p-(amidinophenyl)-methanesulphonyl fluoride, residual factor Va activity was monitored by its ability to enhance prothrombin activation in the presence of excess factor Xa (1 nmol/L), prothrombin (1.4 μmol/L), and 5 mmol/L CaCl2. After 5 minutes of activation, the reaction was stopped by the addition of EDTA and the resultant thrombin was measured in the third stage using a chromogenic assay. Remaining factor Va activity was calculated by dividing the rate of thrombin formation in the presence of factor Va treated with APC in stage 1 by the rate of thrombin formation with untreated factor Va. The validity of this assay with respect to enzyme concentration and time of reaction was presented elsewhere.26 All experiments were repeated at least 2 times on separate days with comparable results.
Clotting assays.
Clotting was initiated at the level of factor X activation by use of X-CP. All reagents were diluted in TBS containing 1 mg/mL gelatin. Assays were performed at room temperature in 96-well plates. To dilutions of APC (20 μL) were added 10 μL of phospholipid, 10 μL of 0.25 nmol/L X-CP, and 20 μL of normal pooled plasma. Final concentrations of reagents are indicated in the figure legends. The entire mixture was incubated for 1 minute. Clotting was initiated by the addition of 25 μL of 20 mmol/L CaCl2. The clotting time was determined on a Vmax Kinetic Microplate Reader (Molecular Devices, Sunnyvale, CA) as the time at which the A405 increased 0.02 above the plasma background level before initiation of clot formation. This value corresponds to ≈10% of the increase in optical density of fully clotted plasma. The progress curves were examined for all reactions to insure that this cut off accurately reflected the clotting process. All experiments were repeated at least 2 times on separate days with comparable results.
Electrophoresis.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was performed with 6% to 20% gradient acrylamide gels with the Laemmli buffer system.30 For Western blots, gels were transferred to polyvinylidene difluoride membranes (PVDF; Millipore, Bedford, MA) in a semidry apparatus (Bio-Rad, Hercules, CA). Membranes were blocked with 2% nonfat dry milk in TBS and reacted with an affinity-purified goat antibovine factor Va heavy chain immunoglobulin preparation. Biotinylated rabbit antigoat IgG antibody was added, followed by streptavidin conjugated alkaline phosphatase followed with Attophos reagent (Vistra fluorescence Western Kit, Amersham, Arlington Heights, IL). The final image was processed on a Storm 860 phosphoimager (Molecular Devices).
Preparation of phospholipid vesicles.
Extruded vesicles were prepared as described29 to insure comparable size between phospholipid types. A 100-nm Nucleopore membrane was used. PS/PC vesicles were 20%PS:80%PC and PE/PS/PC vesicles were 40%PE:20%PS:40%PC. Lipids were mixed in the weight proportions indicated, dried under argon, and lyophilized overnight to remove organic solvents. 14C-PC (Amersham) was included as tracer for the determination of lipid concentrations. The lipids were then reconstituted under argon in TBS to 2 mg total lipid/mL. After extrusion, the vesicles were used immediately or stored at +20°C under argon. Storage did not alter vesicle activity.
RESULTS
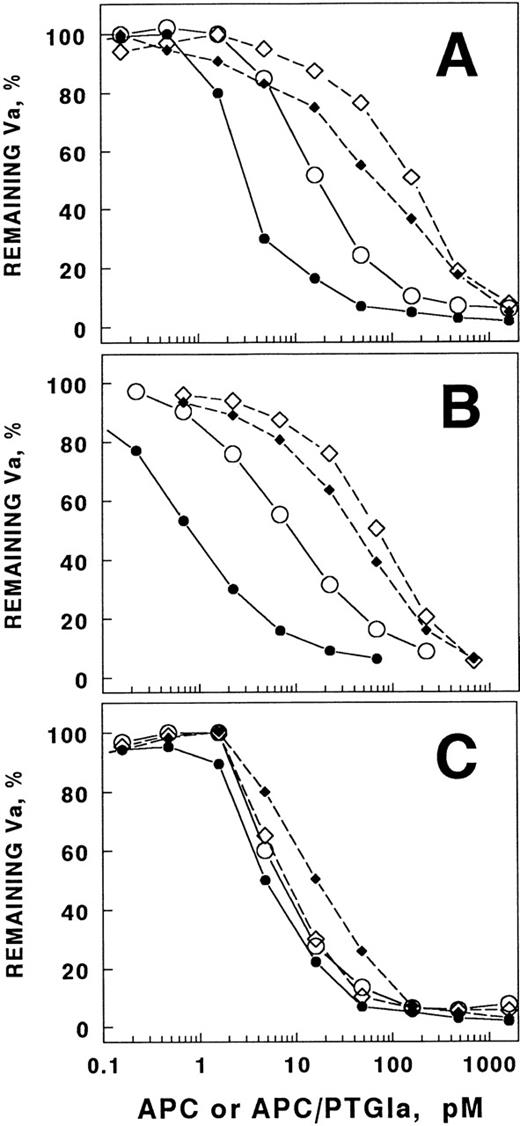
