Abstract
Acute graft-versus-host disease (GVHD) is a rare complication after solid organ transplantation (SOT) that carries high mortality. Caused by immunocompetent donor leukocytes within the transplanted organ, which become activated against recipient tissues, GVHD typically develops 2 to 12 weeks after SOT and can affect the skin, gastrointestinal tract, liver, and bone marrow. Signs and symptoms are nonspecific and include a rash, nausea, appetite loss, diarrhea, and cytopenias. Pancytopenia from marrow-directed GVHD is the primary driver of mortality. The diagnosis of GVHD is often delayed but should be confirmed by biopsy of an affected organ. Evidence of donor chimerism in blood or marrow supports the diagnosis. When GVHD is diagnosed we initiate treatment with systemic corticosteroids. At that time, if GVHD only involves skin or oral mucosa we also decrease maintenance immunosuppression levels to allow the recipient to reject the donor immune cells. For GVHD involving the marrow we initiate an allogeneic hematopoietic cell donor search early. In this article, we describe 3 cases of GVHD after SOT, outline our approach to diagnosis and management, and then provide analysis of the 3 instructive cases.
Introduction
Acute graft-versus-host disease (GVHD), a frequent complication of allogeneic hematopoietic cell transplantation (alloHCT), is a rare but serious complication after solid organ transplantation (SOT). First reported in 1988 by Burdick et al1 in a recipient of orthotopic liver transplantation, GVHD results when donor-derived immunocompetent leukocytes within the transplanted organ evade elimination by the recipient immune system, encounter alloantigens, and mount an immune response against recipient tissues.2,3 The risk of developing GVHD is increased for organs containing greater amounts of viable lymphoid tissue.4,5 Incidence of, and mortality rates from, GVHD are best defined for intestinal and orthotopic liver transplantation (Table 1).4,10,16 Epidemiologic estimates and other information regarding GVHD are derived from small, single institution studies and case reports. In contrast to alloHCT in which prophylaxis to prevent GVHD is routinely administered, prophylaxis to prevent GVHD is not given after SOT.
Our hematology service is consulted approximately once each year over concern for GVHD after SOT. These consults are challenging; the diagnosis is often delayed and there are no rigorous studies to guide management. In this article, we begin by describing 3 cases of GVHD after SOT that demonstrate the diverse presentation and clinical course, as well as highlight the complexity of clinical decision-making. We then review of the literature and outline our approach to diagnosis and management. We finish with insights derived from the 3 cases.
Three examples of SOT recipients who developed GVHD
Case 1: GVHD involving only skin
A 70-year-old man with insulin-dependent diabetes, alcoholic cirrhosis, and hepatocellular carcinoma underwent orthotopic liver transplantation from a 42-year-old male donor. The recipient was blood group A-positive, seronegative for Epstein-Barr virus (EBV), and seropositive for cytomegalovirus (CMV). The donor was blood group A-positive, seronegative for CMV, and his death occurred owing to trauma. Induction immunosuppression consisted of basiliximab on posttransplant days 1 and 4. Tacrolimus was started on day 1 with a goal level of 10 ng/mL. On posttransplant day 20, the recipient developed an erythematous papular rash on his upper chest, which subsequently spread over his torso and extremities. His tacrolimus level was maintained at 10 to 14 ng/mL. Pathology from a skin biopsy on day 22 was consistent with GVHD, and blood chimerism testing showed that 7% of CD3-positive T cells and 1% of CD33-positive myeloid cells were of donor origin. On day 23 treatment with methylprednisolone, 2 mg/kg per day, was initiated. Tacrolimus was continued but the target level was reduced to 7 ng/mL. His skin rash showed no improvement after 3 days; thus, starting on day 26, alemtuzumab 10 mg/d was administered for 5 days. Methylprednisolone 2 mg/kg per day was continued for 7 days, converted to prednisone, and tapered by 10% every 3 days. Tacrolimus levels remained at 5 to 8 ng/mL. His skin rash resolved slowly over 8 months. Blood chimerism testing on day 85 showed that 1% to 5% of CD33-positive myeloid cells were of donor origin. Lymphocyte chimerism could not be measured, presumably owing to the prior treatment with alemtuzumab.
