Key Points
DNA POLθ–mediated DNA repair protects leukemia cells from the toxic effect of metabolically induced DNA damage.
DNA POLθ is a new therapeutic target to eradicate leukemia clones expressing oncogenic tyrosine kinases.
Abstract
Leukemia cells accumulate DNA damage, but altered DNA repair mechanisms protect them from apoptosis. We showed here that formaldehyde generated by serine/1-carbon cycle metabolism contributed to the accumulation of toxic DNA-protein crosslinks (DPCs) in leukemia cells, especially in driver clones harboring oncogenic tyrosine kinases (OTKs: FLT3(internal tandem duplication [ITD]), JAK2(V617F), BCR-ABL1). To counteract this effect, OTKs enhanced the expression of DNA polymerase theta (POLθ) via ERK1/2 serine/threonine kinase-dependent inhibition of c-CBL E3 ligase-mediated ubiquitination of POLθ and its proteasomal degradation. Overexpression of POLθ in OTK-positive cells resulted in the efficient repair of DPC-containing DNA double-strand breaks by POLθ-mediated end-joining. The transforming activities of OTKs and other leukemia-inducing oncogenes, especially of those causing the inhibition of BRCA1/2-mediated homologous recombination with and without concomitant inhibition of DNA-PK–dependent nonhomologous end-joining, was abrogated in Polq−/− murine bone marrow cells. Genetic and pharmacological targeting of POLθ polymerase and helicase activities revealed that both activities are promising targets in leukemia cells. Moreover, OTK inhibitors or DPC-inducing drug etoposide enhanced the antileukemia effect of POLθ inhibitor in vitro and in vivo. In conclusion, we demonstrated that POLθ plays an essential role in protecting leukemia cells from metabolically induced toxic DNA lesions triggered by formaldehyde, and it can be targeted to achieve a therapeutic effect.
Introduction
Alterations in metabolism associated with DNA damage and repair are hallmarks of cancer.1 For example, activating mutations in isocitrate dehydrogenases 1 and 2 (IDH1/2) produce 2-hydroxyglutarate, which downregulates the DNA damage sensor ataxia-telangiectasia mutated and additional repair mediators that suppress homologous recombination (HR) repair.2,3
Leukemia cells are challenged by formaldehyde,4-7 which induces a variety of DNA damage, such as interstrand crosslinks (ICLs) and DNA-protein crosslinks (DPCs).8 We, and others, reported that Fanconi anemia pathway protects normal and leukemia cells from the toxic effect of ICLs.9-13
DPCs are one of the most deleterious types of DNA damage.14 DPCs form when cellular proteins become covalently trapped on DNA strands. The bulkiness of DPCs blocks chromatin-related processes (eg, DNA replication and transcription), and they are not good substrates for any canonical DNA repairs.
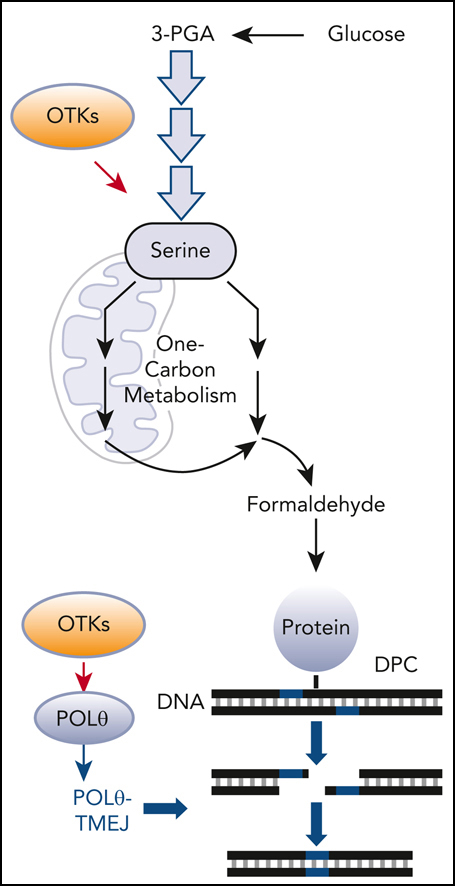
Recently, we suggested that DNA polymerase theta (POLθ)–mediated end-joining (TMEJ) repairs formaldehyde-induced DPCs at DNA double-strand breaks (DSBs) containing 5′ adducts.15 POLθ (encoded by POLQ gene) is a unique but highly error-prone DNA polymerase, capable of exerting helicase and endonuclease activities.16,17 POLθ creates microhomology-dependent synapsis of the 3′ overhanging ssDNA tails during the essential steps of TMEJ.18
POLθ is thought to be important for DSB repair in HR-deficient cancer cells, and its downregulation is synthetically lethal when occurring along with BRCA1/2 mutations.19,20 POLθ is also important for the survival of cells deficient in other DNA damage response (DDR) genes,21 including DNA-PKcs, a key member of DNA-PK–mediated nonhomologous end-joining (D-NHEJ).22 Yet the knockout of Polq in nontransformed cells and mice causes minimal effects.19,20,23 POLθ is therefore a promising drug target in cancers, especially in those deficient in HR or NHEJ.24,25
Methods
Cell lines
Primary cells
FLT3(ITD)-positive acute myeloid leukemia (AML) samples were from the ECOG-ACRIN E1900 clinical trial.33 HR-proficient AML samples, FLT3(ITD)-positive AML primary leukemia xenograft cells, myeloproliferative neoplasm (MPN), chronic myeloid leukemia (CML), and acute lymphoblastic leukemia (ALL) samples were obtained as described.27,28,34 Additional samples were obtained from the department of internal medicine I, Medical University of Vienna (Vienna, Austria). Samples of normal hematopoietic cells were obtained from STEMCELL Technologies (Vancouver, Canada).
Flt3ITD;Polq−/− mice
B6.Cg-Polqtm1Jcs/J mice (Jackson Laboratories, JAX #006194) and B6.129-Flt3tm1Dgg/J mice (Jackson Laboratories, JAX #:011112) were crossbred to generate 1) Flt3ITD/ITD;Polq−/−, 2) Flt3ITD/-;Polq−/−, 3) Flt3ITD/ITD;Polq+/+, and 4) Flt3ITD/-;Polq+/+ animals.
Inhibition of c-CBL
Cells were transfected with 10 nM siRNA c-CBL using the Nucleofector kit (Cat No. VPA-1003, Lonza, Germany). c-CBL inhibition was achieved using pYTPEP peptide.35 Briefly, cells were treated with 100 μM peptide for 12 hours before 2 μM SCH772984 were added for 24 hours.
POLθ inhibitors (POLθi)
Novobiocin (NVB) (NSC 2382) and ART558 (HY-141520) were from Selleckchem and MedChemExpress, respectively.
In vitro sensitivity assays of primary cells
Human primary cells were incubated with the inhibitors in StemSpanSFEM, supplemented with growth factors (CML, AML, and normal counterparts = 100 ng/mL stem cell factor [SCF], 20 ng/mL interleukin-3 (IL-3), 100 ng/mL Flt-3 ligand, 20 ng/mL granulocyte colony stimulating factor [G-CSF], 20 ng/mL IL-6; MPN = 100 ng/mL SCF, 10 ng/mL Flt-3 ligand, 20 ng/mL IL-3, IL-6, G-CSF, granulocyte-macrophage CSF [GM-CSF], 12 U/mL erythropoietin (EPO), and 2.5 ng/mL thrombopoietin (TPO); and Ph+ ALL 10 ng/mL IL-7), as described previously.28,34 Inhibitors were added for 3 days, followed by plating in MethoCult medium (STEMCELL Technologies) when indicated; colonies were scored after 5 to 7 days. Ph+ ALL cells were counted in liquid culture.
Clonogenic assays
Lin-cKit+ murine bone marrow cells (mBMCs) and Lin-CD34+ human cells were maintained in StemSpanSFEM medium supplemented with normal, CML-like, and AML-like 100 ng/mL SCF and 20 ng/mL IL-3; MPN-like 100 ng/mL SCF, 10 ng/mL FLT3 ligand, 20 ng/mL IL-3, IL-6, G-CSF), and GM-CSF, 12 units per mL erythropoietin, and 2.5 ng/ml thrombopoietin, as described before.27,28,34 Clonogenic activity was tested in the presence of these growth factors and threshold concentrations of growth factors (normal and oncogene-transduced cells, respectively), as described previously.30,36
Protein stability assay
Cells were treated with 100 μg cycloheximide (S7418, Selleckchem) for the indicated time periods. Total cell lysates were analyzed via sodium dodecyl sulfate-polyacrylamide gel electrophoresis followed by western blotting.
Formaldehyde and DPCs measurement
Formaldehyde was measured using a formaldehyde assay kit (Cat#:Ab196997, Abcam). DPCs were measured using advanced recovery of K (potassium)-SDS precipitates (ARK) assay.37
TMEJ and MMEJ activities
Cells were cotransfected with either DPC-TMEJ substrate15 or pBABE-MMEJ plasmid38 after digestion with I-SceI and dsRED Mito (control for transfection efficiency) using CD34+ cell Nucleofector kit (Cat No. VPA-1003, Lonza, Germany). Then, 72 hours after transfection, green fluorescent protein–positive (GFP+ and Red+) cells were analyzed via flow cytometry (Facscanto, BD).
5-bromo-2′-deoxyuridine/PCNA assay
DNA end resection was examined as described before.39
Analysis of clonal sensitivity to POLθi
AML primary cells were incubated with ATR558 or diluent for 6 days, as described before34 after the analysis of the frequency of clones with somatic mutations. For measuring the changes in the variant allele frequencies and in the clonal fractions post-ART558 treatment, we used the Tapestri Singe-Cell DNA Myeloid Panel platform (Mission Bio). Single-cell DNA sequencing was performed using Single Cell & Transcriptomics Core at Johns Hopkins University.
In vivo treatment
NOD/RAG1/2-/-IL2Rγ-/–SGM3 (NRGS) mice (The Jackson Laboratories) were total body irradiated (600 cGy) and injected intravenously with 1 × 106 FLT3(ITD)-positive AML primary cells, as described before.27 Treatment started when 3% to 5% of human CD45+ (hCD45+) leukemia cells were detected in peripheral blood. Mice were treated for 14 consecutive days with vehicle (control), NVB (100 mg/kg25), quizartinib (10 mg/kg27), and their combination. Leukemia burden was analyzed 3 days after the end of treatment using flow cytometry detecting hCD45+ cells. The median survival time was determined.