Recent studies of the APC/PTGla chimera showed that the chimera was a much more potent anticoagulant than wild-type APC in plasma, but that the two enzymes had very similar activity in certain purified systems.26 These results suggested the possibility that there might be a plasma component that interfered with the activity of the native APC. Because prothrombin interacts with factor Va and is the most plentiful of the vitamin K–dependent zymogens, we examined the ability of prothrombin to interfere with APC inactivation of factor Va (Fig 1A). It is apparent that the presence of physiological concentrations of prothrombin shifts the APC concentration dependence of factor Va inactivation far to the right (ie, it requires much higher APC concentrations to inhibit equivalent amounts of factor Va in the presence of prothrombin than in its absence). This effect was apparent on phospholipid vesicles with or without phosphatidylethanolamine, a lipid that increases APC activity significantly.8,29 The presence of protein S had little influence on the ability of prothrombin to inhibit factor Va inactivation by APC (Fig 1B). In contrast, the APC/PTGla chimera that exhibits much greater plasma anticoagulant activity was relatively insensitive to prothrombin inhibition (Fig 1C). This suggests that prothrombin might be a physiologically important inhibitor of factor Va inactivation and that this inhibition is not dependent on protein S. The presence of PE had little effect on prothrombin inhibition of the chimera. Because the chimera and wild-type APC bind to phospholipids with similar affinity,26 it is unlikely that the mechanism of prothrombin inhibition of factor Va inactivation is due entirely to competition for the phospholipid surface.
Prothrombin inhibits APC inactivation of factor Va more effectively than it inhibits APC/PTGla. Factor Va (0.2 nmol/L) was incubated for 30 minutes with APC (A and B) or APC/PTGla (C) at the concentrations indicated. Either PS/PC vesicles or PE/PS/PC vesicles were present at a final concentration of 20 μg/mL. Residual factor Va activity was assessed as described in Materials and Methods. (A) Compared with the absence of prothrombin (○) on PS/PC vesicles, physiological concentrations of prothrombin (1.4 μmol/L) shifted the APC concentration dependence of factor Va inactivation about 10-fold (◊). Compared with the absence of prothrombin (•) on PE/PS/PC vesicles, physiological prothrombin concentrations shifted this concentration dependence about 30-fold (⧫). (B) The above experimental conditions were used except that protein S (72 nmol/L) was present. The symbols are the same as in (A). (C) With APC/PTGla, addition of physiological concentrations of prothrombin had little effect on the enzyme concentration dependence of factor Va inactivation. The experiments were performed in the absence of protein S, as it does not influence chimera activity. The symbols are as described in (A).
Prothrombin inhibits APC inactivation of factor Va more effectively than it inhibits APC/PTGla. Factor Va (0.2 nmol/L) was incubated for 30 minutes with APC (A and B) or APC/PTGla (C) at the concentrations indicated. Either PS/PC vesicles or PE/PS/PC vesicles were present at a final concentration of 20 μg/mL. Residual factor Va activity was assessed as described in Materials and Methods. (A) Compared with the absence of prothrombin (○) on PS/PC vesicles, physiological concentrations of prothrombin (1.4 μmol/L) shifted the APC concentration dependence of factor Va inactivation about 10-fold (◊). Compared with the absence of prothrombin (•) on PE/PS/PC vesicles, physiological prothrombin concentrations shifted this concentration dependence about 30-fold (⧫). (B) The above experimental conditions were used except that protein S (72 nmol/L) was present. The symbols are the same as in (A). (C) With APC/PTGla, addition of physiological concentrations of prothrombin had little effect on the enzyme concentration dependence of factor Va inactivation. The experiments were performed in the absence of protein S, as it does not influence chimera activity. The symbols are as described in (A).