Case 2: GVHD involving skin and marrow
A 64-year-old man with atrial fibrillation and hepatitis C–induced cirrhosis complicated by hepatocellular carcinoma underwent orthotopic liver transplantation from a 57-year-old male donor. The recipient was blood group O-positive and seronegative for CMV and EBV. The donor was blood group O-positive, seronegative for CMV and EBV, and his death was due to a cerebrovascular accident. Induction immunosuppression included methylprednisolone and rabbit antithymocyte globulin (ATG); tacrolimus was used for maintenance immunosuppression with a goal level of 5 to 10 ng/mL. On posttransplant day 21, the recipient noted a morbilliform rash on his chest, which was mildly pruritic. Over the next day, the rash became confluent and spread to his abdomen, back, and proximal upper extremities. Pathology from a skin biopsy was consistent with acute GVHD. Blood counts at the time were notable for mild normocytic anemia (hemoglobin, 11 g/dL) and mild thrombocytopenia (platelets, 130 × 109 cells per L). On day 24, a marrow examination showed a normocellular marrow with trilineage hematopoiesis. Blood chimerism testing demonstrated that 67% of CD3-positive T cells, 17% of CD56-positive natural killer (NK) cells, and 77% of CD8-positive T cells were of donor origin. Chimerism testing of the marrow aspirate showed that 44% of CD3-positive T cells were of donor origin. On days 24 and 25, methylprednisolone 1000 mg/d (10 mg/kg per day) was given. This was later reduced to 2 mg/kg per day, converted to an equivalent dose of prednisone, and tapered by 10% every 5 days. On day 26, the rash was progressing, and the patient received a single dose of cyclophosphamide 50 mg/kg with mesna support. Tacrolimus dosing was adjusted to target a level of 10 ng/mL. The recipient’s blood counts declined as expected after cyclophosphamide and he became transfusion dependent. Repeat marrow examination (day 43) showed a recovering marrow with 20% to 30% cellularity. Flow cytometry showed reduced numbers of T-lymphocytes (5.2% of leukocytes). Marrow chimerism, however, showed that 36% of CD3-posiitve T cells were still of donor origin.
The recipient remained pancytopenic and transfusion dependent despite administration of granulocyte-colony stimulating factor and eltrombopag. Blood chimerism on day 87 continued to show significant numbers of donor-origin leukocytes. Tacrolimus levels remained at 10 to 15 ng/mL. Repeat marrow examination (day 120) showed <20% cellularity and <1% blasts. Because of the persisting marrow failure, the recipient underwent alloHCT from an HLA-identical sibling on day 131 after conditioning with fludarabine, equine ATG, cyclophosphamide, and 3 Gy total-body irradiation. Hematopoietic donor cells were collected from the peripheral blood after granulocyte-colony stimulating factor stimulation, and the recipient received GVHD prophylaxis with methotrexate and tacrolimus with goal level of 10 ng/mL. Neutrophil engraftment occurred on day 20 after alloHCT. On day 37 after alloHCT (day 169 post–liver transplant), blood chimerism showed that 99% of both CD3-positive T cells and CD33-positive myeloid cells originated from the alloHCT donor and 1% were from the liver donor. Despite complete recovery of neutrophils and becoming transfusion independent, the recipient developed Nocardia nova pneumonia and died on day 56 after alloHCT (day 194 post–liver transplant).