Statistics
Data are expressed as mean ± standard deviation from at least 3 independent experiments, unless stated otherwise. When conducting subgroup comparisons between 2 groups, a 2-tailed unpaired t test was used for normally distributed variables. 5-bromo-2′-deoxyuridine/PCNA data were analyzed using the Mann-Whitney U test. The response additivity approach was used to study the synergistic effects.40 The P value for the possible synergistic effect is given by the significance of the interaction effect in a factorial analysis of variance by bootstrapping the individual and combination effects. The median survival time ± standard error of the mice was calculated using Kaplan-Meier method and compared using log-rank test. P values less than 0.05 were considered statistically significant. Stata 17 (Stata Corp LLC., College Station, TX) was used to conduct synergistic effects analysis.
Institutional approvals
All mouse experiments were approved by the institutional animal care and use committee review boards at Temple University. Studies involving leukemia samples were not research involving human participants as defined by the Department of Health and Human Services or Food and Drug Administration regulations.
Additional and more detailed methods are given in supplemental Methods, available on the Blood website.
Results
POLθ repairs DPCs caused by exposure to formaldehyde generated by serine/1-carbon (1C) cycle metabolism
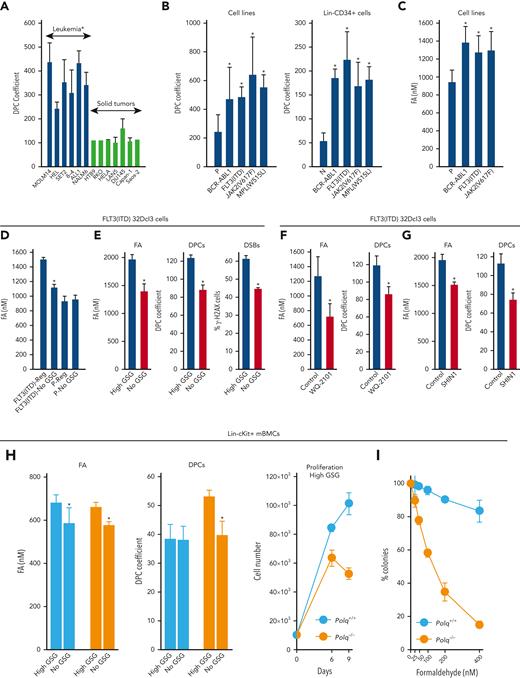
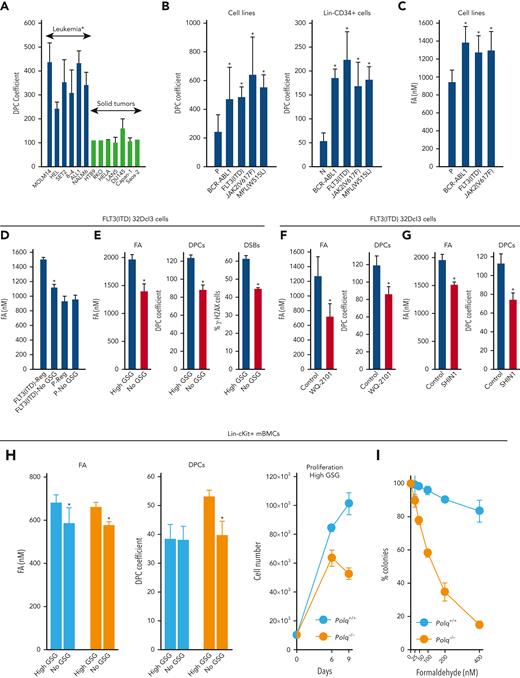
Hematologically malignant cell lines accumulated 2 to 4 times more DPCs than solid tumor cell lines (Figure 1A). The large cohort of hematologically malignant cell lines express oncogenic tyrosine kinases (OTKs).41,42 We detected 2 to 4 times more DPCs in OTK-positive cell lines and Lin-CD34+ stem/progenitor primary cells when compared with those in parental cells and hematopoietic cells harvested from healthy individuals, respectively (Figure 1B). DPCs can be induced by endogenous metabolites, such as reactive aldehydes.14 In concordance, elevated levels of formaldehyde were detected in cells expressing FLT3(ITD), JAK2(V617F), and BCR-ABL1 (Figure 1C).
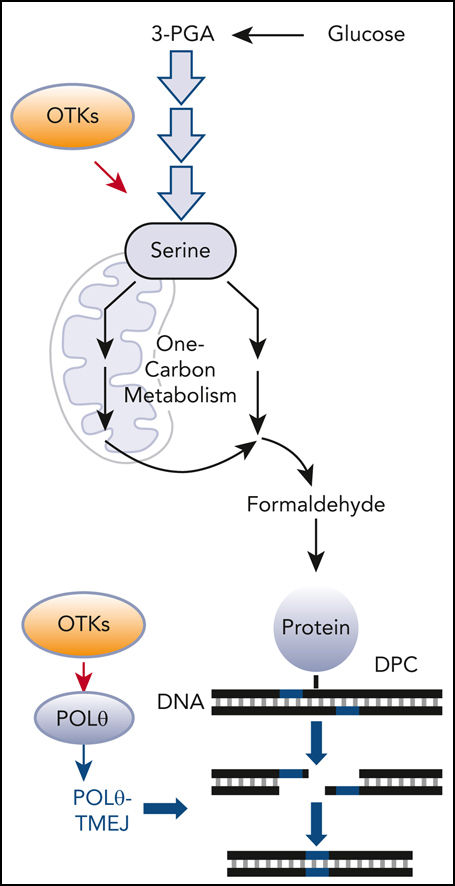
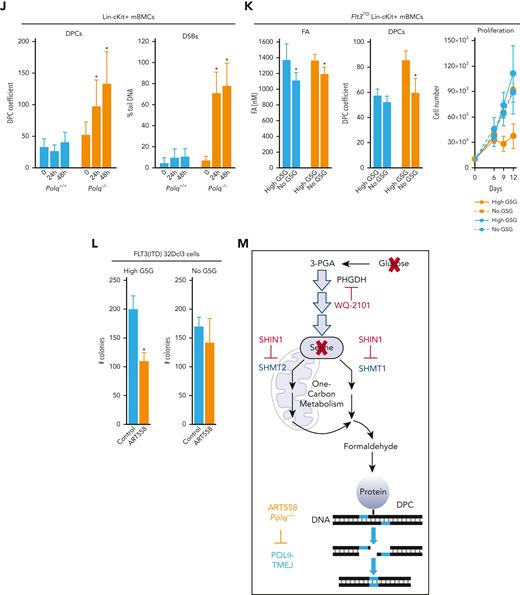
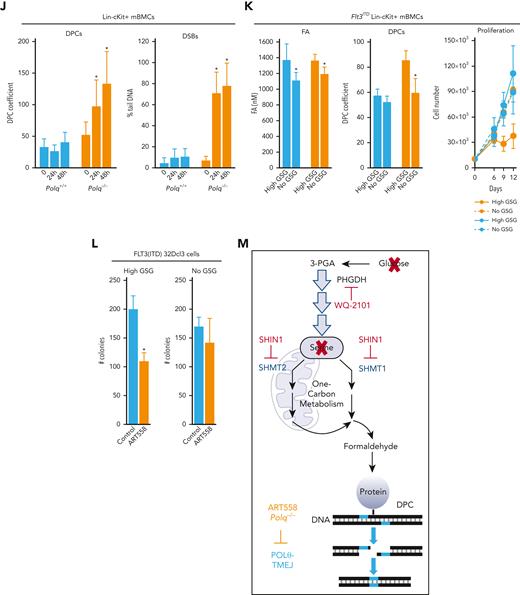
POLθ is required to resolve DPCs caused by formaldehyde generated via serine/1C cycle metabolism in OTKs-positive hematological malignancies. (A) Mean ± SD of DPCs in the indicated cell lines; ∗P < .05 using t test. (B) Mean ± SD of DPCs in: (left) 32Dcl3 parental cells (P) and cells expressing FLT3(ITD), JAK2(V617F), and BCR-ABL1, and (right) human Lin-CD34+ primary cells from leukemias expressing FLT3(ITD), JAK2(V617F), and BCR-ABL1 and from healthy donors. ∗P < .05 when compared with P/N using t test. (C) Mean formaldehyde (FA) levels ± SD in 32Dcl3 parental cells (P) and cells expressing FLT3(ITD), JAK2(V617F), and BCR-ABL1. ∗P < .05 when compared with P using t test. (D) Mean ± SD of FA levels in FLT3(ITD) 32Dcl3 cells cultured in standard medium (Reg) and in medium without glucose, serine, and glycine (no GSG); ∗P < .05 using t test when compared with corresponding Reg. (E) Mean ± SD of FA levels, DPCs, and % of γ-H2AX–positive cells in FLT3(ITD) 32Dcl3 cells cultured in glucose + 5× serine + 5× glycine high (high GSG) medium and in medium without glucose, serine, and glycine (no GSG). ∗P < .05 using t test. (F) Mean ± SD of FA levels and DPCs in FLT3(ITD) 32Dcl3 cells cultured in glucose-supplemented serine + glycine–free medium and treated or not with WQ-2101; ∗P < .05 using t test. (G) Mean ± SD of FA levels and DPCs from 3 experiments in FLT3(ITD) 32Dcl3 cells cultured in standard medium and treated or not with SHIN1. ∗P < .05 using t test. (H) Lin-cKit+ mBMCs from Polq+/+ and Polq−/− mice (blue and orange, respectively) were cultured in high GSG and/or no GSG medium. Results represent mean ± SD of FA levels and DPCs and proliferation rate; ∗P < .05 when compared with corresponding high GSG using t test. (I) Clonogenic activity of Lin-cKit+ BMCs from Polq+/+ and Polq−/− mice treated with the indicated concentrations of formaldehyde for 4 hours after plating in methylcellulose. Results represent the mean percentage of colonies ± SD when compared with untreated counterparts. (J) Lin-cKit+ mBMCs from Polq+/+ and Polq−/− mice were untreated (0) and treated with 400 nM formaldehyde for 24 and 48 hours. Results represent mean ± SD of DPCs and tail DNA percentage from the neutral comet assay. ∗P ≤ .001 when compared with Polq+/+ counterpart using t test. (K) Lin-cKit+ mBMCs from Flt3ITD;Polq−/− (orange) and Flt3ITD;Polq+/+ (blue) mice were cultured in high GSG and/or no GSG medium. Results represent mean ± SD of FA levels and DPCs and proliferation rate; ∗P < .05 when compared with corresponding high GSG using t test. (L) Sensitivity of FLT3(ITD) 32Dcl3 cells to 25 μM ART558 in high GSG and no GSG medium. Results represent the mean percentage of colonies ± SD when compared with untreated counterparts; ∗P < .05 using t test. (M) A scheme illustrating functional pathway where serine/1C cycle–produced formaldehyde induces DPCs, which are likely repaired by POLθ-mediated TMEJ. SD, standard deviation.