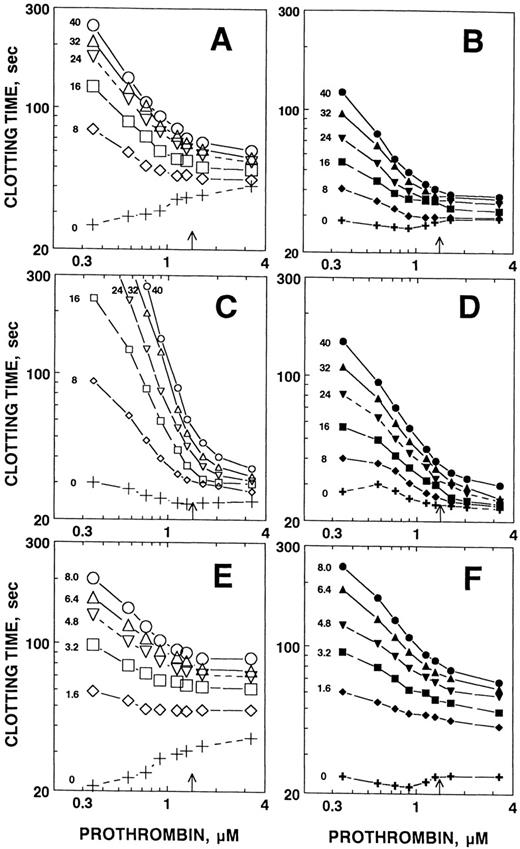
To determine if prothrombin modulates the hemostatic balance in plasma, we analyzed the impact of changing prothrombin concentration on the clotting time of plasma in the presence and absence of APC. Two phospholipid concentrations were used to test whether observed effects were due to membrane competition. In the absence of APC, at the lower concentration of phospholipids containing PE, increasing concentrations of prothrombin inhibited clotting slightly, probably due to competition with factor Va and factor Xa for the membrane surface31(Fig 2A, “+”). In the presence of APC, as the prothrombin concentration increased, the ability of APC to inhibit clotting was depressed. At the lower concentration of phospholipid in the absence of PE, increasing prothrombin concentrations had less effect on the clotting time in the absence of APC (Fig 2B). Again, however, prothrombin potently inhibited the APC anticoagulant effect at all concentrations of APC examined. At higher lipid concentrations where increasing prothrombin concentration decreased the clotting time slightly, increasing prothrombin concentration again potently inhibited APC anticoagulant responses in the presence (Fig 2C) or absence (Fig 2D) of PE. Note that at 25% normal prothrombin levels, roughly the levels achieved during oral anticoagulant therapy,10 APC is at least 5 times more effective than when prothrombin is at physiological concentrations. As was the case in purified systems, the chimera was considerably less sensitive to inhibition by increasing prothrombin levels (Fig 2E and F), suggesting that the decreased sensitivity to prothrombin inhibition is a major mechanism by which the chimera exhibits increased plasma anticoagulant activity. This decreased sensitivity was observed in vesicles that did (Fig 2E) or did not (Fig 2F) contain PE.
Prothrombin inhibits the anticoagulant activity of APC in plasma. To study the influence of prothrombin on APC and APC/PTGla anticoagulant activity in plasma, we initiated clotting at the level of factor X activation using 0.25 nmol/L X-CP. Plasma was diluted to 25%, with the concomitant reduction in prothrombin concentration (to 35 μmol/L final) by the other assay constituents. Prothrombin in TBS was then added to give the final concentrations indicated. The arrow on the x-axis indicates the approximate physiological concentration of prothrombin (1.4 μmol/L). Either PE/PS/PC vesicles (left panels) or PS/PC vesicles (right panels) were used. The PE/PS/PC concentration was adjusted to give approximately the same clotting time in the absence of added prothrombin or APC as the PS/PC vesicles. The PE/PS/PC concentrations were 8 μg/mL in (A), 100 μg/mL in (C), and 8 μg/mL in (E). The PS/PC concentrations were 30 μg/mL in (B), 100 μg/mL in (D), and 30 μg/mL in (F). The prothrombin concentration dependence of the anticoagulant response to APC (A through D) or APC/PTGla (E and F) was then determined. Because of its increased potency as an anticoagulant, the concentrations of the chimera were reduced relative to APC. Final APC or APC/PTGla concentrations are indicated on the figure (nmol/L).