Case 3: GVHD involving gut and marrow
A 63-year-old man with atrial fibrillation, insulin-dependent diabetes, and alcoholic cirrhosis underwent orthotopic liver transplantation from a 51-year-old male donor. Prior to transplantation the patient developed renal failure due to hepatorenal syndrome requiring initiation of hemodialysis. The recipient was blood group A-positive, seropositive for EBV, and seronegative for CMV. The donor was blood group A-positive, seropositive for EBV, seronegative for CMV, and the cause of death was trauma. Induction immunosuppression included methylprednisolone and basiliximab. Mycophenolate mofetil (MMF) and tacrolimus (goal level 5-10 ng/mL) were given as maintenance immunosuppression. After transplantation, the recipient’s thrombocytopenia resolved but anemia persisted. On posttransplant day 21, MMF was stopped, but by day 35, there was no improvement in his anemia (hemoglobin, 8-9 g/dL). On day 45 the recipient developed new pancytopenia with an absolute neutrophil count of <1 × 109 cells per L. Work-up for infection was negative, including blood polymerase chain reaction testing for EBV, CMV, human herpesvirus 6, and parvovirus B19. The patient had no evidence of hemolysis or blood loss, and the absolute reticulocyte count was low at 0.7 × 109 cells per L. Tacrolimus levels were maintained at 8 to 10 ng/mL. On day 55 he developed watery diarrhea with a stool volume of >1.5 L/d. On day 58, he was admitted to the intensive care unit with fever and hypotension and was found to have Klebsiella pneumoniae bacteremia. He also underwent a marrow biopsy, which showed cellularity <10%, blasts <1%, and no evidence of malignancy or dysplasia. Marrow chimerism testing showed that 24% of CD3-positive T cells were of donor origin. To address medications as the cause of his marrow failure, trimethoprim-sulfamethoxazole was stopped and atovaquone started for Pneumocystis jirovecii prophylaxis. Owing to disagreement among the treating clinicians as to whether the marrow failure represented GVHD or ongoing infection, high-dose corticosteroids were not initiated. Ruxolitinib, 5 mg every 12 hours, was started. Despite this, his pancytopenia and diarrhea persisted and repeat marrow on day 72 showed continued hypocellularity (<10%) with 36% of CD3-positive T cells being of donor origin. On day 76, endoscopic biopsies of the duodenum and rectum showed frequent apoptotic epithelial cells with glandular destruction, implying severe GVHD. Methylprednisolone 2 mg/kg per day was started, tacrolimus was continued at 5 to 8 ng/mL, and he received rabbit ATG at 1 mg/kg with plan for a total dose of 5 mg/kg. Given his preexisting comorbidities and current critical illness, he was deemed not to be an alloHCT candidate. On day 82, the recipient had no improvement in his pancytopenia or diarrhea and expressed the wish to stop disease-focused treatments. He died on posttransplant day 84.
GVHD pathophysiology and risk factors
Given the rare occurrence of GVHD after SOT, much of the knowledge of its pathogenesis is based on experience in organ recipients and from animal models of GVHD after alloHCT.3 Solid organ allografts contain differing amounts of donor leukocytes, which are a mixed population including monocytes, NK cells, T lymphocytes, and other hematopoietic progenitors.22 Transplantation of these immunocompetent cells within the organ, along with the immunosuppression to prevent rejection, can create conditions for the development of tolerance and GVHD.23 Owing to the high level of HLA mismatching the recipient immune system eliminates the donor lymphocytes. During this time the donor lymphoid tissues within the transplanted organ are replaced with recipient lymphoid cells.24 More rarely donor lymphocytes can attack the recipient, causing GVHD.
Risk factors for GVHD have been reported for orthotopic liver and intestinal transplantation (Table 2). Common themes among the risk factors include immunodeficiency in the recipient, increased mass of transplanted donor lymphoid tissue, and inflammation in the recipient. Although transplanted organs such as the liver and intestine, which contain substantial amounts of donor lymphoid tissue, carry an increased risk of inducing GVHD in the recipient, it is unknown whether the type of transplanted organ or its composition of passenger lymphocyte subsets contributes to the development of GVHD or its outcome.2 We recently developed a machine-learning approach to predict which recipients of orthotopic liver transplantation are at high-risk for developing GVHD based on donor and recipient characteristics available at the time of transplantation.10 Although this algorithm requires further validation, early data suggest that preemptive monitoring of blood chimerism levels in high-risk recipients could be useful to make an earlier diagnosis of GVHD.33
GVHD clinical features and diagnosis
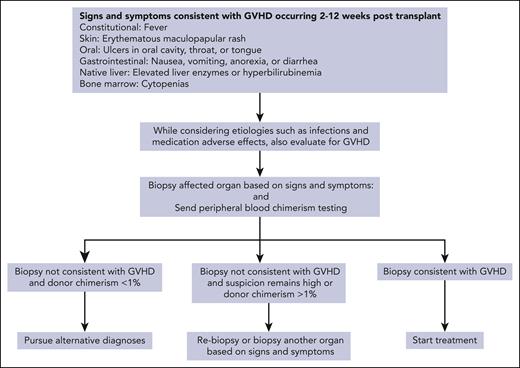
GVHD typically develops 2 to 12 weeks after SOT, although later presentations have been reported.4,10,11,33 Recipient tissues that can be affected by GVHD include the skin, oropharyngeal mucosa, gastrointestinal (GI) tract, liver, and bone marrow.22,33,34 Signs and symptoms are nonspecific and include fever, rash, oral sores, nausea, anorexia, watery or bloody diarrhea, and cytopenias. If the recipient retains their native liver, additional signs of GVHD can include elevated liver enzymes and hyperbilirubinemia. Rash is the most common manifestation, which usually begins as a central erythematous, maculopapular eruption, spreading to the extremities, and, if severe, becoming generalized erythroderma with the formation of bullae.35 Marrow involvement by GVHD and the resulting cytopenias drive the most common causes of death, that is, sepsis and hemorrhage.3,4,10,36,37
The diagnosis of GVHD after SOT is made based on clinical presentation and is confirmed by the presence of histopathologic features in an affected organ. Biopsy should be pursued when suspicion for GVHD is high based on clinical presentation, timing, and the likelihood of competing diagnoses. Morphologic features of GVHD in different organs are shown in Table 3. The presence of infiltrating donor lymphocytes within the affected tissue in the setting of appropriate morphologic features provides additional evidence for the diagnosis.6,40 This is particularly true for the bone marrow in which involvement with GVHD frequently has the morphologic appearance of hypoplasia or aplasia, which is nonspecific and has many causes.
GVHD after SOT has some similarities to transfusion-associated GVHD, including the presence of chimerism as well as cytopenias from marrow involvement with resulting high mortality (Table 1).21 Because of these similar clinical manifestations and the fact that recipients of SOT are frequently receive transfused blood products, cases are reported in which the cause of GVHD (transplanted organ vs nonirradiated blood product) only becomes clear after chimerism testing.41 After SOT, the presence of low-level donor chimerism in blood is a normal finding that can be measurable for months and is associated with the development of tolerance to the organ.23,42,43 Although the presence of macrochimerism, defined as donor T lymphocytes being >1% of total T lymphocytes in blood, has been associated with the development of GVHD after orthotopic liver transplantation, GVHD remains a clinical diagnosis: the presence of donor chimerism contributes along with clinical manifestations and histopathology.33
Unfortunately, the diagnosis of GVHD after SOT is often delayed.3 Because no sign or symptom is pathognomonic, it can be difficult to distinguish GVHD from far more common complications such as infections and the adverse effects of medications. Owing to its rarity and the variable timing of onset, a high level of suspicion is required to make the diagnosis of GVHD among competing diagnoses. When we encounter a clinical presentation, which could be consistent with GVHD, we simultaneously consider multiple etiologies so that we can arrive at a diagnosis quickly and use the algorithm in Figure 1.
How we approach GVHD treatment
Initial considerations
When we encounter a diagnosis of GVHD after SOT, we favor starting treatment, given the high associated mortality. Spontaneous resolution of GVHD without intervention was reported in 4 recipients after intestinal transplantation over a 13-year period.5 Unfortunately, the factors associated with spontaneous resolution are unknown and we do not wait for this possibility when confronted with a new diagnosis of GVHD.
Before starting treatment for GVHD, we consider the following questions:
What are the recipient’s underlying comorbidities?
In addition to GVHD, what other acute illnesses are present?
How is the transplanted organ functioning?
Have other diagnoses been considered and excluded? Do other members of the organ transplant team concur with the diagnosis of GVHD?
The last question is particularly important because many different physicians often care for SOT recipients, including transplant surgeons, infectious disease specialists, and organ transplant specialists. A carefully coordinated management approach with shared decision making should be the rule.
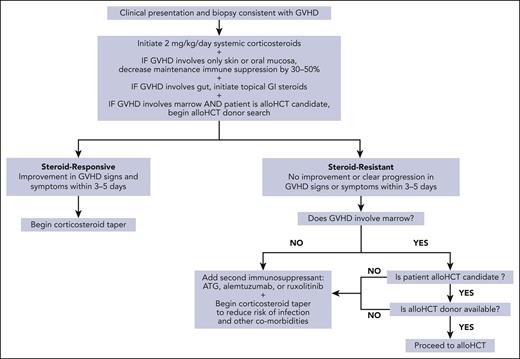
Once GVHD is diagnosed, we start systemic corticosteroids
Once a diagnosis of GVHD is confirmed, we initiate treatment with systemic corticosteroids. This approach is informed through our experiences shared with organ transplant colleagues, guidelines for treatment of acute GVHD after alloHCT, and reports of GVHD treatment after SOT (Figure 2).4,5,10,16,44 There are no formal studies to guide the starting dose of corticosteroids, and reports vary in their dosing with some as high as 15 mg/kg per day. We start with a corticosteroid dose equivalent of 2 mg/kg per day and continue this dose for at least 5 days while monitoring for a response. There are no data describing the use of topical steroids for limited skin GVHD or nonabsorbable GI steroids for upper GI GVHD, as is done after alloHCT. We do not use either of these approaches for limited GVHD after SOT owing to the potential need for decreasing maintenance immunosuppression (see further sections) and the fact that once GVHD is clinically detectable, significant levels of circulating donor lymphocytes are also usually present. At the time we start systemic corticosteroids we base additional treatment decisions on the organs involved, as described in further sections.
As there are no trials to guide infection prophylaxis in SOT recipients being treated for GVHD, our approach is informed by experience in patients with severe aplastic anemia treated with immunosuppression, and guidelines from the Infectious Diseases Society of America and the American Society of Transplantation.45-48 When starting systemic corticosteroids, we recommend that these recipients receive P jirovecii prophylaxis, antiviral prophylaxis against herpes simplex virus (HSV) and varicella-zoster virus (VZV), and mold-active antifungal prophylaxis. We also periodically monitor for viral and breakthrough fungal infections, and base these decisions on recipient risk factors and discussions with our organ transplant colleagues.
For GVHD involving only skin or oral mucosa, we decrease immunosuppression
If GVHD only involves the skin or oral mucosa, we decrease maintenance immunosuppression levels by 30% to 50% at the time we start systemic corticosteroids. There is limited available data to guide decision making on whether, and by how much, to adjust maintenance immunosuppression once corticosteroids are started. Decreasing maintenance immunosuppression is an attempt to improve the ability of the recipient’s immune system to eliminate donor immune cells and alter the pathophysiology of GVHD after SOT. This must be balanced against the risk of rejection. A practical example of decreasing immunosuppression would be to reduce the target blood tacrolimus level. The successful treatment of GVHD through decreasing tacrolimus levels after starting systemic corticosteroids have been reported in 4 recipients after liver transplantation and in 7 recipients after intestinal transplantation.5,49-51 Decreasing immunosuppression carries the risk of initially worsening symptoms of GVHD, which may limit its use.4,52,53 When we decrease maintenance immunosuppression levels, we aim to accomplish this within the initial 3 to 5 days of corticosteroid exposure to quickly determine GVHD responsiveness and whether additional immunosuppression is needed (refer to “Steroid-resistant GVHD”).
For GVHD involving the gut, we add nonabsorbable oral steroids
If GVHD involves the gut and local infections have been ruled out, at the time we start systemic corticosteroids we also add topically active nonabsorbable oral steroids, such as beclomethasone and budesonide, for their steroid-sparing potential. There are limited data on the use of nonabsorbable oral steroids for GVHD after SOT and our use of these agents draws from experience post-alloHCT in which the addition of beclomethasone to prednisone for patients with GI GVHD in a randomized, placebo-controlled trial allowed for a rapid taper of prednisone starting 10 days after initiation, significantly reducing the risk of GVHD-treatment failure, and reducing the risk of mortality.54
When GVHD involves bone marrow, we begin alloHCT donor search early
If GVHD involves the bone marrow we quickly determine whether the recipient is an alloHCT candidate and, if so, begin a donor search. GVHD of the marrow can lead to marrow failure resembling aplastic anemia with associated high mortality, and we treat SOT recipients with GVHD involving the marrow in a manner similar to aplastic anemia.55 alloHCT has been performed successfully in SOT recipients and it is notable that 12 of 14 SOT recipients who underwent alloHCT for aplastic anemia that developed after SOT were alive at 0.5 to 23 years after alloHCT.56 To evaluate whether the SOT recipient is a candidate for alloHCT, we consider their acute illnesses at the time of GVHD, preexisting comorbidities, and the opinions of our alloHCT and organ transplant colleagues. When searching for an alloHCT donor, we consider the HLA type of the organ recipient but not the HLA type of the organ donor. Based on the limited reports of alloHCT after SOT, the degree of HLA mismatch between the organ recipient, organ donor, and hematopoietic cell donor did not significantly affect outcomes via post-alloHCT GVHD or organ rejection.56
Steroid-resistant GVHD
We define GVHD after SOT as steroid-resistant if there is no clinical improvement or clear progression within 3 to 5 days of starting corticosteroids at 2 mg/kg per day. The rate of steroid responsiveness for GVHD may vary by transplanted organ and is reported to be 40% to 50% for GVHD after intestinal transplantation and as high as 83% for GVHD after orthotopic liver transplantation.16 The data underlying these statistics are limited, however, and the high mortality associated with GVHD after SOT suggests that many cases of GVHD progress despite treatment with corticosteroids and additional immunosuppression.
When we encounter steroid-resistant GVHD after SOT we pursue additional treatment in 1 of 2 ways: (1) add another immune suppressant or (2) pursue early alloHCT if the recipient has GVHD involving the marrow, is a candidate for alloHCT, and has an alloHCT donor identified. For recipients with steroid-resistant GVHD who do not meet criteria for pursuit of early alloHCT, we add another immune suppressant that targets T lymphocytes and NK cells, such as ATG or alemtuzumab, or interferes with inflammatory cytokines, such as ruxolitinib. Determining which immune suppressant to add relies on a combination of pathophysiology and experience treating GVHD in post-alloHCT and post-SOT settings. The pathophysiology of GVHD involves 2 primary pathways: (1) activation of donor T lymphocytes in response to host antigen–presenting cells and (2) generation of inflammatory cytokines, which stimulate donor effector CD8-positive T cells and NK cells.57 Immune suppressants that have been added to corticosteroids to treat GVHD after SOT are shown in Table 4, and many of these agents target 1 or both of these pathways. Murali et al4 identified immunosuppressive regimens that successfully treated GVHD after orthotopic liver transplantation. In addition to corticosteroids and calcineurin inhibitors, they found that successful immunosuppressive regimens included ATG, muromonab-CD3, interleukin-2 receptor antagonists, alefacept, tumor necrosis factor alpha inhibitors, and rituximab. More recently, Jacobs et al87 reported the efficacy of adding ruxolitinib to corticosteroids in 3 cases of GVHD after SOT. For steroid-resistant GVHD in the alloHCT setting, ATG and alemtuzumab were shown to be beneficial and the Janus kinase inhibitor ruxolitinib demonstrated an overall response rate of 73% and a complete response rate of 53%.97-99
The major risk with intensifying immunosuppression is an increased susceptibility to infections, particularly after T lymphocyte–directed agents and broader immunosuppressants such as high-dose cyclophosphamide. As described in previous sections, our approach to infection prophylaxis is extrapolated from patients with aplastic anemia treated with immunosuppression and subspecialty guidelines.45-48 We recommend that these recipients receive P jirovecii prophylaxis, and antiviral prophylaxis against HSV and VZV, and mold-active antifungal prophylaxis. We also periodically monitor for viral and breakthrough fungal infections and base these decisions on recipient risk factors and discussions with our organ transplant colleagues.
For recipients with steroid-resistant GVHD involving the marrow, there is high associated mortality. Treatment of GVHD-associated marrow failure after SOT includes supportive care with transfusions and hematopoietic growth factor support, however, these therapies are temporary. For these reasons, as soon as it is known that GVHD involves the marrow we begin searching for an alloHCT donor based on the HLA typing of the SOT recipient. Doney et al56 reported of 8 cases of alloHCT after SOT performed at the Fred Hutchinson Cancer Center and reviewed 27 cases from the literature and found no evidence that the prior SOT increased the risk of hematopoietic cell graft failure or that the new hematopoietic cell graft increased the risk of rejection of the transplanted organ. Although most reported cases of alloHCT after SOT involved the use of HLA-matched sibling donors, HLA-mismatched related and unrelated donors, as well as cord blood units, have also been utilized. Because both GVHD-associated marrow failure and immune-mediated aplastic anemia are diseases primarily driven by non-neoplastic, functionally activated cytotoxic T cells, we use the alloHCT experience for aplastic anemia to inform conditioning preferences.100 As such, we prefer nonmyeloablative or reduced-intensity conditioning regimens, which include a T-cell directed agent such as ATG, because these have demonstrated high cure rates for aplastic anemia while limiting alloHCT-related morbidity and mortality.101 GVHD prophylaxis regimens for alloHCT after SOT will typically contain a calcineurin inhibitor to accommodate the long-term immunosuppression needs of the solid organ graft. Infection prophylaxis and surveillance in the post-alloHCT setting follows our institutional norms and typically includes antiviral prophylaxis for HSV and VZV, P jirovecii prophylaxis, antifungal prophylaxis, and routine blood CMV polymerase chain reaction monitoring.
Comments on the 3 case examples of GVHD
Case 1: GVHD involving only skin
For the first case, the diagnosis of GVHD was made via skin biopsy; significant donor chimerism was also noted in the blood. Treatment was promptly initiated with high-dose corticosteroids. Because a rash was the only manifestation of GVHD, tacrolimus levels were reduced to allow the patient’s immune system to eliminate the donor immune cells. When the rash did not improve after 72 hours, GVHD was felt to be steroid resistant, and alemtuzumab was added with eventual good response. Alemtuzumab, a humanized anti-CD52 monoclonal antibody, has been shown to be efficacious in steroid-refractory acute GVHD after alloHCT.102 The diagnosis of GVHD was made and treatment was started early before development of cytopenias to suggest marrow involvement.
Case 2: GVHD involving skin and marrow
For the second case, the diagnosis of GVHD was made by skin biopsy. Blood chimerism studies showed high proportions of donor T cells and NK cells. The initial marrow showed no morphologic evidence of GVHD but had high proportions of donor chimerism. Treatment with high-dose corticosteroids was promptly initiated. When the rash progressed after 48 hours, GVHD was deemed steroid resistant, and a dose of cyclophosphamide was given. The rash resolved but marrow involvement by GVHD persisted, although the extent of this was difficult to determine given the marrow-suppressive effects of cyclophosphamide. Once it became clear that the recipient’s marrow failure reflected GVHD, an alloHCT was pursued. Although there was successful engraftment and the possibility of an excellent outcome, the recipient died of an opportunistic infection from prolonged immunosuppression. In hindsight alloHCT should have been pursued earlier, once cytopenias developed and significant donor chimerism was noted in the blood and marrow.
Case 3: GVHD involving gut and marrow
The third case illustrates the significant challenges of diagnosing GVHD, as in this case the diagnosis was delayed for 2 reasons. First, there was an absence of a rash, which is the most common manifestation of GVHD. Second, there was disagreement among the treating clinicians as to whether the pancytopenia and diarrhea were because of GVHD or to other more common complications, such as infections and medications. Cytopenias can be caused by multiple etiologies including viral infections (EBV, CMV, human herpesvirus 6, parvovirus B19) and medication toxicities (trimethoprim-sulfamethoxazole, MMF, antivirals). This disagreement persisted despite the early marrow biopsy showing marrow failure with elevated donor chimerism. Multiple treatments were attempted without effect, including initiation of ruxolitinib, which is not first-line treatment for GVHD. Once the diagnosis of GVHD was confirmed via gut biopsies, treatment with high-dose corticosteroids and ATG were initiated, but the recipient opted not to continue GVHD-directed therapy. The recipient likely needed an alloHCT owing to marrow involvement but was deemed not to be a candidate because of comorbidities and critical illness. Comorbidities and concurrent acute illnesses in SOT recipients who develop GVHD-associated marrow failure can make alloHCT a pursuit requiring careful consideration.
Conclusion
Acute GVHD is a rare but often fatal complication after SOT. The signs and symptoms include fever, rash, oral sores, nausea, anorexia, watery or bloody diarrhea, elevated liver enzymes, hyperbilirubinemia, and cytopenias. Cytopenias from marrow involvement drive the high mortality. Signs or symptoms of GVHD should prompt consideration of the diagnosis and biopsy of an affected organ. Chimerism studies of blood or marrow can inform the likelihood of a GVHD diagnosis. A team-oriented approach to diagnosis and management is a must. Once the diagnosis of GVHD is confirmed, treatment should be initiated with high-dose systemic corticosteroids. For steroid-resistant GVHD not involving the marrow, salvage therapy includes agents targeting T cells or Janus kinases. For steroid-resistant GVHD involving the marrow, alloHCT should be pursued.
Acknowledgments
The authors thank Joachim Deeg, Jorge Reyes, and Scott Biggins for reviewing the manuscript and providing helpful suggestions.
This work was supported by funding from T32 HL007093 from the National Institutes of Health, National Heart, Lung, and Blood Institute.
Authorship
Contribution: J.P.C. and J.L.A. contributed equally to the writing of this manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Jason P. Cooper, University of Washington, 1959 NE Pacific St, Box 357710, Seattle, WA 98195-7710; e-mail: jasonc8@uw.edu.
References
Author notes
∗J.P.C. and J.L.A. contributed equally to this work.