POLθ is required to resolve DPCs caused by formaldehyde generated via serine/1C cycle metabolism in OTKs-positive hematological malignancies. (A) Mean ± SD of DPCs in the indicated cell lines; ∗P < .05 using t test. (B) Mean ± SD of DPCs in: (left) 32Dcl3 parental cells (P) and cells expressing FLT3(ITD), JAK2(V617F), and BCR-ABL1, and (right) human Lin-CD34+ primary cells from leukemias expressing FLT3(ITD), JAK2(V617F), and BCR-ABL1 and from healthy donors. ∗P < .05 when compared with P/N using t test. (C) Mean formaldehyde (FA) levels ± SD in 32Dcl3 parental cells (P) and cells expressing FLT3(ITD), JAK2(V617F), and BCR-ABL1. ∗P < .05 when compared with P using t test. (D) Mean ± SD of FA levels in FLT3(ITD) 32Dcl3 cells cultured in standard medium (Reg) and in medium without glucose, serine, and glycine (no GSG); ∗P < .05 using t test when compared with corresponding Reg. (E) Mean ± SD of FA levels, DPCs, and % of γ-H2AX–positive cells in FLT3(ITD) 32Dcl3 cells cultured in glucose + 5× serine + 5× glycine high (high GSG) medium and in medium without glucose, serine, and glycine (no GSG). ∗P < .05 using t test. (F) Mean ± SD of FA levels and DPCs in FLT3(ITD) 32Dcl3 cells cultured in glucose-supplemented serine + glycine–free medium and treated or not with WQ-2101; ∗P < .05 using t test. (G) Mean ± SD of FA levels and DPCs from 3 experiments in FLT3(ITD) 32Dcl3 cells cultured in standard medium and treated or not with SHIN1. ∗P < .05 using t test. (H) Lin-cKit+ mBMCs from Polq+/+ and Polq−/− mice (blue and orange, respectively) were cultured in high GSG and/or no GSG medium. Results represent mean ± SD of FA levels and DPCs and proliferation rate; ∗P < .05 when compared with corresponding high GSG using t test. (I) Clonogenic activity of Lin-cKit+ BMCs from Polq+/+ and Polq−/− mice treated with the indicated concentrations of formaldehyde for 4 hours after plating in methylcellulose. Results represent the mean percentage of colonies ± SD when compared with untreated counterparts. (J) Lin-cKit+ mBMCs from Polq+/+ and Polq−/− mice were untreated (0) and treated with 400 nM formaldehyde for 24 and 48 hours. Results represent mean ± SD of DPCs and tail DNA percentage from the neutral comet assay. ∗P ≤ .001 when compared with Polq+/+ counterpart using t test. (K) Lin-cKit+ mBMCs from Flt3ITD;Polq−/− (orange) and Flt3ITD;Polq+/+ (blue) mice were cultured in high GSG and/or no GSG medium. Results represent mean ± SD of FA levels and DPCs and proliferation rate; ∗P < .05 when compared with corresponding high GSG using t test. (L) Sensitivity of FLT3(ITD) 32Dcl3 cells to 25 μM ART558 in high GSG and no GSG medium. Results represent the mean percentage of colonies ± SD when compared with untreated counterparts; ∗P < .05 using t test. (M) A scheme illustrating functional pathway where serine/1C cycle–produced formaldehyde induces DPCs, which are likely repaired by POLθ-mediated TMEJ. SD, standard deviation.
We, and others, reported that serine/1C cycle metabolism is reprogrammed in OTK-positive leukemia cells and can generate formaldehyde.29,43-45 To investigate the role of serine/1C cycle metabolism in the production of formaldehyde, FLT3(ITD)-positive and parental 32Dcl3 cells were cultured in glucose-, serine-, and glycine-free medium (no GSG) and in standard RPMI medium supplemented with glucose, serine, and glycine (Reg) to abrogate or maintain serine/1C cycle metabolism, respectively. No GSG diminished formaldehyde in FLT3(ITD) cells almost to the levels detected in parental cells cultured in regular medium (Figure 1D), suggesting that serine/1C cycle metabolism is responsible for the accumulation of formaldehyde in FLT3(ITD) cells.
In addition, to study the role of serine/1C cycle metabolism in the production of formaldehyde-mediated DPCs, we applied a protocol described previously.29 FLT3(ITD)-positive 32Dcl3 cells were cultured in no GSG medium and in medium supplemented with glucose and 5× serine (1430 μM) and 5× glycine (665 μM) (high GSG) to abrogate or enhance serine/1C cycle metabolism, respectively. No GSG reduced intracellular formaldehyde and accumulation of DPCs and DSBs (Figure 1E). Cell proliferation rate was not significantly affected by these culture conditions.
The role of de-novo serine synthesis in the production of formaldehyde and DPCs was tested in the presence of glucose and the absence of serine and glycine. An inhibitor of serine synthesis (WQ-2101, a negative allosteric modulator of 3-phosphoglycerate dehydrogenase to abrogate the committed step in serine biosynthesis43) diminished formaldehyde and DPCs in FLT3(ITD)-positive cells (Figure 1F).
The role of production of 1C units in the accumulation of formaldehyde and DPCs was examined in a standard RPMI medium containing glucose, serine, and glycine (Reg). SHIN1 (inhibitor of serine hydroxymethyltransferase 1 and 2 (SHMT1/2)46 inactivates both cytosolic SHMT1 and mitochondrial SHMT2 to block the production of 1C units from serine) reduced formaldehyde and DPCs levels in FLT3(ITD)-positive cells (Figure 1G).
In hematopoietic cells, formaldehyde is usually degraded by aldehyde dehydrogenase 2 (ALDH2) and/or alcohol dehydrogenase 5.11 In concordance with previous reports,47,48 FLT3(ITD)-positive Lin-CD34+ AML cells and FLT3(ITD)-positive 32D cell line cells displayed reduced ALDH activity when compared with normal and parental cells, respectively, whereas alcohol dehydrogenase activity was similar in both normal and leukemia cells (supplemental Figure 1A-B).
The accumulation of DPCs and DSBs and proliferation activity were examined in stem/early progenitor Lin-cKit+Polq−/− and Polq+/+ mBMCs cultured in high GSG and/or no GSG conditions. Although formaldehyde levels were elevated in Polq−/− and Polq+/+ cells maintained in high GSG medium, DPCs accumulated exclusively in Polq−/− cells, which was associated with proliferation arrest (Figure 1H). Moreover, Lin-cKit+Polq−/− and Polq+/+ mBMCs were treated with formaldehyde. The absence of POLθ abrogated the clonogenic activity of formaldehyde-treated cells (Figure 1I), which was preceded by the abundant accumulation of DPCs and DSBs (Figure 1J). Interestingly, POLθ did not affect the sensitivity of mBMCs to reactive oxygen species hydrogen peroxide (supplemental Figure 1C).
To provide a more direct link between POLθ and serine/1C cycle metabolism–generated formaldehyde-induced DPCs, Lin-cKit+Flt3ITD;Polq−/− and Flt3ITD;Polq+/+ mBMCs were cultured in no GSG and in high GSG medium. We observed that formaldehyde levels were elevated in both Flt3ITD;Polq−/− and Flt3ITD;Polq+/+ cells maintained in high GSG medium, but DPCs accumulated exclusively in Flt3ITD;Polq−/− cells in high GSG medium, which was associated with proliferation disadvantage (Figure 1K). Moreover, FLT3(ITD)-positive 32Dcl3 cells maintained in No GSG and High GSG medium were treated with POLθi ART558.24 Although the presence/absence of glucose, serine, and glycine did not significantly affect the clonogenic activity of FLT3(ITD)-positive 32Dcl3 cells, ART558 exerted a selective inhibitory effect only in high GSG medium (Figure 1L), in which the formaldehyde generated by serine/1C cycle metabolism resulted in the accumulation of DPCs and DSBs (Figure 1E).
Altogether, elevated levels of DPCs in OTK-positive hematological malignancies may result from enhanced production of formaldehyde via serine/1C cycle metabolism (Figure 1M). Moreover, we postulate that POLθ-mediated TMEJ plays a key role in protecting OTK-positive cells from the toxic effect of formaldehyde-induced DNA damage.
OTKs induce abundant expression of POLθ
TCGA database analysis revealed that AML cells express the highest levels of POLQ messenger RNA (mRNA) among all tumors (supplemental Figure 2A). POLQ expression did not differ between AMLs in Caucasian and African-American, or in male and female patients (supplemental Figure 2B). In addition, POLQ mRNA was elevated in normal spleen cells, suggesting that its expression is higher in hematopoietic tissues when compared with other organs (supplemental Figure 2C).
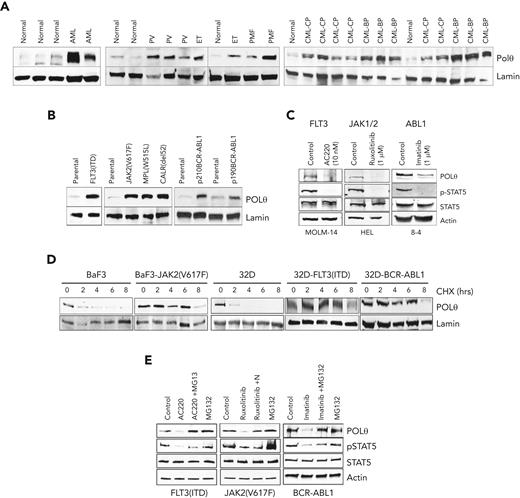
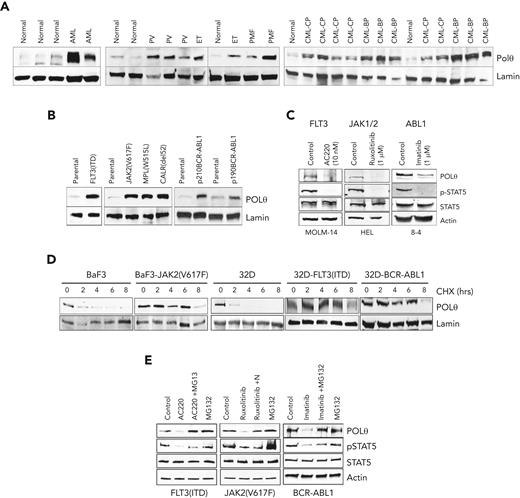
Western analysis using prevalidated anti-POLθ antibody (supplemental Figure 2D) revealed abundant overexpression of POLθ in OTK-positive primary leukemia cells and leukemia cell lines carrying OTKs when compared with their normal counterparts (Figure 2A and B, respectively). The magnitude of overexpression of POLθ depended on the expression levels of OTK (supplemental Figure 2E). Using kinase-specific inhibitors (TKi), we showed that elevated levels of POLθ were stimulated by OTK enzymatic activities (Figure 2C). However, OTKs did not increase POLQ transcription and mRNA expression (supplemental Figure 2F-H).