Prothrombin inhibits the anticoagulant activity of APC in plasma. To study the influence of prothrombin on APC and APC/PTGla anticoagulant activity in plasma, we initiated clotting at the level of factor X activation using 0.25 nmol/L X-CP. Plasma was diluted to 25%, with the concomitant reduction in prothrombin concentration (to 35 μmol/L final) by the other assay constituents. Prothrombin in TBS was then added to give the final concentrations indicated. The arrow on the x-axis indicates the approximate physiological concentration of prothrombin (1.4 μmol/L). Either PE/PS/PC vesicles (left panels) or PS/PC vesicles (right panels) were used. The PE/PS/PC concentration was adjusted to give approximately the same clotting time in the absence of added prothrombin or APC as the PS/PC vesicles. The PE/PS/PC concentrations were 8 μg/mL in (A), 100 μg/mL in (C), and 8 μg/mL in (E). The PS/PC concentrations were 30 μg/mL in (B), 100 μg/mL in (D), and 30 μg/mL in (F). The prothrombin concentration dependence of the anticoagulant response to APC (A through D) or APC/PTGla (E and F) was then determined. Because of its increased potency as an anticoagulant, the concentrations of the chimera were reduced relative to APC. Final APC or APC/PTGla concentrations are indicated on the figure (nmol/L).
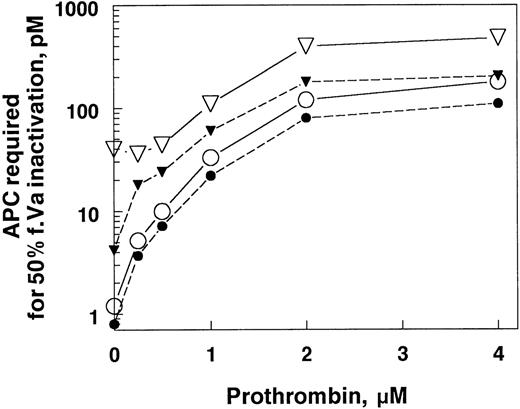
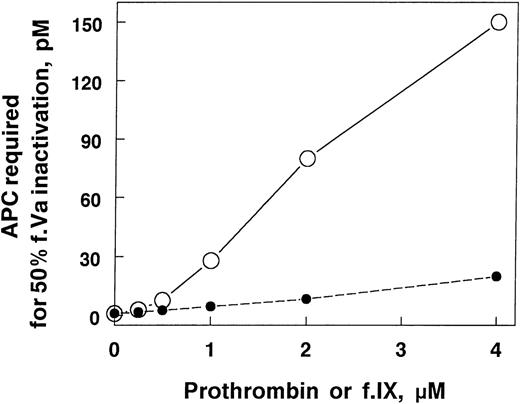
Plasma from factor V Leiden patients is poorly anticoagulated by APC. In factor Xa–initiated clotting, the APC concentration required to achieve equivalent anticoagulant responses is only about 4 to 5 times higher than normal plasma. In contrast, in purified systems in the absence of prothrombin, factor Va Leiden requires almost 30 times more APC to achieve comparable inactivation relative to wild-type factor Va. To determine if prothrombin might be responsible for some of the differences in the plasma and purified systems, we added increasing concentrations of prothrombin to factor Va Leiden and normal factor Va and then measured their sensitivity to APC (Fig 3). The concentration of APC required to achieve 50% factor Va inactivation increased rapidly with increasing prothrombin concentrations. The APC concentration dependence of factor Va Leiden inhibition was increased also, but to a much lesser extent. As a result, the very large difference in inactivation rates observed between normal and factor Va Leiden in the absence of prothrombin became much smaller as the prothrombin concentration approached physiological levels. This phenomenon may explain part of the reason that factor Va Leiden appears much more resistant to APC in purified systems than in plasma. In addition, some of this difference between the purified system with APC alone and plasma is mediated by protein S.
The influence of prothrombin on the inactivation of factor Va and factor Va Leiden. Factor Va or factor Va Leiden (0.2 nmol/L) in TBS, 5 mmol/L CaCl2, 5 mg/mL BSA, pH 7.4 were incubated for 30 minutes at room temperature with APC at different concentrations in the presence of the prothrombin concentrations indicated. The reactions contained 40 μg/mL PE/PS/PC with or without 72 nmol/L protein S. The APC concentration required for 50% factor Va inactivation (y-axis) was calculated from the APC concentration dependence curve of factor Va inactivation under each condition. The reaction mixtures were: •, factor Va, APC, and protein S; ○, factor Va, APC, and no protein S; ▾, factor Va Leiden, APC, and protein S; ▿, factor Va Leiden, APC, and no protein S.
The influence of prothrombin on the inactivation of factor Va and factor Va Leiden. Factor Va or factor Va Leiden (0.2 nmol/L) in TBS, 5 mmol/L CaCl2, 5 mg/mL BSA, pH 7.4 were incubated for 30 minutes at room temperature with APC at different concentrations in the presence of the prothrombin concentrations indicated. The reactions contained 40 μg/mL PE/PS/PC with or without 72 nmol/L protein S. The APC concentration required for 50% factor Va inactivation (y-axis) was calculated from the APC concentration dependence curve of factor Va inactivation under each condition. The reaction mixtures were: •, factor Va, APC, and protein S; ○, factor Va, APC, and no protein S; ▾, factor Va Leiden, APC, and protein S; ▿, factor Va Leiden, APC, and no protein S.
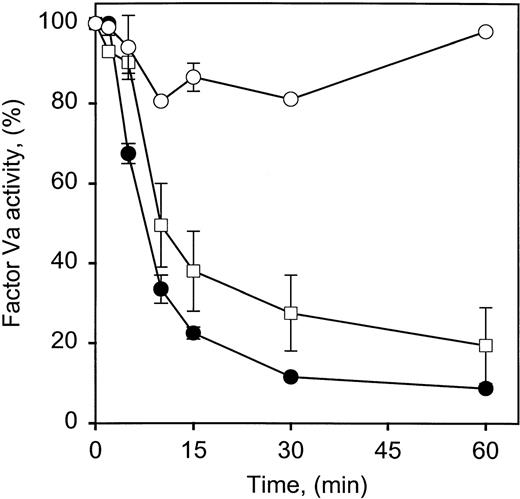
Although changes in prothrombin concentration in the physiological range clearly reduce the ability of APC to inactivate factor Va in a concentration-dependent fashion, the mechanism is unclear. One possibility is that prothrombin is simply competing with APC for binding to the membrane surface and because it is the most plentiful of the vitamin K–dependent factors, changes in the physiological range would have the greatest effect on factor Va inactivation. The observation that APC inactivation of factor Va Leiden was less sensitive to the presence of prothrombin than factor Va (Fig 3) and the differences in prothrombin inhibition of factor Va inactivation by the chimera (Figs 1C, 2E, and 2F) both argue against this conclusion. Multiple approaches were taken to evaluate this possibility. In the first, factor Va inactivation by APC was monitored in the absence of phospholipid. Although phospholipid accelerates factor Va inactivation, it is not required for the initial cleavage of factor Va at Arg506,32 a cleavage that reduces factor Va activity in plasma-based assays. In this phospholipid-free system, there was a prothrombin concentration-dependent decrease in the rate of factor Va inactivation (Fig 4). The prothrombin concentrations required for inhibition are approximately in the range of the binding constant observed for a prothrombin derivative interacting with the prothrombin binding subunit of factor Va (10 to 15 μmol/L) in solution.18 The much lower concentration of prothrombin required in the membrane system presumably reflects its increased local prothrombin concentration.31 In addition, the presence of factor Va has been shown to increase the affinity of prothrombin for the membrane surface approximately 50-fold,33 further facilitating complex assembly between factor Va and prothrombin.
Factor Va inactivation by APC is inhibited by prothrombin in the absence of phospholipid. Factor Va (100 nmol/L) was incubated at 37°C in 0.15 mol/L NaCl, 5 mmol/L CaCl2, 20 mmol/L HEPES, pH 7.5, 0.2 mg/mL BSA with 10 nmol/L APC in the absence or presence of different prothrombin concentrations. The reaction was stopped at the times indicated by addition of 10 mmol/L benzamidine HCl. The residual factor Va was measured with a 1-stage clotting assay using factor V–deficient plasma. The prothrombin concentrations present were: •, no prothrombin; □, 1.4 μmol/L prothrombin; ▵, 5 μmol/L prothrombin; and ○, 10 μmol/L prothrombin. Bars represent the standard deviation of 2 experiments, duplicate samples.
Factor Va inactivation by APC is inhibited by prothrombin in the absence of phospholipid. Factor Va (100 nmol/L) was incubated at 37°C in 0.15 mol/L NaCl, 5 mmol/L CaCl2, 20 mmol/L HEPES, pH 7.5, 0.2 mg/mL BSA with 10 nmol/L APC in the absence or presence of different prothrombin concentrations. The reaction was stopped at the times indicated by addition of 10 mmol/L benzamidine HCl. The residual factor Va was measured with a 1-stage clotting assay using factor V–deficient plasma. The prothrombin concentrations present were: •, no prothrombin; □, 1.4 μmol/L prothrombin; ▵, 5 μmol/L prothrombin; and ○, 10 μmol/L prothrombin. Bars represent the standard deviation of 2 experiments, duplicate samples.