OTKs enhance the expression of POLθ. (A-C) Western analysis of POLθ expression in nuclear lysates from Lin-CD34+ cells from healthy donors (normal) and patients with FLT3(ITD)-positive AML, Jak2(V617F)-positive MPN (PV, ET, and PMF), and BCR-ABL1–positive CML (CP and BP) (A) and parental (32Dcl3, BaF3) cells and these expressing the indicated oncogenes (B). Lamin served as loading control. (C) Western blot of indicated proteins in total cell lysates from cells expressing OTKs and treated with specific TKi (FLT3 inhibitor AC220, JAK1/2 inhibitor ruxolitinib, and ABL1 inhibitor imatinib). (D) CHX chase assay: nuclear cell lysates obtained from indicated cells after CHX treatment (hours) were analyzed using western blot to detect POLθ and lamin (loading control). (E) Western blot of indicated proteins in total cell lysates from cells expressing OTKs and treated with specific TKi (FLT3 inhibitor AC220, JAK1/2 inhibitor ruxolitinib, ABL1 inhibitor imatinib) and proteasome inhibitor MG132. BP, blast phase; CHX, cycloheximide; CP, chronic blast; ET, essential thrombocythemia; PMF, primary myelofibrosis; PV, polycythemia vera.
OTKs enhance the expression of POLθ. (A-C) Western analysis of POLθ expression in nuclear lysates from Lin-CD34+ cells from healthy donors (normal) and patients with FLT3(ITD)-positive AML, Jak2(V617F)-positive MPN (PV, ET, and PMF), and BCR-ABL1–positive CML (CP and BP) (A) and parental (32Dcl3, BaF3) cells and these expressing the indicated oncogenes (B). Lamin served as loading control. (C) Western blot of indicated proteins in total cell lysates from cells expressing OTKs and treated with specific TKi (FLT3 inhibitor AC220, JAK1/2 inhibitor ruxolitinib, and ABL1 inhibitor imatinib). (D) CHX chase assay: nuclear cell lysates obtained from indicated cells after CHX treatment (hours) were analyzed using western blot to detect POLθ and lamin (loading control). (E) Western blot of indicated proteins in total cell lysates from cells expressing OTKs and treated with specific TKi (FLT3 inhibitor AC220, JAK1/2 inhibitor ruxolitinib, ABL1 inhibitor imatinib) and proteasome inhibitor MG132. BP, blast phase; CHX, cycloheximide; CP, chronic blast; ET, essential thrombocythemia; PMF, primary myelofibrosis; PV, polycythemia vera.
A cycloheximide chase assay revealed that POLθ protein half-life was prolonged by ≥3 times in the presence of OTKs (Figure 2D). Numerous elements of proteasomal machinery were detected in anti-POLθ immunoprecipitates (supplemental Figure 2I), suggesting that proteasomal degradation regulates POLθ expression. In concordance, the proteasomal inhibitor MG132 rescued overexpression of POLθ when OTK was inhibited by specific TKi (Figure 2E), indicating that OTKs prevented proteasomal degradation of POLθ. In addition, using a plasmid containing POLQ 3’UTR, we showed that translation of POLθ was modestly enhanced by OTKs (supplemental Figure 2J, left panel).
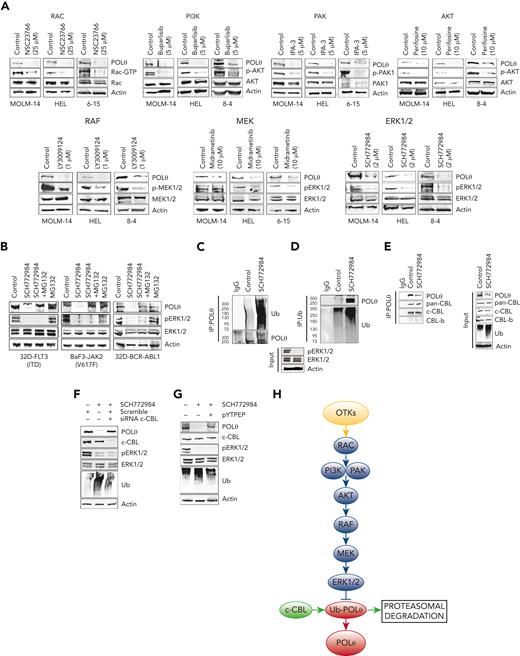
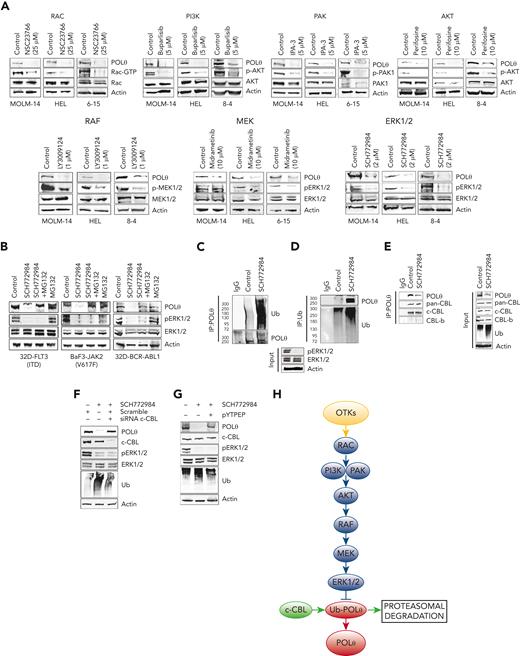
OTKs induce intracellular signaling pathways to promote cell transformation and regulate DDR.49 Using selective inhibitors of OTKs downstream effectors, we determined that OTKs activated the RAC-PI3K/PAK-AKT-RAF-MEK-ERK1/2 pathway to enhance the expression of POLθ (Figure 3A). PLCγ, PKC, p38MAPK, and mTOR signaling proteins were not involved in this process, and RAS might (FLT3(ITD) JAK2(V617F)) or might not (BCR-ABL1) play a role (supplemental Figure 3).
OTKs→RAC→PI3K/PAK→AKT→RAF→MEK→ERK/c-CBL pathway enhances the expression of POLθ. (A) FLT3(ITD)-positive MOLM14 cells, JAK2(V617F)-positive HEL cells, and BCR-ABL1–positive 32Dcl3 cells4-15 were treated with PI3K inhibitor buparlisib, AKT inhibitor perifosine, RAF1 inhibitor LY3009124, RAC inhibitor NSC23766, MEK inhibitor midrametinib, ERK inhibitor SCH772984, PAK1/2 inhibitor IPA-3 after western analysis of the total cell lysates. (B) Western blot of total cell lysates from 32D-FLT3(ITD), BAF3-JAK2(V617F), and 32D-BCR-ABL1 cells untreated (control) and treated with MG132 and/or SCH772984. (C-G) Total cell lysates were obtained from 32D-FLT3(ITD) cells treated with SCH772984 or diluent (control). (C) Western blot detecting ubiquitinated proteins in anti-POLθ immunoprecipitates. (D) Western blot detecting POLθ in antiubiquitin (Ub) immunoprecipitates. (E) Western blot detecting CBL proteins in anti-POLθ immunoprecipitates (IP:POLθ) and in total cell lysates (input) using pan-CBL and c-CBL and CBL-b specific antibodies. (F) Western blot of total cell lysates from 32D-FLT3(ITD) cells treated or not with SCH772984 and c-CBL siRNA or scrambled RNA. (G) Western blot of total cell lysates from 32D-FLT3(ITD cells treated or not with SCH772984 and pYTPEP CBL inhibitory peptide. (H) A diagram illustrating OTK-induced signaling pathways responsible for overexpression of POLθ.
OTKs→RAC→PI3K/PAK→AKT→RAF→MEK→ERK/c-CBL pathway enhances the expression of POLθ. (A) FLT3(ITD)-positive MOLM14 cells, JAK2(V617F)-positive HEL cells, and BCR-ABL1–positive 32Dcl3 cells4-15 were treated with PI3K inhibitor buparlisib, AKT inhibitor perifosine, RAF1 inhibitor LY3009124, RAC inhibitor NSC23766, MEK inhibitor midrametinib, ERK inhibitor SCH772984, PAK1/2 inhibitor IPA-3 after western analysis of the total cell lysates. (B) Western blot of total cell lysates from 32D-FLT3(ITD), BAF3-JAK2(V617F), and 32D-BCR-ABL1 cells untreated (control) and treated with MG132 and/or SCH772984. (C-G) Total cell lysates were obtained from 32D-FLT3(ITD) cells treated with SCH772984 or diluent (control). (C) Western blot detecting ubiquitinated proteins in anti-POLθ immunoprecipitates. (D) Western blot detecting POLθ in antiubiquitin (Ub) immunoprecipitates. (E) Western blot detecting CBL proteins in anti-POLθ immunoprecipitates (IP:POLθ) and in total cell lysates (input) using pan-CBL and c-CBL and CBL-b specific antibodies. (F) Western blot of total cell lysates from 32D-FLT3(ITD) cells treated or not with SCH772984 and c-CBL siRNA or scrambled RNA. (G) Western blot of total cell lysates from 32D-FLT3(ITD cells treated or not with SCH772984 and pYTPEP CBL inhibitory peptide. (H) A diagram illustrating OTK-induced signaling pathways responsible for overexpression of POLθ.
Proteasomal inhibitor MG132 prevented the downregulation of POLθ also upon inhibition of ERK1/2 in OTK-positive cells (Figure 3B), suggesting that OTK-mediated upregulation of POLθ depends on ERK1/2-mediated inhibition of proteasomal degradation. In concordance, the inhibition of ERK1/2 kinase promoted ubiquitination of POLθ (Figure 3C-D). c-CBL and CBL-b E3 ligases have been reported to be key components of OTK-mediated transforming machinery.50,51 We show that c-CBL but not CBL-b were detected in anti-POLθ immunoprecipitates (Figure 3E), and downregulation of c-CBL rescued POLθ overexpression in FLT3(ITD)-positive cells after the inhibition of ERK1/2 kinase (Figure 3F). This observation was validated by the rescued overexpression of POLθ by c-CBL inhibitory peptide (Figure 3G).
Altogether, we postulate that OTK-activated ERK1/2 kinase inhibited c-CBL–mediated ubiquitination and proteasomal degradation of POLθ, resulting in the overexpression of POLθ protein (Figure 3H). Additional mechanisms, for example, enhanced translation but not transcription, might also contribute to enhanced POLθ expression in these leukemia cells.
POLθ-mediated TMEJ repairs DPCs in OTK-positive leukemia
Because OTKs do not enhance the expression of SPRTN protease and TDP1 and TDP2 hydrolases (supplemental Figure 4), the proteins directly involved in DPCs removal,14 it is unlikely that they provide sufficient protection against abundant DPCs in OTK-positive cells. Unresolved DPCs will ultimately generate DSBs.
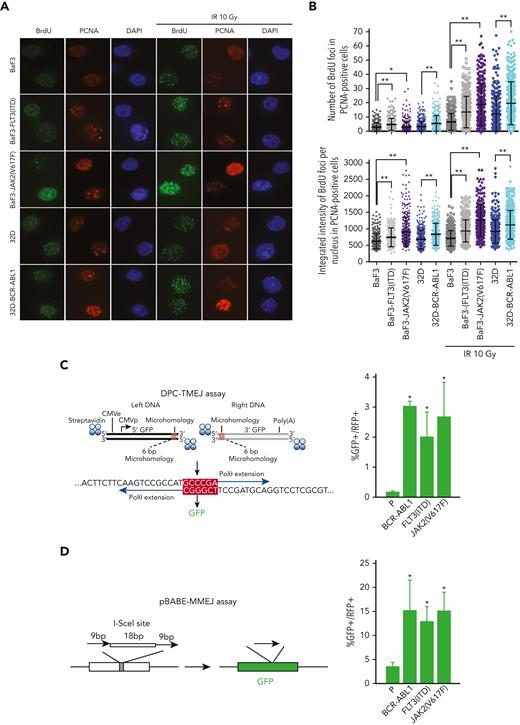
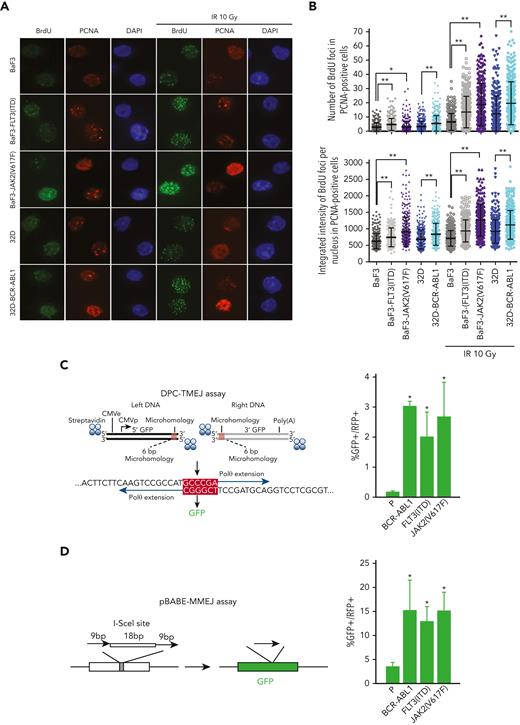
We observed that the presence of OTK promoted DSB-end resection (Figure 4A-B). This observation is consistent with the overexpression of CtIP (facilitates end resection,52) in OTK-positive cells (supplemental Figure 4A).53
OTKs-positive cells stimulate DNA resection and TMEJ/MMEJ. (A-B) BaF3 and 32D parental cells and the cells expressing JAK2(V617F), FLT3(ITD), or BCR-ABL1 were mock-treated or irradiated (10 Gy). (A) Immunofluorescence against BrdU and PCNA is shown. (B) The data show the mean ± SD; ∗P ≤ .05, ∗∗P ≤ .0001 using Mann-Whitney U test. (C) DPC-TMEJ assay and (D) pBABE-MMEJ assay. DPC-TMEJ or pBABE-MMEJ reporters and DsRed plasmid were transiently transfected in to parental 32Dcl3 cells (P) and cells expressing FLT3(ITD), JAK2(V617F), and BCR-ABL1 followed by flow cytometry. Results represent the mean percentage of GFP+ cells in DsRed+ population ± SD. ∗P < .05 when compared with P using t test. BrdU, 5-bromo-2′-deoxyuridine; SD, standard deviation.
OTKs-positive cells stimulate DNA resection and TMEJ/MMEJ. (A-B) BaF3 and 32D parental cells and the cells expressing JAK2(V617F), FLT3(ITD), or BCR-ABL1 were mock-treated or irradiated (10 Gy). (A) Immunofluorescence against BrdU and PCNA is shown. (B) The data show the mean ± SD; ∗P ≤ .05, ∗∗P ≤ .0001 using Mann-Whitney U test. (C) DPC-TMEJ assay and (D) pBABE-MMEJ assay. DPC-TMEJ or pBABE-MMEJ reporters and DsRed plasmid were transiently transfected in to parental 32Dcl3 cells (P) and cells expressing FLT3(ITD), JAK2(V617F), and BCR-ABL1 followed by flow cytometry. Results represent the mean percentage of GFP+ cells in DsRed+ population ± SD. ∗P < .05 when compared with P using t test. BrdU, 5-bromo-2′-deoxyuridine; SD, standard deviation.
Overexpression of POLθ and enhanced end resection at DSBs may favor TMEJ over HR and D-NHEJ.19,20 Thus, POLθ-mediated TMEJ activity was examined in leukemia cells using a previously characterized DPC-TMEJ substrate that models 5′ DPC-associated DSBs15 (Figure 4C, left panel). In addition, the pBABE-MMEJ reporter plasmid was used, with which the I-SceI–induced DSB in GFP sequence is repaired by POLθ38 (Figure 4D, left panel). Both DPC-TMEJ and pBABE-MMEJ activities were elevated 4 to 20 times in OTKs-positive cells when compared with their parental counterparts (Figure 4C-D, right panels). Enhanced DPC-TMEJ and pBABE-MMEJ in OTKs-positive cells were likely dependent on POLθ because these activities were downregulated by POLθi to the levels observed in nontransformed cells (supplemental Figure 4B).
POLθ plays an important role in leukemogenesis
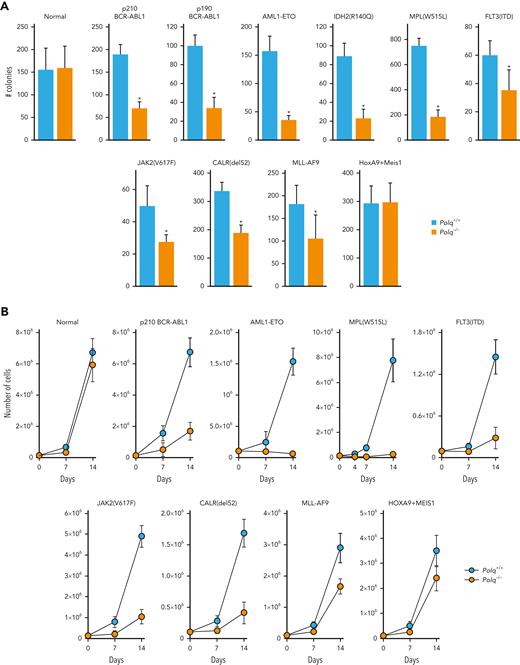
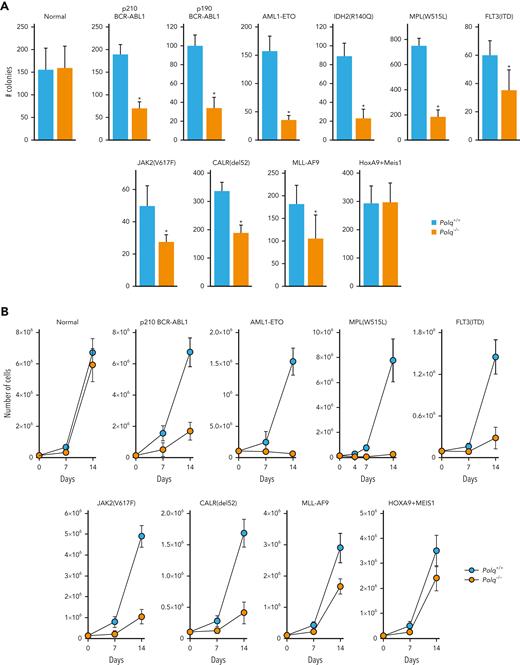
Polq−/− mice did not display significant defects in hematopoietic stem cell and peripheral blood compartments when compared with Polq+/+ counterparts (supplemental Figure 5A-B). Moreover, no difference in clonogenic activity and proliferation was observed, and similar numbers of DSBs were observed in Lin-cKit+ Polq−/− and Polq+/+ GFP+ mBMCs (Figure 5A-C, normal).
POLθ promoted leukemogenesis of HR/D-NHEJ–proficient and HR/D-NHEJ–deficient cells. (A) Clonogenic activity of Lin-cKit+Polq−/− and Polq+/+ GFP+ bone marrow cells expressing GFP (normal) and the indicated oncogenes. Results represent mean ± SD number of colonies; ∗P < .05 when compared with Polq+/+ counterparts using t test. (B) Proliferation of Lin-cKit+ Polq−/− and Polq+/+ bone marrow cells expressing GFP (normal) and the indicated oncogenes. Results represent mean ± SD number of cells; ∗P < .05 using t test. (C) DSBs were assessed by neutral comet assay in Polq+/+ (blue bars) and Polq−/− (orange bars) Lin- mBMCs expressing GFP (normal) and the indicated oncogenes. Results represent mean olive tail moment ± SD; ∗P < .05 when compared with Polq+/+ counterparts using t test. (D) Mean ± SD number of colonies from Lin- bone marrow cells harvested from Flt3ITD;Polq−/− (n=2) and Flt3ITD;Polq+/+ (n=4) mice; ∗P < .05 when compared with Polq+/+ counterparts using t test. (E) Engraftment of BCR-ABL1–positive Polq+/+ and Polq−/− cells in sublethally irradiated syngeneic mice. Results represent the mean percentage ± SD of GFP+ cells detected 4 weeks after transplantation in 10 mice per group. ∗P < .05 using t test. SD, standard deviation.
POLθ promoted leukemogenesis of HR/D-NHEJ–proficient and HR/D-NHEJ–deficient cells. (A) Clonogenic activity of Lin-cKit+Polq−/− and Polq+/+ GFP+ bone marrow cells expressing GFP (normal) and the indicated oncogenes. Results represent mean ± SD number of colonies; ∗P < .05 when compared with Polq+/+ counterparts using t test. (B) Proliferation of Lin-cKit+ Polq−/− and Polq+/+ bone marrow cells expressing GFP (normal) and the indicated oncogenes. Results represent mean ± SD number of cells; ∗P < .05 using t test. (C) DSBs were assessed by neutral comet assay in Polq+/+ (blue bars) and Polq−/− (orange bars) Lin- mBMCs expressing GFP (normal) and the indicated oncogenes. Results represent mean olive tail moment ± SD; ∗P < .05 when compared with Polq+/+ counterparts using t test. (D) Mean ± SD number of colonies from Lin- bone marrow cells harvested from Flt3ITD;Polq−/− (n=2) and Flt3ITD;Polq+/+ (n=4) mice; ∗P < .05 when compared with Polq+/+ counterparts using t test. (E) Engraftment of BCR-ABL1–positive Polq+/+ and Polq−/− cells in sublethally irradiated syngeneic mice. Results represent the mean percentage ± SD of GFP+ cells detected 4 weeks after transplantation in 10 mice per group. ∗P < .05 using t test. SD, standard deviation.
Polq−/− and Polq+/+ mBMCs were infected with retroviral particles encoding GFP and oncogenes associated with HR and/or D-NHEJ deficiency (OTKs: BCR-ABL1 and MPL(W515L) and other oncogenes: AML1-ETO, IDH2(R140Q)) and HR and D-NHEJ proficiency (OTKs: FLT3(ITD) and JAK2(V617F) and other oncogenes: MLL-AF9, HOXA9+MEIS1, and CALR(del52)).3,27,28,34,61,62
The impaired clonogenic activity (Figure 5A) and long-term proliferation (Figure 5B) of all (except HOXA9+MEIS1) oncogene-transduced Polq−/− GFP+Lin-cKit+ mBMCs were evident. The clonogenic activity and proliferation of Polq−/− cells transduced with oncogenes associated with HR and/or D-NHEJ deficiency were reduced by ∼3 to 4 times and ∼4 to 35 times, respectively, when compared with those of the Polq+/+ counterparts. Interestingly, Polq−/− cells transduced with oncogenes promoting HR and D-NHEJ proficiency demonstrated a modest 1.5 to 2 times reduction of clonogenic activity, and their long-term proliferation was reduced by twofold to fivefold in comparison to that of Polq+/+ cells.
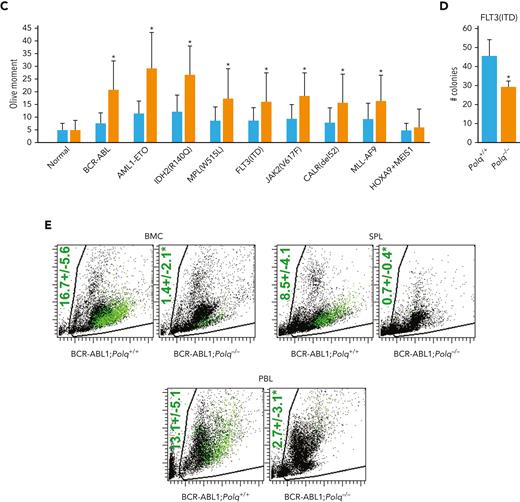
Reduced proliferation potential of Polq−/− GFP+Lin- mBMCs transduced with all oncogenes (except HOXA9+MEIS1) was accompanied by an accumulation of DSBs when compared with that of Polq+/+ counterparts (Figure 5C). This effect was also associated with elevation of formaldehyde levels (supplemental Figure 5C) and the accumulation of DPCs (supplemental Figure 5D) in OTK-positive Polq−/− cells.
We also tested the proliferation capacity of Lin- mBMCs obtained from Flt3ITD;Polq−/− and Flt3ITD;Polq+/+ mice. In the absence of Polq, Flt3ITD cells displayed reduced clonogenic potential (Figure 5D).
To study the impact of POLθ on leukemogenesis, BCR-ABL1–positive Polq−/− and BCR-ABL1–positive Polq+/+ GFP+Lin- mBMCs were injected intravenously into sublethally irradiated syngeneic mice, and the engraftment was assessed by detecting GFP+ cells in the peripheral blood, spleen, and bone marrow. We found that the engraftment of BCR-ABL1–positive Polq−/− cells was reduced by 5 to 10-fold when compared with that in Polq+/+ counterparts (Figure 5E).
Genetic and biochemical targeting of POLθ in leukemia
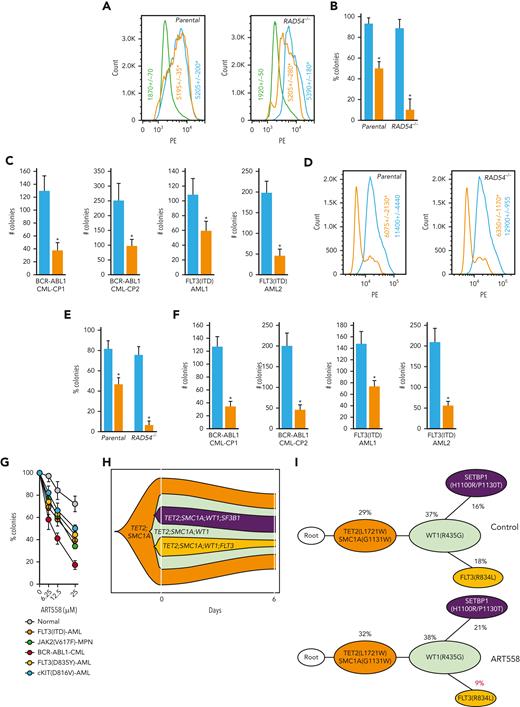
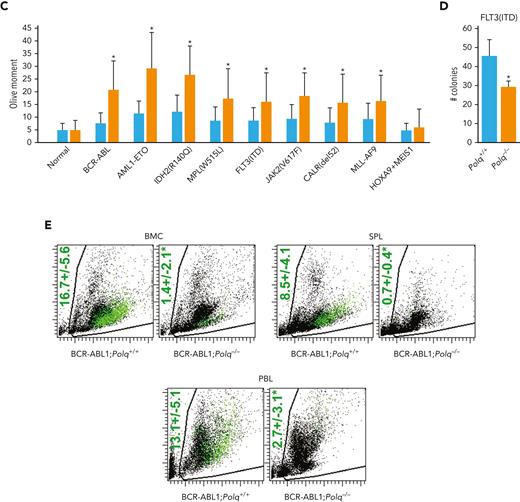
Expression of Flag-tagged POLθ-deficient dominant-negative mutant (D2230A+Y2231A) and POLθ wild-type proteins were confirmed via anti-Flag immunofluorescent staining in the transfected GFP+ cells (Figure 6A). The POLθ(D2230A+Y2231A) mutant, when compared with the POLθ wild-type, reduced the clonogenic activity of HR-deficient Nalm6(RAD54−/−) and HR-proficient Nalm6 parental cells by approximately ninefold and twofold, respectively (Figure 6B). Moreover, The POLθ(D2230A+Y2231A) mutant reduced colony formation of GFP+Lin-CD34+ BCR-ABL1–positive CML-CP primary cells by 2.5-fold to 3.5-foldfold and GFP+Lin-CD34+ FLT3(ITD)-positive AML primary cells by 1.5-fold to fourfold (Figure 6C).
Genetic and biochemical targeting of POLθ exerted antileukemia effect in HR-deficient and HR-proficient leukemias. (A) Representative anti-Flag flow cytometry profiles in Nalm6 (parental) and Nalm6(RAD54−/−) B-ALL cells infected with Flag-POLθ (D2230A+Y2231A) DNA polymerase–inactive mutant (orange), Flag- POLθ wild-type (blue), or GFP only (green). Geo mean ± SD of anti-Flag immunofluorescence is shown; ∗P < .001 when compared with wild-type using t test. (B) Results represent the percentage of colonies ± SD of GFP+ cells expressing POLθ (D2230A+Y2231A) mutant (orange) or POLθ wild-type (blue), when compared with cells expressing GFP only; ∗P < .01 using t test. (C) Results represent number of colonies ± SD from Lin-CD34+GFP+ BCR-ABL1–positive CML-CP and FLT3(ITD)-positive AML cells expressing POLθ (D2230A+Y2231A) mutant (orange) or POLθ wild-type (blue); ∗P < .01 using t test. (D) Representative anti-POLθ flow cytometry analyses showing expression of POLθ in GFP+ cells transduced with POLQ shRNA (orange) or scrambled RNA (blue). Results show Geo mean ± SD of anti-POLθ immunofluorescence; ∗P < .05 using t test. (E) Results represent the percentages of colonies ± SD from GFP+ cells transduced with POLQ shRNA (orange) and scrambled RNA (blue) when compared with cells expressing GFP only; ∗P < .01 using t test. (F) Results represent number of colonies ± SD from Lin-CD34+GFP+ BCR-ABL1–positive CML-CP and FLT3(ITD)-positive AML cells transduced with POLQ shRNA (orange) or scrambled RNA (blue); ∗P < .01 using t test. (G) Results represent the percentages of colonies ± SD from Lin-CD34+ cells isolated from FLT3(ITD)-positive AMLs (n = 3), FLT3(D835Y)-positive AML (n = 1), cKIT(D816V)-positive AML (n = 1), JAK2(V617F) MPN (n = 1), BCR-ABL1–positive CML-CP (n = 3), and normal healthy donors (n = 3) incubated with POLθi (ART558). (H-I) Cells from patients with Lin- AML were treated with ART558 for 6 days after SC-tDNAseq. (H) The fish plot reflects number of cells before (0 days) and 6 days after the treatment and the inferred clonal evolution pattern based on SC-tDNAseq data. (I) The phylogenic tree visualizes the predicted order of mutation acquisition and the proportion of subclones with a different combination of mutations in control and ART558-treated cells: ADO rate = 3.9%, FPR = 1.0%. ADO, allele dropout; FPR, false positive rate; SC-tDNAseq, single-cell targeted DNA sequencing; SD, standard deviation.
Genetic and biochemical targeting of POLθ exerted antileukemia effect in HR-deficient and HR-proficient leukemias. (A) Representative anti-Flag flow cytometry profiles in Nalm6 (parental) and Nalm6(RAD54−/−) B-ALL cells infected with Flag-POLθ (D2230A+Y2231A) DNA polymerase–inactive mutant (orange), Flag- POLθ wild-type (blue), or GFP only (green). Geo mean ± SD of anti-Flag immunofluorescence is shown; ∗P < .001 when compared with wild-type using t test. (B) Results represent the percentage of colonies ± SD of GFP+ cells expressing POLθ (D2230A+Y2231A) mutant (orange) or POLθ wild-type (blue), when compared with cells expressing GFP only; ∗P < .01 using t test. (C) Results represent number of colonies ± SD from Lin-CD34+GFP+ BCR-ABL1–positive CML-CP and FLT3(ITD)-positive AML cells expressing POLθ (D2230A+Y2231A) mutant (orange) or POLθ wild-type (blue); ∗P < .01 using t test. (D) Representative anti-POLθ flow cytometry analyses showing expression of POLθ in GFP+ cells transduced with POLQ shRNA (orange) or scrambled RNA (blue). Results show Geo mean ± SD of anti-POLθ immunofluorescence; ∗P < .05 using t test. (E) Results represent the percentages of colonies ± SD from GFP+ cells transduced with POLQ shRNA (orange) and scrambled RNA (blue) when compared with cells expressing GFP only; ∗P < .01 using t test. (F) Results represent number of colonies ± SD from Lin-CD34+GFP+ BCR-ABL1–positive CML-CP and FLT3(ITD)-positive AML cells transduced with POLQ shRNA (orange) or scrambled RNA (blue); ∗P < .01 using t test. (G) Results represent the percentages of colonies ± SD from Lin-CD34+ cells isolated from FLT3(ITD)-positive AMLs (n = 3), FLT3(D835Y)-positive AML (n = 1), cKIT(D816V)-positive AML (n = 1), JAK2(V617F) MPN (n = 1), BCR-ABL1–positive CML-CP (n = 3), and normal healthy donors (n = 3) incubated with POLθi (ART558). (H-I) Cells from patients with Lin- AML were treated with ART558 for 6 days after SC-tDNAseq. (H) The fish plot reflects number of cells before (0 days) and 6 days after the treatment and the inferred clonal evolution pattern based on SC-tDNAseq data. (I) The phylogenic tree visualizes the predicted order of mutation acquisition and the proportion of subclones with a different combination of mutations in control and ART558-treated cells: ADO rate = 3.9%, FPR = 1.0%. ADO, allele dropout; FPR, false positive rate; SC-tDNAseq, single-cell targeted DNA sequencing; SD, standard deviation.
Downregulation of POLθ by specific shRNA was confirmed by immunofluorescence in the transfected GFP+ cells (Figure 6D) and resulted in a 15-fold and a twofold reduction of the clonogenic activity of Nalm6(RAD54−/−) and Nalm6 parental cells, respectively (Figure 6E). In addition, POLQ shRNA inhibited colony formation of GFP+Lin-CD34+ BCR-ABL1–positive CML-CP and GFP+Lin-CD34+ FLT3(ITD)–positive AML primary cells by fourfold and twofold to fourfold, respectively (Figure 6F). Moreover, knocking down POLQ using CRISPR/Cas9 resulted in reduced colony formation by FLT3(ITD)-positive 32Dcl3 cells (supplemental Figure 6A-C).
In addition, FLT3(ITD/TKD) AML, cKIT(D816V) AML, JAK2(V617F) MPN, and BCR-ABL1 CML Lin-CD34+ primary stem/progenitor cells were more sensitive to POLθi ART558 than their normal counterparts (Figure 6G). Of note, BCRA1-deficient BCR-ABL1 cells61 were more sensitive to ART558 than cells expressing other OTKs.27,28 Moreover, BaF3 cells transformed by FLT3-activating mutations in the juxtamembrane domain (p.Glu598_Tyr599del = delEY; p.Phe590_Asp593delinsLeuTyr = delIns) and in tyrosine kinase domain 1 (E611V) were sensitive to ART558 when compared with parental cells (supplemental Figure 6D). However, mutation in the spliceosome regulator SRSF2 did not affect the response of AML cell lines to ART558 (supplemental Figure 6E).
We applied single-cell targeted DNA sequencing using the Missionbio/Tapestri single-cell DNA myeloid panel to detect genetic alterations associated with sensitivity to POLθi in cells from patients with AML carrying FLT3(R834L), TET2(L1721W), SMC1A(G1131W), WT1(R435G), and SETBP1(H1100R/P1130T) mutants. We observed that although ART558 treatment reduced the total cell number only by 30%, the clone carrying FLT3(R834L) variant63 was selectively targeted by POLθi with a remarkable threefold efficiency (the clone cell number reduced from 18% to 6%) in just 6 days (Figure 6H). Moreover, the analysis of clonal composition revealed specific twofold relative reduction of that clone upon treatment with ART558, whereas clones not carrying FLT3 mutation did not shrink proportionally (Figure 6I). This observation supports the hypothesis that OTK-positive clones are sensitive to POLθi, especially those displaying HR-deficient phenotype (WT1 inactivation caused HR deficiency in FLT3(ITD) cells31).
Therapeutic effect of POLθi combined with standard drugs against HR and D-NHEJ–proficient leukemia
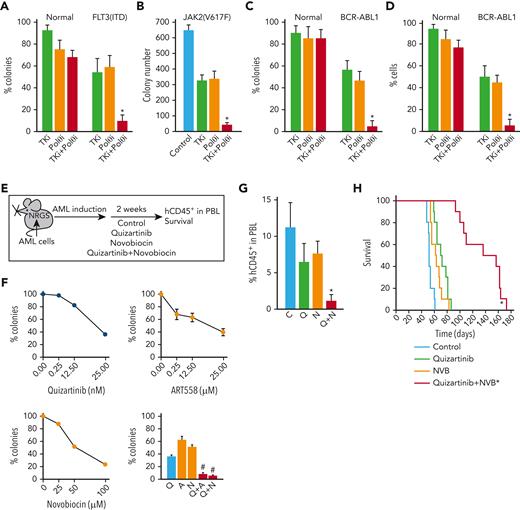
Because TKis targeting FLT3(ITD), JAK2(V617F), and BCR-ABL1 induced acute HR and D-NHEJ deficiency,27,28,34 we hypothesized that they would sensitize OTK-positive cells to POLθi. In concordance, we show that POLθi ART558 combined with OTK-specific TKi exerts 5 to 10 times stronger effect against FLT3(ITD)-positive AML, JAK2(V617F)-positive MPN, BCR-ABL1–positive CML, and BCR-ABL1–positive ALL primary cells in vitro with minimal or no effect on normal primary hematopoietic cells (Figure 7A-D). In addition, POLθi combined with FLT3i exerted 2 to 3 times stronger effect than individual agents against BaF3 cells transformed by FLT3(delEY), FLT3(delIns), and FLT3(E611V) mutants (supplemental Figure 7A). The synergy between OTKi and POLθi may result from a combination of the incomplete effects of each inhibitor used individually. In addition, OTKi abrogates the antiapoptotic protection provided by OTK, which might amplify the potentially lethal effect of DSBs accumulated after inhibition of POLθ.24,25,64
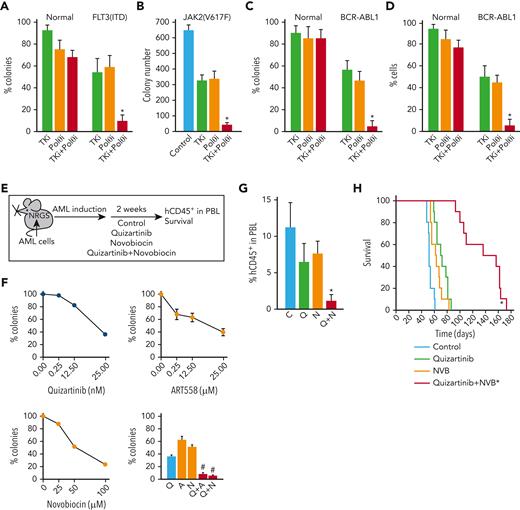
TKi enhanced the effect of POLθI against OTK-positive hematological malignancies. (A-C) Lin-CD34+ cells from FLT3(ITD)-positive AMLs (n = 3), JAK2(V617F)-positive MPN (n = 1), BCR-ABL1–positive CML-CPs (n = 3) and normal healthy donors (n = 3), and (D) mononuclear cells from Ph+ ALL (n = 3) and normal healthy donors (n = 3) were incubated with OTK-specific TKi (panel A, 10 nM FLT3 kinase inhibitor quizartinib; panel B, 25 nM JAK1/2 kinase inhibitor ruxolitinib; panels C,D, 1 μM ABL1 kinase inhibitor imatinib) and/or POLθi (25 μM ART558). Results represent the mean percentage of colonies ± SD compared with untreated controls (A,C), mean number of colonies ± SD (B), and mean percentage of living cells ± SD compared with untreated controls (D) ∗P < .05 when compared with individual treatments using two-tailed unpaired t test. (E) PLX treatment experimental diagram. (F) Sensitivity of Lin-CD34+ PLX patient cells to the indicated inhibitors. Results represent the mean percentage of colonies ± SD compared with untreated controls. ∗P < .05 when compared with individual treatments using response additivity approach. (G) Mean number of hCD45+ AML cells ± SD in peripheral blood lymphocyte of PLX-bearing NRGS mice untreated (C) and treated with treated with quizartinib (Q), NVB (N), and the combination (Q + N). ∗P < .05 when compared with individual treatments using two-tailed unpaired t test. (H) Survival curves and MST of the mice. ∗P < .001 in comparison to quizartinib and NVB using Kaplan-Meier log-rank test. MST, median survival time; SD, standard deviation.
TKi enhanced the effect of POLθI against OTK-positive hematological malignancies. (A-C) Lin-CD34+ cells from FLT3(ITD)-positive AMLs (n = 3), JAK2(V617F)-positive MPN (n = 1), BCR-ABL1–positive CML-CPs (n = 3) and normal healthy donors (n = 3), and (D) mononuclear cells from Ph+ ALL (n = 3) and normal healthy donors (n = 3) were incubated with OTK-specific TKi (panel A, 10 nM FLT3 kinase inhibitor quizartinib; panel B, 25 nM JAK1/2 kinase inhibitor ruxolitinib; panels C,D, 1 μM ABL1 kinase inhibitor imatinib) and/or POLθi (25 μM ART558). Results represent the mean percentage of colonies ± SD compared with untreated controls (A,C), mean number of colonies ± SD (B), and mean percentage of living cells ± SD compared with untreated controls (D) ∗P < .05 when compared with individual treatments using two-tailed unpaired t test. (E) PLX treatment experimental diagram. (F) Sensitivity of Lin-CD34+ PLX patient cells to the indicated inhibitors. Results represent the mean percentage of colonies ± SD compared with untreated controls. ∗P < .05 when compared with individual treatments using response additivity approach. (G) Mean number of hCD45+ AML cells ± SD in peripheral blood lymphocyte of PLX-bearing NRGS mice untreated (C) and treated with treated with quizartinib (Q), NVB (N), and the combination (Q + N). ∗P < .05 when compared with individual treatments using two-tailed unpaired t test. (H) Survival curves and MST of the mice. ∗P < .001 in comparison to quizartinib and NVB using Kaplan-Meier log-rank test. MST, median survival time; SD, standard deviation.
To test the potential clinical application of such a strategy, NRGS mice bearing FLT3(ITD)-positive primary AML xenograft cells were treated with FLT3i quizartinib and/or POLθi NVB,25 (Figure 7E). NVB was used for in vivo studies because of the unfavorable pharmacokinetics of ART558 in mice.25 NVB has not been optimized as a POLθ helicase inhibitor and is, therefore, likely to exhibit off-target effects. Nevertheless, this is the only POLθi with acceptable liver microsome stability available to us for in vivo studies.
AML primary cells were sensitive to both the POLθis, and the combination of FLT3i+POLθi exerted synergistic effects in vitro (Figure 7F). In concordance with in vitro results, the combination of quizartinib and NVB reduced AML xenograft cells in peripheral blood by fourfold to fivefold when compared with single treatment (Figure 7G). Moreover, although quizartinib and NVB modestly prolonged the median survival time of primary AML-bearing NRGS mice (72 ± 3 and 63 ± 3 days, respectively, vs control 53 ± 1 days), animals treated with quizartinib + NVB survived twice longer (136.3 ± 9.8 days) than those treated with a single drug (Figure 7H). No obvious toxicity was attributed to the treatment with quizartinib + NVB.
Our report indicated that the repair of etoposide-induced DPCs is promoted by POLθ.15 Therefore, the combination of etoposide and ART558 was tested against FLT3(ITD)-negative, HR-proficient AMLs characterized previously34 and against AML carrying NRAS(G12D) mutant activating ERK,65 which elevates the expression of POLθ (Figure 3H). Suboptimal concentrations of etoposide and ART558, when compared with individual treatments, exerted 4 to 6 and 4 to 5 times stronger effect in vitro against Lin-CD34+, HR-proficient AML and NRAS(G12D)-positive cells, respectively (supplemental Figure 7B).
Discussion
OTK-positive leukemia cells accumulate high levels of reactive oxygen species-induced oxidative DNA damage, but altered nucleotide excision repair, mismatch repair, HR, and/or NHEJ, but not TMEJ, activities protect them from apoptosis (57,64,66-68 and this work). However, endogenous aldehydes, for example, formaldehyde (the most abundant endogenous human carcinogen), represent another class of metabolically derived, highly active chemicals that cause DNA damage.69
We report here that serine synthesis/1C cycle metabolism produced excess formaldehyde, which generated numerous DPCs in OTK-positive cells. Oxidative protein demethylation associated with hematopoietic differentiation is unlikely to be the source of formaldehyde in differentiation-arrested leukemia cells.70 Because histone demethylases (eg, LSD1, JMJ family) were also implicated in generation of formaldehyde,71-74 we cannot exclude them as potential sources of DPCs. Moreover, ALDH-mediated decay of formaldehyde may be reduced in leukemia cells, further contributing to the accumulation of DPCs.47,48
We discovered that OTK-positive cells display unusually high levels of POLθ because of the prevention of c-CBL–mediated ubiquitination and proteasomal degradation of POLθ. c-CBL appears to be inhibited by other OTKs (eg, SRC and EGFR), therefore inhibition of proteasomal degradation may represent a major mechanism responsible for elevated expression of POLθ in tumor cells.75 We also show that enhanced POLθ-mediated TMEJ in OTK-positive leukemia cells plays a critical role in the repair of DSBs triggered by DPCs. Intriguingly, in the absence of POLθ, cells accumulated more formaldehyde-induced DPCs, suggesting the role of POLθ in earlier stages of resolving DPCs (eg, by stimulating protein displacement and/or DNA end-trimming).76,77
The protective effect of POLθ in leukemias may extend beyond TMEJ-dependent repair of DPC-induced DSBs. For example, OTKs are known to cause replication stress and fork stalling,78 and POLθ as a multifunctional enzyme can be involved in repair of these lesions, too.79 It has been suggested that the upregulation of POLθ may be crucial to tolerate replication stress better in HR-proficient breast, lung, and colorectal cancers.80
Patients with leukemias overexpressing POLθ may benefit from therapies targeting POLθ because its elevated expression is a predictive marker of sensitivity to POLθi.25 The antileukemia effect of targeting POLθ in cells displaying HR deficiency with or without concomitant D-NHEJ deficiency most likely depends on synthetic lethality resulting from inadequate DNA repair.22,24,25 Although inactivating mutations in HR and D-NHEJ are rare in leukemias,81 HR and/or D-NHEJ deficiency could be induced by mutations in genes whose products regulate metabolism (eg, IDH1/23 and this work), transcription (eg, AML1-ETO and IGH-MYC34,62,82), kinase activity (eg, BCR-ABL161), and DNA methylation (eg, TET2 and WT131). An exceptionally strong antileukemic effect of POLθi could also be achieved by the pharmacological induction of the HR and/or D-NHEJ–deficient phenotype by Food and Drug Administration–approved OTK inhibitors, for example, FLT3i quizartinib and JAK1/2i ruxolitinib.27,28
PARP1i olaparib and talazoparib were also very effective against FLT3i-treated HR/D-NHEJ–deficient AML cells.27 However, PARP1 (and other PARP proteins sensitive to the inhibitors) regulates not only DDR but also other fundamental intracellular activities (eg, chromatin remodeling, epigenome and transcription regulation, ribosome biogenesis, and RNA processing), whereas POLθ relates to DDR.83,84 Thus, POLθi may be less toxic than PARPi. Moreover, the interplay between PARP1 and Polθ is not fully understood, and their functions in hematopoietic cells may be interdependent and independent,85 thus suggesting that POLθi could be combined with PARPi to achieve better antileukemia effect.
In addition, Polθi could be effective against PARPi-resistant cells.24,25 Remarkably, we showed that POLθi exerted inhibitory effects against HR and D-NHEJ–proficient PARPi-resistant leukemia cells, albeit not as pronounced as in HR ± D-NHEJ–deficient cells. This effect was accompanied by extensive DNA end processing. It has been reported that TMEJ and HR are antagonistic,19,20 thus overexpression of POLθ in leukemia cells accumulating DPCs and displaying excessive DNA end processing may favor TMEJ over HR and D-NHEJ. This hypothesis is supported by this work showing that the effect of POLθi against HR and D-NHEJ–proficient leukemia is greatly enhanced by DPC-inducing drug etoposide.
In conclusion, we postulate that POLθ-mediated TMEJ protects OTK-positive cells against the toxicity of DPCs caused by the formaldehyde generated by serine/1C cycle metabolism. Leukemia cells are vulnerable to the inhibition of POLθ, especially when combined with drugs that induce DSB repair deficiencies (TKi) and DPCs (etoposide). Because POLθ-mediated TMEJ is highly mutagenic,60 inhibition of TMEJ by POLθi should not induce genomic instability and secondary tumors.
Acknowledgments
This work was funded by the National Institutes of Health (NIH), National Cancer Institute grants R01CA244179, CA237286, CA186238, and CA247707; and from Leukemia and Lymphoma Society grants TRP 6565-19 and TRP 6628-21 (T. Skorski). J.-Y.M was funded by the Canadian Institutes of Health Research FDN-388879 and is a Tier I Canada Research Chair in DNA Repair and Cancer Therapeutics. G.P.G. was funded by NIH, National Cancer Institute grant P01 CA247773 and a grant from the University Cancer Research Fund. P.V. was supported by the Austrian Science Fund (FWF) grant P4704-B20. R.L.L. was supported by NIH, National Cancer Institute grants 2P30CA008748-48 (Memorial Sloan Kettering [MSK] Cancer Center Support Grant) and UG1CA233332 (ECOG Leukemia Translational Science Center). This study was conducted in part by the ECOG-ACRIN Cancer Research Group supported by NIH, National Cancer Institute grants U10CA180820, UG1CA189859, UG1CA233290, UG1CA232760, and UG1CA233332.
Authorship
Contribution: U.V., K.S.-R., M.T., M.N.-S., M.-C.C., A.-M.K., P.P.-B., J.C., K.G., J.A., M.D., W.F., S.C., and R.N. performed experiments; J.J., K.N.C., and K.M.-W. did bioinformatic analyses; H.Z. analyzed statistical significance; W.C. and G.M. synthesized POLθ inhibitor ART558; G.C. synthesized POLθ substrate; J.G. supervised J.C.; M.W. supervised R.N.; T. Sliwinski supervised M.D.; K.P. supervised P.P.-B.; R.T.P. supervised G.C.; J.-Y.M. supervised M.-C.C.; G.P.G. supervised W.F.; E.M.P. provided genetically characterized AML samples from E1900; H.F.F. led trial E1900, M.S.T. was ECOG-ACRIN Leukemia Committee chair when E1900 was activated; M.R.L. is the current ECOG-ACRIN Leukemia Committee chair; R.L.L. performed molecular analyses of samples from E1900 clinical trial; P.V. and E.H. provided leukemia samples after NGS; J.G., M.W., S.M.S., R.P., J.-Y.M., and G.P.G. contributed to the final version of the manuscript; T. Skorski conceived the idea and supervised U.V., K.S.-R., M.T., M.N.-S., B.V.L., A-.M.K., K.G., M.D., and S.C., and wrote the manuscript.
Conflict-of-interest disclosure: H.F.F. serves on the advisory board of Jazz Pharmaceuticals and Incyte. R.T.P. is a co-founder and chief scientific officer of Recombination Therapeutics, LLC. M.S.T. received research funding from AbbVie, Orsenix, BioSight, Glycomimetics, Rafael Pharmaceuticals, and Amgen; is on advisory boards for AbbVie, Daiichi-Sankyo, Orsenix, KAHR, Jazz Pharma, Roche, Novartis, Innate Pharmaceuticals, Kura, Syros Pharmaceuticals, Ipsen Biopharmaceuticals, and Cellularity; and received royalties from UpToDate, the Data and Safety Monitoring Board (DSMB) HOVON protocol Ho156, and the Adjudication Committee Foghorn Therapeutics protocol FHD-286. R.L.L. is on the supervisory board of Qiagen; is a scientific advisor to Imago, Mission Bio, Zentalis, Ajax, Auron, Prelude, C4 Therapeutics and Isoplexis; receives research support from Ajax, Zentalis, and Abbvie; has consulted for Incyte, Janssen, and Astra Zeneca; and has received honoraria from Astra Zeneca for invited lectures. The remaining authors declare no competing financial interests.
Correspondence: Tomasz Skorski, Fels Cancer Institute for Personalized Medicine, Lewis Katz School of Medicine, Temple University, Philadelphia, PA 19140; e-mail: tskorski@temple.edu.
References
Author notes
Data are available on request from the corresponding author, Tomasz Skorski (tskorski@temple.edu).
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.