To rule out the possibility that the observed inhibition was mediated by contaminating phospholipid in one of the reagents, 0.1% Tween 20 was added to the reaction mixture. The detergent did not prevent prothrombin inhibition of APC inactivation of factor Va (data not shown). To rule out phospholipid contamination in the purified system further, we used Gla domainless activated protein C, which cannot bind to phospholipid. Prothrombin still inhibited the inactivation of factor Va by this form of APC (Fig 5). Addition of phospholipid to this inactivation mixture slowed factor Va inactivation without altering the prothrombin inhibition appreciably (data not shown).
Factor Va inactivation by GD-APC is inhibited by prothrombin in the absence of phospholipid. Factor Va (100 nmol/L) was incubated at 37°C in 0.15 mol/L NaCl, 5 mmol/L CaCl2, 20 mmol/L HEPES, pH 7.5, 0.1% gelatin with 10 nmol/L GD-APC in the absence or presence of different prothrombin concentrations. The reaction was stopped at the times indicated by addition of 10 mmol/L benzamidine HCl. The residual factor Va was measured with a 1-stage clotting assay using factor V–deficient plasma. The prothrombin concentrations present were: •, no prothrombin; □, 1.4 μmol/L prothrombin; ○, 10 μmol/L prothrombin. Bars represent the standard deviation of 2 experiments, duplicate samples.
Factor Va inactivation by GD-APC is inhibited by prothrombin in the absence of phospholipid. Factor Va (100 nmol/L) was incubated at 37°C in 0.15 mol/L NaCl, 5 mmol/L CaCl2, 20 mmol/L HEPES, pH 7.5, 0.1% gelatin with 10 nmol/L GD-APC in the absence or presence of different prothrombin concentrations. The reaction was stopped at the times indicated by addition of 10 mmol/L benzamidine HCl. The residual factor Va was measured with a 1-stage clotting assay using factor V–deficient plasma. The prothrombin concentrations present were: •, no prothrombin; □, 1.4 μmol/L prothrombin; ○, 10 μmol/L prothrombin. Bars represent the standard deviation of 2 experiments, duplicate samples.
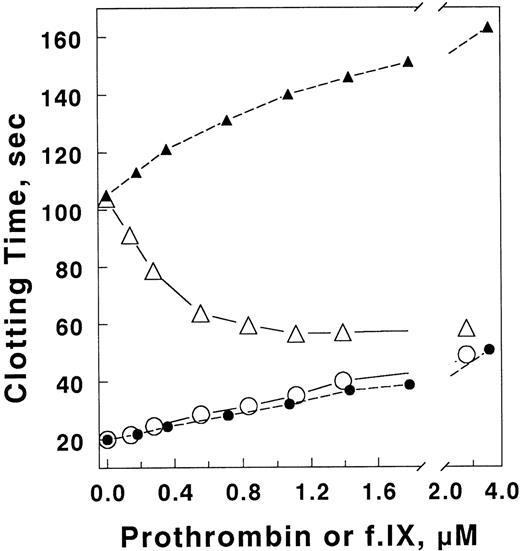
To determine the specificity of the prothrombin inhibition, factor IX, which has been reported to bind phospholipid with an affinity similar to prothrombin (2 v 0.6 μmol/L, respectively34), was used as a potential inhibitor and the dose dependence of the inhibition compared with that of prothrombin (Fig 6). Both factors reduced the factor Va inactivation. However, prothrombin was much more effective, consistent with a portion of the inhibition being due to competition for phospholipid surface. In plasma, human factor IX has no significant effect on the inhibition of APC anticoagulant activity, but inhibits coagulation slightly in either the presence or absence of APC (Fig 7). Although not definitive mechanistically in itself, when taken together with the inhibition of factor Va inactivation by prothrombin in solution, these results suggest that the factor Va–prothrombin complex is resistant to inhibition by APC.
Comparison of the ability of prothrombin and factor IX to alter the APC concentration dependence of factor Va inactivation in the purified system. The APC concentration dependence of factor Va (0.2 nmol/L) inactivation was determined with varying concentrations of prothrombin or factor IX as indicated. PE/PS/PC vesicles (40 μg/mL) were used. The reactions were performed in TBS, 5 mmol/L CaCl2, 5 mg/mL BSA, pH 7.4 at room temperature. Residual factor Va activity was determined using the prothrombinase assay described in Materials and Methods (•, with added factor IX; ○, with added prothrombin).
Comparison of the ability of prothrombin and factor IX to alter the APC concentration dependence of factor Va inactivation in the purified system. The APC concentration dependence of factor Va (0.2 nmol/L) inactivation was determined with varying concentrations of prothrombin or factor IX as indicated. PE/PS/PC vesicles (40 μg/mL) were used. The reactions were performed in TBS, 5 mmol/L CaCl2, 5 mg/mL BSA, pH 7.4 at room temperature. Residual factor Va activity was determined using the prothrombinase assay described in Materials and Methods (•, with added factor IX; ○, with added prothrombin).
Factor IX does not inhibit the anticoagulant activity of APC in plasma. The anticoagulant activity of APC in plasma was determined in the presence of varying concentrations of prothrombin or factor IX as indicated. Clotting was initiated with 0.25 nmol/L X-CP in the presence of 8 μg/mL PE/PS/PC. Final plasma dilution was 25%, with the concomitant reduction in prothrombin concentration to 0.35 μmol/L. Prothrombin (open symbols) or factor IX (closed symbols) was added at the concentrations indicated in the presence (▵, ▴) or absence (○, •) of 16 nmol/L APC.
Factor IX does not inhibit the anticoagulant activity of APC in plasma. The anticoagulant activity of APC in plasma was determined in the presence of varying concentrations of prothrombin or factor IX as indicated. Clotting was initiated with 0.25 nmol/L X-CP in the presence of 8 μg/mL PE/PS/PC. Final plasma dilution was 25%, with the concomitant reduction in prothrombin concentration to 0.35 μmol/L. Prothrombin (open symbols) or factor IX (closed symbols) was added at the concentrations indicated in the presence (▵, ▴) or absence (○, •) of 16 nmol/L APC.
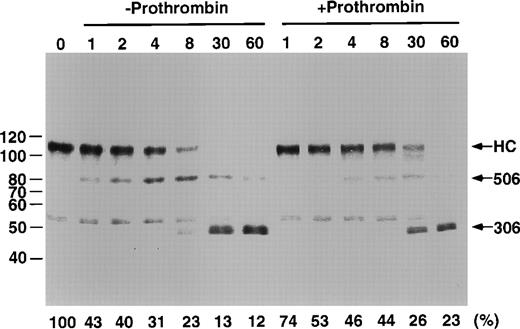
To further analyze the mechanism of inhibition, we examined the cleavage products of factor Va in the presence and absence of prothrombin. For this purpose, Western blots were run and probed with affinity-purified polyclonal anti–factor Va heavy chain antibodies. Although prothrombin slowed cleavage, there was no obvious change in the relative distribution of the cleavage products, suggesting that the presence of prothrombin inhibits both the cleavage at Arg506 and Arg306 (Fig 8). A minor, unidentified cleavage product was present in the original factor Va preparation.
Proteolytic processing of factor Va by APC in the presence and absence of prothrombin. A total of 500 nmol/L factor Va, 120 μg/mL PE/PS/PC with or without 1.4 μmol/L prothrombin was incubated in 150 NaCl, 20 mmol/L HEPES, pH 7.4, 5 mmol/L CaCl2, 0.2% BSA at 37°C for the times indicated. The reaction was stopped by addition of 10 mmol/L benzamidine HCl. At this point, prothrombin was added to the samples from the time course performed in the absence of prothrombin. This was to insure that the differences observed in the blots were not due to a prothrombin effect on the electrophoretic pattern. Gradient 5% to 15% SDS-PAGE gels were run, blotted, and probed with an affinity-purified, goat anti–factor Va heavy chain antibody. The blot was developed as described in Materials and Methods. Residual Va activity is indicated along the bottom of the gel. Molecular weight marker locations are indicated on the left of the figure. The locations of the factor Va heavy chain (HC), the product of cleavage at Arg506 (506), and the product of cleavage at Arg306 (306) recognized by this antiserum are indicated along the right side of the figure.
Proteolytic processing of factor Va by APC in the presence and absence of prothrombin. A total of 500 nmol/L factor Va, 120 μg/mL PE/PS/PC with or without 1.4 μmol/L prothrombin was incubated in 150 NaCl, 20 mmol/L HEPES, pH 7.4, 5 mmol/L CaCl2, 0.2% BSA at 37°C for the times indicated. The reaction was stopped by addition of 10 mmol/L benzamidine HCl. At this point, prothrombin was added to the samples from the time course performed in the absence of prothrombin. This was to insure that the differences observed in the blots were not due to a prothrombin effect on the electrophoretic pattern. Gradient 5% to 15% SDS-PAGE gels were run, blotted, and probed with an affinity-purified, goat anti–factor Va heavy chain antibody. The blot was developed as described in Materials and Methods. Residual Va activity is indicated along the bottom of the gel. Molecular weight marker locations are indicated on the left of the figure. The locations of the factor Va heavy chain (HC), the product of cleavage at Arg506 (506), and the product of cleavage at Arg306 (306) recognized by this antiserum are indicated along the right side of the figure.
DISCUSSION
The present studies indicate that the prothrombin-factor Va complex is resistant to APC inactivation whether the complex is formed on the membrane surface or in solution. The major evidence in favor of this mechanism is that the concentrations of prothrombin required to inhibit factor Va inactivation by APC in solution are very similar to the Kd for the prothrombin-factor Va interaction.18 Complex formation between factor Va and prothrombin could slow factor Va inactivation by APC either through steric hindrance or due to conformational changes in the factor Va. The latter possibility is supported by the observation that formation of the factor Va-meizothrombin complex results in conformational changes in meizothrombin,35 suggesting the possibility of reciprocal changes in the factor Va.
Our data appear to conflict with the earlier report from Mitchell et al,17 which indicated that prothrombin inhibition of factor Va inactivation was due to inhibition of protein S function, whereas our data clearly indicate that prothrombin inhibition of this reaction occurs in the absence of protein S. It is possible that their plasma was from an individual with factor V Leiden, a mutation not appreciated at that time. Supporting this possibility, as seen in Fig 3, in the absence of protein S, APC inactivation of factor Va Leiden is relatively insensitive to prothrombin.
The present findings provide a rational explanation for the clinical observation that prothrombin levels strongly influence thrombotic tendency. In addition to the very modest impact of increasing prothrombin concentration on the rate of thrombin formation, there is a very potent inhibition on factor Va inactivation by APC. It is now well established that heterozygous protein C deficiency is a significant risk factor for venous thrombosis. The basis for this observation is almost certainly due to the fact that protein C circulates at concentrations well below the levels required for maximal activation and in fact well below the Km6 for activation. Thus, as the level of protein C decreases, there is a proportionate decrease in APC generated in response to a given level of thrombin generation. Direct measurement of APC levels in plasma has shown a correlation with the protein C concentration.36 The present findings suggest that elevated prothrombin levels in concert with decreased protein C levels should greatly increase thrombotic risk. To our knowledge, this combination has not been studied systematically, but at least one case report of ischemic stroke in a young individual with prothrombin G20210A gene mutation and protein C deficiency has been presented.37
On the other hand, the risk of thrombosis increases significantly when individuals carry the factor V Leiden and prothrombin G20210A dimorphisms.38-41 The observations that prothrombin is somewhat less effective in inhibiting factor Va Leiden inactivation by APC than wild-type factor Va may make the risk of the combined inheritance of these dimorphisms less severe than might have been anticipated. Our data would suggest that an even more severe risk might be associated with simultaneous protein C deficiency and prothrombin G20210A dimorphism.
Perhaps more intriguing is the relationship between low prothrombin levels and the efficacy of APC as an anticoagulant. The observation that the efficacy of APC as an anticoagulant is much greater at prothrombin concentrations near those observed in stably anticoagulated patients than at normal levels provides an explanation for the effectiveness of oral anticoagulants despite the fact that they severely depress protein C levels. In this case, the efficacy of any APC that is generated is apparently enhanced by almost the same degree that the activation of protein C is inhibited. Specifically, at 20% of the normal prothrombin and protein C concentrations, levels that are common in stably anticoagulated individuals,10 12 the protein C activation rate would drop about 5-fold. The efficacy reported here suggests a 5-fold increase in anticoagulant function, resulting in maintenance of the natural anticoagulant function despite reduction in circulating APC. This phenomenon may be offset somewhat by the reduction in protein S levels. These observations suggest further that supplementation with relatively small amounts of protein C should enhance the antithrombotic effects of coumadin anticoagulants more substantially than previously appreciated. They further suggest that for patients difficult to manage on oral anticoagulants, the hemostatic balance might be better preserved with decreased warfarin doses and relatively minor protein C supplementation.
Supported in part by a project of a Specialized Center of Research grant from the National Heart, Lung, and Blood Institute (to N.L.E.) Grant No. P50 HL54502.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Charles T. Esmon, PhD, Oklahoma Medical Research Foundation, 825 NE 13th St, Oklahoma City, OK 73104; e-mail:Charles-Esmon@omrf.ouhsc.edu.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal