Key Points
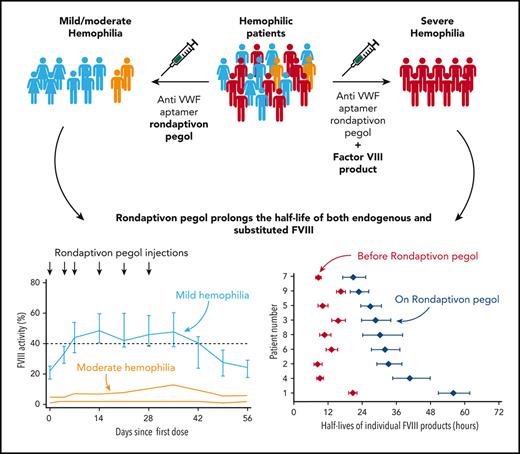
Rondaptivon pegol is a first-in-class prohemostatic molecule that prolongs the half-life of both endogenous FVIII and substituted FVIII.
Rondaptivon pegol could be used to enable once-weekly substitution therapy in severe hemophilia A or as prophylaxis in nonsevere hemophilia A.
Abstract
Factor VIII (FVIII) circulates in a noncovalent complex with von Willebrand Factor (VWF), the latter determining FVIII half-life. The VWF-binding aptamer rondaptivon pegol (BT200) increases plasma levels of VWF/FVIII in healthy volunteers. This trial assessed its safety, pharmacokinetics, and pharmacodynamics in hemophilia A. Nineteen adult patients (ages 20-62 years, 4 women) with hemophilia A (8 mild, 2 moderate, and 9 severe) received subcutaneous injections of rondaptivon pegol. After an initial fixed dose of 3 mg on days 0 and 4, patients received weekly doses of 2 to 9 mg until day 28. Severe hemophilia A patients underwent sparse-sampling population pharmacokinetics individual profiling after the final dose of rondaptivon pegol. Adverse events, pharmacokinetics, and pharmacodynamics were assessed. FVIII activity and VWF levels were measured. All patients tolerated rondaptivon pegol well. The geometric mean half-life of rondaptivon pegol was 5.4 days and rondaptivon pegol significantly increased VWF levels. In severe hemophilia A, 6 doses of rondaptivon pegol increased the half-lives of 5 different FVIII products from a median of 10.4 hours to 31.1 hours (range, 20.8-56.0 hours). Median FVIII increased from 22% to 48% in mild hemophilia A and from 3% to 7.5% in moderate hemophilia A. Rondaptivon pegol is a first-in-class prohemostatic molecule that extended the half-life of substituted FVIII approximately 3-fold and increased endogenous FVIII levels approximately 2-fold in hemophilia patients. This trial was registered at www.clinicaltrials.gov as #NCT04677803.
Introduction
Hemophilia A is a rare X-chromosomal inherited bleeding disorder caused by a deficiency or functional defect of coagulation factor VIII (FVIII). Usually, classification of severity is based on residual endogenous FVIII activity levels, categorizing hemophilia into severe (<1%) and nonsevere (ie, moderate [1%-5%] and mild [>5%]) hemophilia.1 Recurrent joint bleeds are the hallmark of severe hemophilia.2 Hemarthrosis leads to damage and inflammation in affected joints, which eventually progress to irreversible, disabling joint arthropathy. Moreover, hemophilia can also cause intracranial hemorrhage3 or premature mortality when critical organs are involved.4 Although bleeding frequency decreases with increasing endogenous FVIII levels, patients with moderate and even mild hemophilia5 also frequently have arthropathy.6 Regular prophylactic substitution of FVIII has become a mainstay to prevent bleeding in severe hemophilia,7 but it is costly. Moreover, FVIII has a limited half-life, which is largely determined by the clearance of its chaperone, von Willebrand factor (VWF).8 Over the past decade, new therapies have been developed for severe hemophilia, most notably non–FVIII replacement therapies and extended half-life products.9-12 However, available extended half-life FVIII concentrates extend the half-life by only approximately 50%13 because they cannot overcome the physiologic clearance of VWF, which imposes a ceiling on FVIII replacement therapy. Less progress has been made in the treatment of nonsevere hemophilia, which still largely relies on the VWF/FVIII releasing agent desmopressin and on-demand FVIII replacement therapy, the latter mainly used for treatment of bleeding rather than prophylaxis. Even in nonsevere hemophilia, annual bleeding rates range from 0.5 to 4.5.14,15 Of note, FVIII replacement may lead to the development of FVIII inhibitors in severe and nonsevere hemophilia.16,17
Recently, rondaptivon pegol (previously rondoraptivon pegol, BT200), a VWF-binding aptamer, was found to increase VWF/FVIII plasma levels in healthy volunteers,18 an effect that could be beneficial for patients with hereditary bleeding disorders. Rondaptivon pegol is a fully 2′-O-methylated RNA aptamer conjugated to a 40-kD polyethylene glycol (PEG); the aptamer portion binds to the A1 region of VWF.19 Pegylation of the A1 domain reduces clearance of VWF by the macrophage low-density lipoprotein receptor–related protein (LRP-1),20 and the high-affinity binding of rondaptivon pegol to the A1 domain can be regarded as reversible pegylation of the A1 domain. Thus, it is possible that rondaptivon pegol dose-dependently decreases the clearance of VWF and thereby elevates VWF/FVIII levels severalfold in healthy volunteers. However this putative mechanism of action still has to be proven.
We hypothesized that rondaptivon pegol would increase FVIII and VWF antigen levels in hemophilia A patients. The present trial investigated the pharmacokinetics, effects, and safety of rondaptivon pegol in patients with hemophilia A of any severity.
Methods
Population and study design
This phase 2 trial (EUDRA-CT 2020-003807-32 and NCT04677803) was approved by the national competent authority and the Ethics Committee of the Medical University of Vienna and was conducted at the Department of Clinical Pharmacology from January to May 2021. All authors had access to primary clinical trial data and analyzed the data. Patients with hereditary bleeding disorders (FVIII deficiency without inhibitors, or VWF deficiency) were eligible for this basket trial. The present report focuses on the hemophilia A cohort. Every study participant provided written informed consent and had to be at least 18 years old (supplemental Figure 1, available on the Blood website). Key exclusion criteria were clinically significant medical history or ongoing chronic illness that might jeopardize the safety of the patient or compromise the quality of the data, previous spontaneous thromboembolic events, significant drug allergies or anaphylactic reactions, substance abuse, mental illness, or childbearing potential.
Study treatment
Rondaptivon pegol was supplied at a concentration of 15 mg/mL in sterile saline solution for injection (all doses refer to the core nonpegylated aptamer). The structure and function of the VWF A1 domain–binding aptamer were described recently.19 The core aptamer has a molecular mass of 10 kDa, which is pegylated (40 kDa) and binds at multiple binding sites (amino acids 1367-1402, old nomenclature 604-639) of the botrocetin-binding region, which is also in close proximity to lysine 1408, a critical binding site for LRP-1.21 It is possible that the large hydrodynamic radius of the PEG molecule of rondaptivon pegol interferes with LRP-1 binding and hence inhibition of VWF/FVIII clearance; however, this putative mechanism of action remains to be proven. Patients were to receive subcutaneous injections of 3 mg rondaptivon pegol on days 0, 4, and 7 followed by weekly injections of 3 to 9 mg until day 28. All injections (maximum volume of 0.6 mL) were delivered as a bolus over a few seconds into the subcutaneous fat tissue of the upper thigh or abdomen. Inhibition of VWF was monitored for safety with the use of multiple electrode aggregometry, because 5 patients already had prolonged closure times as measured with a platelet function analyzer at baseline. The dose was decreased from 3 mg to 2 mg in 1 patient with mild hemophilia, who had impaired platelet function at baseline. For the 1 severe hemophilia patient who practiced on-demand therapy, participation was limited to the initial phase of dosing (3 doses × 3 mg). One patient with mild hemophilia received an increased dose of 6 mg of rondaptivon pegol on day 7.
The follow-up period consisted of weekly blood sampling until day 56. An example of trough FVIII values and FVIII pharmacokinetics relative to the timing of prophylactic injections for one patient is shown in supplemental Figure 2. Patients with severe hemophilia were invited to participate in a sparse-sampling population pharmacokinetics substudy to determine the half-life of their usual FVIII product at the end of rondaptivon pegol treatment. This substudy followed the principal recommendations for population pharmacokinetics assessment of FVIII.22-24 Although only 6 severe hemophilia patients received regular prophylactic treatment before the study (Table 1), the investigators invited 2 additional patients with on-demand treatment to switch to regular prophylaxis during the study (Table 2). This was done because rondaptivon pegol may inhibit VWF-dependent platelet function at higher doses. We wanted to avoid inhibition of VWF-dependent platelet function in the absence of regular FVIII substitution. For the same reason, the only patient who remained with on-demand therapy received only 3 doses of 3 mg rondaptivon pegol. Based on the results of the phase 1 trial, we estimated that this abbreviated and reduced dose regimen would be safe even in the absence of any FVIII activity.
Demographic and clinical characteristics at baseline
| Characteristic . | Severe (n = 9) . | Nonsevere (n = 10) . | Overall (n = 19) . |
|---|---|---|---|
| Age, y | 44 (40-44) | 40 (27-47) | 42 (38-46) |
| Sex | |||
| Male | 9 (100%) | 6 (60.0%) | 15 (78.9%) |
| Female | 0 (0%) | 4 (40.0%) | 4 (21.1%) |
| Race, Caucasian | 9 (100%) | 10 (100%) | 19 (100%) |
| Weight, kg | 80 (74-88) | 76 (66-95) | 80 (70-94) |
| Height, cm | 180 (175-180) | 172 (167-186) | 176 (172-182) |
| Body mass index, kg/m2 | 25.5 (22.8-29.4) | 24.9 (23.2-28.4) | 25.5 (23.0-29.3) |
| Comorbidities | |||
| Hypertension | 1 (11.1%) | 2 (20.0%) | 3 (15.8%) |
| Cardiomyopathy | 0 (0%) | 1 (10.0%) | 1 (5.3%) |
| Aortic valve insufficiency | 0 (0%) | 1 (10.0%) | 1 (5.3%) |
| Wolff-Parkinson-White syndrome | 0 (0%) | 1 (10.0%) | 1 (5.3%) |
| Raynaud syndrome | 0 (0%) | 1 (10.0%) | 1 (5.3%) |
| Diabetes mellitus | 1 (11.1%) | 1 (10.0%) | 2 (10.5%) |
| Mixed connective tissue disease | 0 (0%) | 1 (10.0%) | 1 (5.3%) |
| Thyroiditis | 0 (0%) | 2 (20.0%) | 2 (10.5%) |
| Asthma | 0 (0%) | 1 (10.0%) | 1 (5.3%) |
| Depression | 2 (22.2%) | 1 (10.0%) | 3 (15.8%) |
| History of hepatitis B | 4 (44.4%) | 0 (0%) | 4 (21.1%) |
| History of hepatitis C | 6 (66.7%) | 0 (0%) | 6 (31.6%) |
| Human immunodeficiency virus | 0 (0%) | 1 (10.0%) | 1 (5.3%) |
| Blood group | |||
| A | 6 (66.7%) | 2 (20.0%) | 8 (42.1%) |
| B | 2 (22.2%) | 0 (0%) | 2 (10.5%) |
| O | 1 (11.1%) | 8 (80.0%) | 9 (47.4%) |
| Factor VIII, at baseline, % | 1 (1-1) | 19 (10-25) | 2 (1-19) |
| Factor VIII, lowest in history, % | <1 (<1- <1) | 12 (5-21) | <1 (<1-11.5) |
| Hemophilic arthropathy | |||
| Yes | 8 (88.9%) | 1 (10.0%) | 9 (47.4%) |
| No | 1 (11.1%) | 9 (90.0%) | 10 (52.6%) |
| Previous regimen | |||
| On-demand | 3 (33.3%) | 10 (100%) | 13 (68.4%) |
| Prophylaxis | 6 (66.7%) | 0 (0%) | 6 (31.6%) |
| Characteristic . | Severe (n = 9) . | Nonsevere (n = 10) . | Overall (n = 19) . |
|---|---|---|---|
| Age, y | 44 (40-44) | 40 (27-47) | 42 (38-46) |
| Sex | |||
| Male | 9 (100%) | 6 (60.0%) | 15 (78.9%) |
| Female | 0 (0%) | 4 (40.0%) | 4 (21.1%) |
| Race, Caucasian | 9 (100%) | 10 (100%) | 19 (100%) |
| Weight, kg | 80 (74-88) | 76 (66-95) | 80 (70-94) |
| Height, cm | 180 (175-180) | 172 (167-186) | 176 (172-182) |
| Body mass index, kg/m2 | 25.5 (22.8-29.4) | 24.9 (23.2-28.4) | 25.5 (23.0-29.3) |
| Comorbidities | |||
| Hypertension | 1 (11.1%) | 2 (20.0%) | 3 (15.8%) |
| Cardiomyopathy | 0 (0%) | 1 (10.0%) | 1 (5.3%) |
| Aortic valve insufficiency | 0 (0%) | 1 (10.0%) | 1 (5.3%) |
| Wolff-Parkinson-White syndrome | 0 (0%) | 1 (10.0%) | 1 (5.3%) |
| Raynaud syndrome | 0 (0%) | 1 (10.0%) | 1 (5.3%) |
| Diabetes mellitus | 1 (11.1%) | 1 (10.0%) | 2 (10.5%) |
| Mixed connective tissue disease | 0 (0%) | 1 (10.0%) | 1 (5.3%) |
| Thyroiditis | 0 (0%) | 2 (20.0%) | 2 (10.5%) |
| Asthma | 0 (0%) | 1 (10.0%) | 1 (5.3%) |
| Depression | 2 (22.2%) | 1 (10.0%) | 3 (15.8%) |
| History of hepatitis B | 4 (44.4%) | 0 (0%) | 4 (21.1%) |
| History of hepatitis C | 6 (66.7%) | 0 (0%) | 6 (31.6%) |
| Human immunodeficiency virus | 0 (0%) | 1 (10.0%) | 1 (5.3%) |
| Blood group | |||
| A | 6 (66.7%) | 2 (20.0%) | 8 (42.1%) |
| B | 2 (22.2%) | 0 (0%) | 2 (10.5%) |
| O | 1 (11.1%) | 8 (80.0%) | 9 (47.4%) |
| Factor VIII, at baseline, % | 1 (1-1) | 19 (10-25) | 2 (1-19) |
| Factor VIII, lowest in history, % | <1 (<1- <1) | 12 (5-21) | <1 (<1-11.5) |
| Hemophilic arthropathy | |||
| Yes | 8 (88.9%) | 1 (10.0%) | 9 (47.4%) |
| No | 1 (11.1%) | 9 (90.0%) | 10 (52.6%) |
| Previous regimen | |||
| On-demand | 3 (33.3%) | 10 (100%) | 13 (68.4%) |
| Prophylaxis | 6 (66.7%) | 0 (0%) | 6 (31.6%) |
Data presented as median (IQR) or n (%).
Population pharmacokinetics terminal half-life estimates
| Patient ID . | Before rondaptivon pegol . | On rondaptivon pegol . | ||||||
|---|---|---|---|---|---|---|---|---|
| INN . | IU . | IU/kg . | Terminal half-life, h (95% CrI) . | IU . | IU/kg . | Terminal half-life, h (95% CrI) . | Fold increase . | |
| 1 | Efmoroctocog alfa | 4000 | 54.1 | 20.6 (19.2-22.1) | 4000 | 54.1 | 56.0 (50.8-61.8) | 2.72 |
| 2 | Lonoctocog alfa | 4000 | 34.8 | 8.3 (7.0-9.9) | 4000 | 36.4 | 33.2 (29.1-38.0) | 3.99 |
| 3 | Lonoctocog alfa | 3000 | 31.9 | 15.5 (13.3-18.2) | 3000 | 31.2 | 28.5 (23.8-34.1) | 1.83 |
| 4 | Moroctocog alfa | 2000 | 25.0 | 9.1 (8.0-10.3) | 2000 | 23.0 | 40.7 (34.6-47.9) | 4.48 |
| 5 | Moroctocog alfa | 1500 | 21.4 | 10.0 (8.4-12.0) | 2000 | 25.0 | 26.8 (23.4-30.7) | 2.67 |
| 6 | Moroctocog alfa | 1000 | 17.2 | 13.1 (11.2-15.3) | 2000 | 33.9 | 31.9 (27.3-37.2) | 2.43 |
| 7 | Octocog alfa | 2000 | 25.6 | 8.6 (7.7-9.6) | 2000 | 24.8 | 20.8 (17.2-25.1) | 2.42 |
| 8 | Octocog alfa | 3000 | 42.3 | 10.7 (8.9-12.9) | 3000 | 42.3 | 30.3 (24.0-38.2) | 2.82 |
| 9 | Octocog alfa | 3900 | 50.2 | 16.4 (14.9-18.1) | 2000 | 24.4 | 22.8 (19.8-26.3) | 1.39 |
| Median fold increase: | 2.67 (IQR, 2.42-2.82) | |||||||
| Patient ID . | Before rondaptivon pegol . | On rondaptivon pegol . | ||||||
|---|---|---|---|---|---|---|---|---|
| INN . | IU . | IU/kg . | Terminal half-life, h (95% CrI) . | IU . | IU/kg . | Terminal half-life, h (95% CrI) . | Fold increase . | |
| 1 | Efmoroctocog alfa | 4000 | 54.1 | 20.6 (19.2-22.1) | 4000 | 54.1 | 56.0 (50.8-61.8) | 2.72 |
| 2 | Lonoctocog alfa | 4000 | 34.8 | 8.3 (7.0-9.9) | 4000 | 36.4 | 33.2 (29.1-38.0) | 3.99 |
| 3 | Lonoctocog alfa | 3000 | 31.9 | 15.5 (13.3-18.2) | 3000 | 31.2 | 28.5 (23.8-34.1) | 1.83 |
| 4 | Moroctocog alfa | 2000 | 25.0 | 9.1 (8.0-10.3) | 2000 | 23.0 | 40.7 (34.6-47.9) | 4.48 |
| 5 | Moroctocog alfa | 1500 | 21.4 | 10.0 (8.4-12.0) | 2000 | 25.0 | 26.8 (23.4-30.7) | 2.67 |
| 6 | Moroctocog alfa | 1000 | 17.2 | 13.1 (11.2-15.3) | 2000 | 33.9 | 31.9 (27.3-37.2) | 2.43 |
| 7 | Octocog alfa | 2000 | 25.6 | 8.6 (7.7-9.6) | 2000 | 24.8 | 20.8 (17.2-25.1) | 2.42 |
| 8 | Octocog alfa | 3000 | 42.3 | 10.7 (8.9-12.9) | 3000 | 42.3 | 30.3 (24.0-38.2) | 2.82 |
| 9 | Octocog alfa | 3900 | 50.2 | 16.4 (14.9-18.1) | 2000 | 24.4 | 22.8 (19.8-26.3) | 1.39 |
| Median fold increase: | 2.67 (IQR, 2.42-2.82) | |||||||
Eight patients with severe hemophilia received 6 doses of rondaptivon pegol plus their usual FVIII replacement therapy. Patient 9 was treated on-demand and received only 3 doses of 3 mg rondaptivon pegol.
CrI, credible interval; INN, international nonproprietary name.
Objectives and end points
The primary objective was to assess the safety of subcutaneous rondaptivon pegol injections and effects on FVIII levels (the primary end point). As a secondary objective, we investigated the pharmacokinetics and pharmacodynamics of rondaptivon pegol and half-lives of substituted FVIII products in patients with severe hemophilia. Secondary end points included rondaptivon pegol concentrations and multiple interdependent assays of VWF and FVIII.
Analytical assays
Plasma rondaptivon pegol concentrations were measured under Good Laboratory Practice at QPS (Newark, DE) by high-performance liquid chromatography after hybridization of rondaptivon pegol to a fluorescent-labeled complementary oligonucleotide.18 The method was qualified and fully validated according to regulatory standards with a lower limit of quantification of 1 ng/mL. Analytical assays for the other biomarkers are commercially available. FVIIIc activity was determined by means of a 1-stage clotting assay using soy phosphatides and rabbit brain phosphatides in ellagic acid as an activator (Actin FS reagent; Siemens Dade, Marburg, Germany) to avoid putative interference of PEG with silica-based clotting assays. VWF antigen (Ag; STA Liatest VWF:Ag; Diagnostica Stago, Asnières-sur-Seine, France), ristocetin cofactor (RCo), and glycoprotein IbM (GpIbM) activity (Siemens Healthcare Diagnostics, Erlangen, Germany), VWF propeptide (Haemochrom Diagnostics, Essen, Germany), VWF collagen-binding activity (Technoclone, Vienna, Austria), ristocetin-induced platelet aggregation with multiple electrode aggregometry (Multiplate; Roche, Vienna, Austria), and the collagen adenosine diphosphate–induced closure time (CADP-CT) were measured by shear-induced platelet function with a platelet function analyzer (PFA200; Siemens Healthcare Diagnostics) that is sensitive to rondaptivon pegol.18 Immunogenicity was assessed by measuring antidrug antibodies with the use of an enzyme-linked immunosorbent assay–based assay.
Population pharmacokinetics of different FVIII products
Blood samples were drawn weekly and additionally on day 4 ± 1 after the first dose. Patients with severe hemophilia could participate in an optional population pharmacokinetics assessment to study the effect of rondaptivon pegol on the half-lives of their respective FVIII products. The population pharmacokinetics sparse sampling approach was deemed to be preferable to pharmacokinetics tight sampling because it facilitated participation during the COVID-19 period. Intervals between blood samples followed applicable recommendations22,24 but were individually adapted according to the emerging estimates of half-lives from increasing trough FVIII concentrations. A minimum of 3 blood samples were obtained during the terminal elimination phase. In individual cases, a fourth sample was taken; in 1 patient, pharmacokinetics estimates were derived from 2 subsequent FVIII infusions. The half-life during rondaptivon pegol therapy was compared with either half-life determinations performed before rondaptivon pegol dosing or the patients’ historical half-life estimates under the same product. The FVIII pharmacokinetics data were plotted individually and entered into the Web Accessible Population Pharmacokinetics Service (WAPPS) for final estimation of half-lives.25,26
Study oversight
The clinical trial was conducted in accordance with the principles set forth in the Declaration of Helsinki and local drug laws. This investigator-initiated trial was sponsored by the Medical University of Vienna and received financial support from Band Therapeutics. All authors had access to the primary clinical trial data and approved the decision to submit the manuscript for publication.
Statistical analysis
The sample size estimation was based on a 100% increase in FVIII levels in healthy volunteers after 6 mg rondaptivon pegol18 and day-to-day variability of 22% in FVIII levels, indicating that 8 patients would be required to achieve 90% power (alpha error, 0.01). Pharmacokinetics parameters of rondaptivon pegol were calculated with the use of Phoenix WinNonlin version 8.3. All statistical analyses were performed with the use of SAS (version 9.2; SAS Institute, Cary, NC). Data were summarized by visit. Descriptive statistics (number of observations, minimum, median, maximum, 25th and 75th percentiles) were provided for continuous variables. Frequency counts and percentages were presented for categoric variables. Missing values were not imputed. Correlations were calculated by means of the Spearman rank correlation test (rS).
The primary outcome for the proof of concept is FVIII activity. The log-transformed value of FVIII activity was used as the primary end point in a mixed model for repeated measurements (MMRM; 0.5 was imputed for <1). We used visit (day 0, day 4, … day 56) and hemophilia A group (nonsevere [mild or moderate] vs severe) as fixed factors (supplemental Table 1A-C). The same analysis strategy was used for secondary end points (supplemental Table 2A-I). Patient was included as random effect with the use of an unstructured covariance matrix. The main test for the proof of concept was the difference from baseline to day 35 (which was 1 week after the last injection) based on the contrast test (2-sided alpha, 5%) in the MMRM as defined above. Least square mean differences are provided. In addition, other pairwise comparison of the visit days to baseline were performed for exploratory purposes (results provided in the supplementary Tables labeled with “differences of least square means”).
Furthermore, we performed subgroup analyses separately for nonsevere (mild or moderate) and severe hemophilia A patients. In the MMRM using severe hemophilia A patients only, we also adjusted for the time interval between the last substitution and the respective visit (covariate last substitution; n = 8).
For all secondary end points, the same MMRM as described for the primary end point was performed based on all hemophilia A patients.
Results
All 19 patients received all planned doses, except 1 patient with nonsevere hemophilia who missed his day 14 dose for personal reasons. He is included in the intention-to-treat analysis, but pharmacokinetics results are presented separately. All patients with severe hemophilia were Caucasian men, with a median age of 44 years (range, 24-62 years) and median weight of 80 kg (range, 60-115 kg) (Table 1). Eight patients were on prophylaxis and 1 patient was treated on-demand (Table 2). All patients with nonsevere hemophilia were Caucasian and 4 (40%) were female; median age was 40 years (range, 19-57 years) and median weight was 76 kg (range, 56-143 kg) (Table 1). The female patients were 53, 46, 47, and 57 years of age and were postmenopausal, surgically sterilized, or hysterectomized.
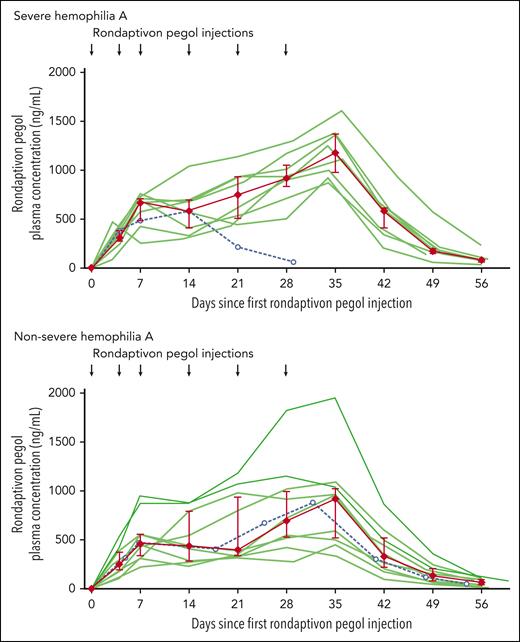
Median rondaptivon pegol doses were 7 mg (range, 2-9 mg, corresponding to 0.03-0.16 mg/kg) on day 28. Plasma levels of rondaptivon pegol increased in a time- and dose-dependent manner, resulting in median rondaptivon pegol trough plasma levels of 1.00 μg/mL (range, 0.34-1.95 μg/mL; n = 17) on day 35 (Figure 1). The geometric mean half-life of rondaptivon pegol was 5.4 days (range, 4.2-9.4 days). Furthermore, there was a moderate cumulative increase of rondaptivon pegol concentrations in plasma up to 35 days. This was anticipated owing to the combined effect of a 2- to 3-fold increase in doses over the study time and the fact that the administration interval was similar to the elimination half-life (which would induce an approximately 2-fold increase in drug concentrations over the study time period). Antibodies against rondaptivon pegol were not detected in any of the analyzed samples. Therefore, similarly to other aptamers,18 rondaptivon pegol does not appear to be immunogenic and an influence of antidrug antibodies on the pharmacokinetics of rondaptivon pegol can be ruled out.
Pharmacokinetics of rondaptivon pegol in hemophilia. One severe hemophilia A patient received only 3 doses, and 1 nonsevere hemophilia A patient missed a dose on day 14 (dashed lines with open circles; data included in the median and interquartile range only until day 14). Individual patient data are depicted with semitransparent green lines and summarized by median (red diamonds) with interquartile range (error bars).
Pharmacokinetics of rondaptivon pegol in hemophilia. One severe hemophilia A patient received only 3 doses, and 1 nonsevere hemophilia A patient missed a dose on day 14 (dashed lines with open circles; data included in the median and interquartile range only until day 14). Individual patient data are depicted with semitransparent green lines and summarized by median (red diamonds) with interquartile range (error bars).
Primary end point
The proof of concept was established because the contrast test for FVIII levels (day 35 minus baseline) was significant (P < .0001). Similar results were obtained when performing separate analyses for the subgroups (nonsevere and severe hemophilia A). In the MMRM using severe hemophilia A patients only, we also adjusted for the time interval between the last FVIII substitution and the respective visit. Again, a significant result was found for the log FVIII value (day 35 minus baseline: P < .0001).
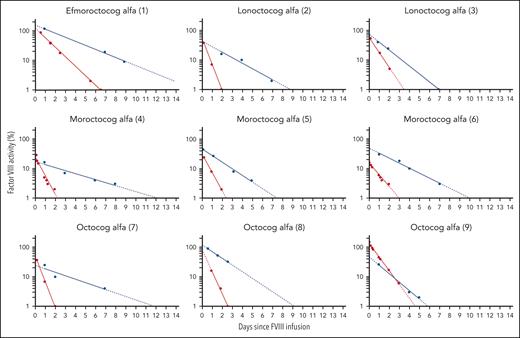
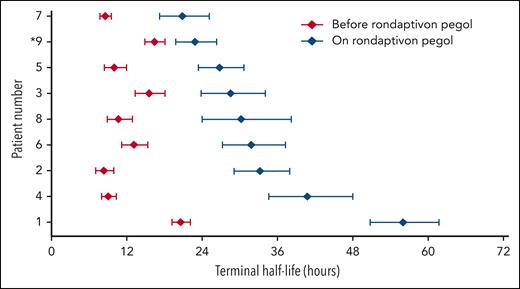
Table 2 compares the half-life estimates of the substituted FVIII products. Half-lives of FVIII products increase from a median of 10.4 hours (range, 8.3-20.6 hours) to 31.1 hours (range, 20.8-56.0 hours) in patients receiving rondaptivon pegol for 28 days. In the on-demand patient who received the abbreviated protocol of 3 doses of 3 mg rondaptivon pegol, the half-life increase was less pronounced: from 16.4 hours to 22.8 hours. Figure 2 shows FVIII elimination curves fitted by linear regression before and after rondaptivon pegol treatment, and Figure 3 shows an example of the rise in FVIII trough levels. Six doses of rondaptivon pegol prolonged the estimated median time to 1% FVIII from 2.8 days (range, 2.2-6.8 days) to 9.3 days (range, 5.7-18.9 days) (supplemental Table 4C).
Rondaptivon pegol–induced changes in FVIII elimination curves. Original elimination curves (fitted by linear regression) of different FVIII products are depicted as red lines and terminal elimination curves under combined therapy of FVIII concentrates and the VWF-binding aptamer rondaptivon pegol as blue lines. The half-lives as estimated by WAPPS population pharmacokinetics are presented in Table 2 and time to 1% or 5% in the supplemental tables. Patient 9 received only 3 doses of rondaptivon pegol because he did not practice regular prophylaxis.
Rondaptivon pegol–induced changes in FVIII elimination curves. Original elimination curves (fitted by linear regression) of different FVIII products are depicted as red lines and terminal elimination curves under combined therapy of FVIII concentrates and the VWF-binding aptamer rondaptivon pegol as blue lines. The half-lives as estimated by WAPPS population pharmacokinetics are presented in Table 2 and time to 1% or 5% in the supplemental tables. Patient 9 received only 3 doses of rondaptivon pegol because he did not practice regular prophylaxis.
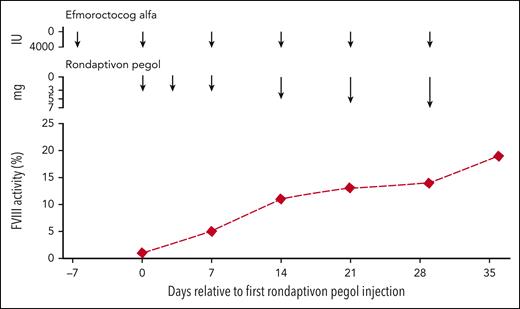
Effect of rondaptivon pegol on trough FVIII levels. Example of increasing FVIII trough levels under treatment with the VWF-binding aptamer rondaptivon pegol in a severe hemophilia patient on once-weekly prophylaxis with 4000 IU efmoroctocog alfa.
Effect of rondaptivon pegol on trough FVIII levels. Example of increasing FVIII trough levels under treatment with the VWF-binding aptamer rondaptivon pegol in a severe hemophilia patient on once-weekly prophylaxis with 4000 IU efmoroctocog alfa.
In all patients with nonsevere hemophilia, median endogenous FVIII levels increased from 19% at baseline to 41% 1 week after the last rondaptivon pegol injection on treatment (P < .0001) (Figure 4). This was reflected by a shortening of the activated partial thromboplastin time from a median of 43 seconds to 37 seconds on day 35 (normal range, 27-41 seconds). In 2 patients with moderate disease, FVIII levels increased from 1% to 2% and from 5% to 13% on day 35, respectively. In patients with mild hemophilia A, FVIII increased from 22% at baseline to 48% on treatment (95% CI for relative change, 92%-185%). Three patients with mild hemophilia A achieved normal FVIII levels of 59%-75%, representing a doubling or tripling of their baseline levels of 24%-26%.
Effects of rondaptivon pegol on FVIII activity levels in nonsevere hemophilia. Effects of the VWF-binding aptamer rondaptivon pegol on FVIII activity levels in 8 patients with mild (blue lines) and 2 patients with moderate (red lines) hemophilia A. Data for patients with mild hemophilia are additionally summarized as median (blue diamonds) with interquartile range (error bars). Contrast test: P < .0001.
Effects of rondaptivon pegol on FVIII activity levels in nonsevere hemophilia. Effects of the VWF-binding aptamer rondaptivon pegol on FVIII activity levels in 8 patients with mild (blue lines) and 2 patients with moderate (red lines) hemophilia A. Data for patients with mild hemophilia are additionally summarized as median (blue diamonds) with interquartile range (error bars). Contrast test: P < .0001.
One week after the last subcutaneous injection of rondaptivon pegol, plasma levels of VWF antigen increased from a median of 97% at baseline to 323% on day 35 and returned to normal on day 56. Similar changes were seen for VWF:GpIbM and VWF collagen-binding activity (Figure 5). In contrast to VWF:Ag, VWF:RCo was minimally affected, which is likely the net result of an increase in VWF mass with increasing concentrations of rondaptivon pegol and a simultaneous but limited inhibition of the A1 domain of VWF by the aptamer. The changes in the VWF:RCo/VWF:Ag ratio are driven by the increase of VWF antigen levels and the relatively steady VWF:RCo values (supplemental Figure 3). The VWF:Ag levels exceeded the maximum measurable value (>420%) in 3 patients on day 35, which slightly underestimates this ratio.
Effect of rondaptivon pegol on VWF parameters. The VWF-binding aptamer rondaptivon pegol increases VWF (A) Ag levels (P < .0001), (B) GpIbM activity (P < .0001), and (D) collagen-binding activity (VWF:CB; P < .0001), whereas C) RCo activity (P = .485) remains mostly at baseline levels. Individual patient data are depicted with semitransparent lines and summarized by median (red diamonds) with interquartile range (error bars). The VWF:Ag and VWF:CB assays could not measure values higher than 420% and 324%, respectively. Therefore all points that exceeded these values were plotted as 421% and 325%, respectively.
Effect of rondaptivon pegol on VWF parameters. The VWF-binding aptamer rondaptivon pegol increases VWF (A) Ag levels (P < .0001), (B) GpIbM activity (P < .0001), and (D) collagen-binding activity (VWF:CB; P < .0001), whereas C) RCo activity (P = .485) remains mostly at baseline levels. Individual patient data are depicted with semitransparent lines and summarized by median (red diamonds) with interquartile range (error bars). The VWF:Ag and VWF:CB assays could not measure values higher than 420% and 324%, respectively. Therefore all points that exceeded these values were plotted as 421% and 325%, respectively.
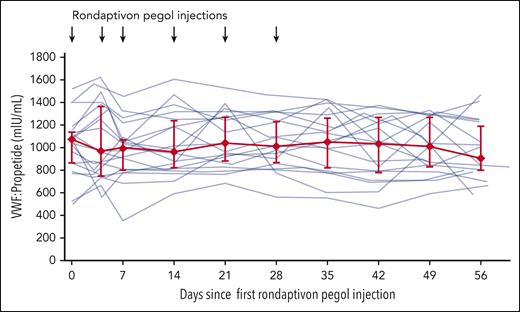
Measuring VWF activity with the VWF:GPIbM assay and the VWF:RCo assay provided complementary information, but unfortunately neither test is physiologic. The VWF:GPIbM uses GPIb with 2 gain-of-function mutations27 that allow it to bind VWF without high shear stress. In contrast, the VWF:RCo assay uses ristocetin, which unfolds VWF and exposes the A1 domain binding to GpIb.28 The binding region for rondaptivon pegol on VWF (1367-1402) overlaps with many amino acids important for ristocetin binding.29 A competitive inhibition of ristocetin binding to VWF is therefore possible. As the drug dose dependently inhibits VWF:RCo and ristocetin-induced aggregation,18,30 the VWF:RCo assay likely reflects the VWF occupancy by rondaptivon pegol. The VWF:GpIbM assay more closely tracked the increase in VWF:Ag (rS = 0.94; P < .0001) and the increase in collagen-binding activity (rS = 0.83; P < .001) on day 35, and may therefore be a better reflection of efficacy. In contrast, VWF pro-peptide (VWF:pp) remained stable (Figure 6). As a consequence, the VWF:pp/VWF:Ag ratio decreased from a median of 11 at baseline to 3 on day 35 and returned to 8 at the end of the study.
The effect of rondaptivon pegol on VWF propeptide levels. The VWF-binding aptamer rondaptivon pegol does not alter VWF synthesis or secretion, as indicated by stable VWF propeptide levels. Individual patient data are depicted with semitransparent lines and summarized by median (red diamonds) with interquartile range (error bars; P = .531).
The effect of rondaptivon pegol on VWF propeptide levels. The VWF-binding aptamer rondaptivon pegol does not alter VWF synthesis or secretion, as indicated by stable VWF propeptide levels. Individual patient data are depicted with semitransparent lines and summarized by median (red diamonds) with interquartile range (error bars; P = .531).
There were 5 patients suffering from nonsevere hemophilia with inexplicably prolonged CADP-CT at baseline and that remained prolonged throughout the study. The cause of their baseline prolongation was not readily apparent to us. It was not due to coexisting VWF:RCo levels, but possibly it indicates a second underlying hemostatic functional defect, for example, of platelet function, in addition to FVIII deficiency. CADP-CT values fluctuated in these patients: CADP-CT reached 300 seconds on days 21 and 28 in 2 of them, but their values dropped by 40 seconds and returned to within the normal range on day 35. On day 35, the median change of CADP-CT was a shortening of CADP-CT by 15 seconds (range, 68 seconds shortening to 39 seconds prolongation; n = 5) Because 3 of the 5 patients had improved (shortened) CADP-CT on day 35, it is difficult to attribute those changes to rondaptivon pegol and therefore to call them treatment-emergent PFA prolongation.
One patient had a normal PFA closure time at baseline and showed treatment-emergent PFA prolongation to 300 seconds while on rondaptivon pegol. This was the lightest patient in the trial (56 kg) who was dosed up to 9 mg rondaptivon pegol. She had a single CADP-CT value of >300 seconds at day 28 which decreased to 193 seconds on day 35 despite repeated doses of 9 mg on day 21 and day 28. In contrast, PFA prolongation was not observed in 2 other (heavier) patients dosed up to 9 mg rondaptivon pegol, and body weight did not emerge as an important covariate related to rondaptivon pegol pharmacokinetics parameters in the integrated pharmacokinetics/pharmacodynamics analysis of this trial.
Consistent with the phase 1 trial in healthy volunteers, the local tolerability was good in patients with hemophilia, which can be explained by the lack of stabilizers and excellent biocompatibility of aptamers. Systemically, rondaptivon pegol was also well tolerated, with no specific drug-related events, and there were no serious adverse events or thrombotic events. Patient satisfaction was invariably good. A list of all reported adverse events is provided in Table 3. Seven treatment-related adverse events (4 possibly drug related) occurred in severe patients and 6 (one possibly drug related) occurred in nonsevere patients. All adverse events were mild or moderate. Three patients with severe hemophilia reported bleeding (1 on day 2, 1 on day 7, and 1 on day 28) and 1 had repeated target joint bleeding; all events were rated as moderate because they triggered substitution of FVIII. Three patients with nonsevere hemophilia had mild bleeding, which included 2 injection-site hematomas (both at day 21), 1 small hematoma on the upper arm (day 1), and 1 nose and 1 gingival bleed (day 28). Rondaptivon pegol did not alter platelet counts but slightly and insignificantly prolonged CADP-CT from a baseline of 100 seconds (range, 74-222 s) to 126 seconds (range, 82-261 seconds) on day 35 (supplemental Figure 4). The overall drug effect did not reach statistical significance, but rondaptivon pegol inhibited ristocetin-induced aggregation by 19% on day 35 (supplemental Figure 4). None of the bleeding events except for gum bleeding was associated with a decreased VWF-dependent platelet function. There was no evidence of an increase in spontaneous in vivo clotting as measured by prothrombin fragment or D-dimer levels (data not presented).
Adverse events
| Adverse event . | Severe (n = 9) . | Nonsevere (n = 10) . | Overall (n = 19) . |
|---|---|---|---|
| Patients with at least 1 event | 6 (66.7%) | 7 (70.0%) | 13 (68.4%) |
| Eye disorders | 1 (11.1%) | 0 | 1 (5.3%) |
| Conjunctivitis, allergic | 1 (11.1%) | 0 | 1 (5.3%) |
| Gastrointestinal disorders | 1 (11.1%) | 2 (20.0%) | 3 (15.79%) |
| Dyspepsia | 1 (11.1%) | 0 | 1 (5.3%) |
| Gingival bleeding | 0 | 1 (10.0%) | 1 (5.3%) |
| Nausea | 1 (11.1%) | 0 | 1 (5.3%) |
| Toothache | 0 | 1 (10.0%) | 1 (5.3%) |
| General disorders and administration site conditions | 1 (11.1%) | 2 (20.0%) | 3 (15.79%) |
| Injection site bruising | 0 | 1 (10.0%) | 1 (5.3%) |
| Pyrexia | 1 (11.1%) | 1 (10.0%) | 2 (10.53%) |
| Infections and infestations | 1 (11.1%) | 1 (10.0%) | 2 (10.53%) |
| Conjunctivitis | 1 (11.1%) | 0 | 1 (5.3%) |
| Nasopharyngitis | 0 | 1 (10.0%) | 1 (5.3%) |
| Injury, poisoning, and procedural complications | 0 | 1 (10.0%) | 1 (5.3%) |
| Subcutaneous hematoma | 0 | 1 (10.0%) | 1 (5.3%) |
| Musculoskeletal and connective tissue disorders | 5 (55.6%) | 1 (10.0%) | 1 (5.3%) |
| Arthralgia | 1 (11.1%) | 0 | 1 (5.3%) |
| Hemarthrosis | 3 (33.3%) | 0 | 3 (15.79%) |
| Muscle hemorrhage | 1 (11.1%) | 0 | 1 (5.3%) |
| Pain in extremity | 1 (11.1%) | 1 (10.0%) | 2 (10.53%) |
| Soft tissue hemorrhage | 1 (11.1%) | 0 | 1 (5.3%) |
| Nervous system disorders | 2 (22.2%) | 0 | 2 (10.53%) |
| Headache | 2 (22.2%) | 0 | 2 (10.53%) |
| Psychiatric disorders | 1 (11.1%) | 0 | 1 (5.3%) |
| Sleep disorder | 1 (11.1%) | 0 | 1 (5.3%) |
| Respiratory, thoracic, and mediastinal disorders | 2 (22.2%) | 1 (10.0%) | 3 (15.79%) |
| Cough | 1 (11.1%) | 0 | 1 (5.3%) |
| Dysphonia | 1 (11.1%) | 0 | 1 (5.3%) |
| Epistaxis | 0 | 1 (10.0%) | 1 (5.3%) |
| Hemoptysis | 1 (11.1%) | 0 | 1 (5.3%) |
| Rhinitis, allergic | 1 (11.1%) | 0 | 1 (5.3%) |
| Skin and subcutaneous tissue disorders | 0 | 1 (10.0%) | 1 (5.3%) |
| Eczema | 0 | 1 (10.0%) | 1 (5.3%) |
| Vascular disorders | 1 (11.1%) | 1 (10.0%) | 2 (10.53%) |
| Hematoma | 0 | 1 (10.0%) | 1 (5.3%) |
| Hemorrhage | 1 (11.1%) | 0 | 1 (5.3%) |
| Adverse event . | Severe (n = 9) . | Nonsevere (n = 10) . | Overall (n = 19) . |
|---|---|---|---|
| Patients with at least 1 event | 6 (66.7%) | 7 (70.0%) | 13 (68.4%) |
| Eye disorders | 1 (11.1%) | 0 | 1 (5.3%) |
| Conjunctivitis, allergic | 1 (11.1%) | 0 | 1 (5.3%) |
| Gastrointestinal disorders | 1 (11.1%) | 2 (20.0%) | 3 (15.79%) |
| Dyspepsia | 1 (11.1%) | 0 | 1 (5.3%) |
| Gingival bleeding | 0 | 1 (10.0%) | 1 (5.3%) |
| Nausea | 1 (11.1%) | 0 | 1 (5.3%) |
| Toothache | 0 | 1 (10.0%) | 1 (5.3%) |
| General disorders and administration site conditions | 1 (11.1%) | 2 (20.0%) | 3 (15.79%) |
| Injection site bruising | 0 | 1 (10.0%) | 1 (5.3%) |
| Pyrexia | 1 (11.1%) | 1 (10.0%) | 2 (10.53%) |
| Infections and infestations | 1 (11.1%) | 1 (10.0%) | 2 (10.53%) |
| Conjunctivitis | 1 (11.1%) | 0 | 1 (5.3%) |
| Nasopharyngitis | 0 | 1 (10.0%) | 1 (5.3%) |
| Injury, poisoning, and procedural complications | 0 | 1 (10.0%) | 1 (5.3%) |
| Subcutaneous hematoma | 0 | 1 (10.0%) | 1 (5.3%) |
| Musculoskeletal and connective tissue disorders | 5 (55.6%) | 1 (10.0%) | 1 (5.3%) |
| Arthralgia | 1 (11.1%) | 0 | 1 (5.3%) |
| Hemarthrosis | 3 (33.3%) | 0 | 3 (15.79%) |
| Muscle hemorrhage | 1 (11.1%) | 0 | 1 (5.3%) |
| Pain in extremity | 1 (11.1%) | 1 (10.0%) | 2 (10.53%) |
| Soft tissue hemorrhage | 1 (11.1%) | 0 | 1 (5.3%) |
| Nervous system disorders | 2 (22.2%) | 0 | 2 (10.53%) |
| Headache | 2 (22.2%) | 0 | 2 (10.53%) |
| Psychiatric disorders | 1 (11.1%) | 0 | 1 (5.3%) |
| Sleep disorder | 1 (11.1%) | 0 | 1 (5.3%) |
| Respiratory, thoracic, and mediastinal disorders | 2 (22.2%) | 1 (10.0%) | 3 (15.79%) |
| Cough | 1 (11.1%) | 0 | 1 (5.3%) |
| Dysphonia | 1 (11.1%) | 0 | 1 (5.3%) |
| Epistaxis | 0 | 1 (10.0%) | 1 (5.3%) |
| Hemoptysis | 1 (11.1%) | 0 | 1 (5.3%) |
| Rhinitis, allergic | 1 (11.1%) | 0 | 1 (5.3%) |
| Skin and subcutaneous tissue disorders | 0 | 1 (10.0%) | 1 (5.3%) |
| Eczema | 0 | 1 (10.0%) | 1 (5.3%) |
| Vascular disorders | 1 (11.1%) | 1 (10.0%) | 2 (10.53%) |
| Hematoma | 0 | 1 (10.0%) | 1 (5.3%) |
| Hemorrhage | 1 (11.1%) | 0 | 1 (5.3%) |
All adverse events were mild or moderate. The first number is the number of patients who suffered an adverse event and the number in the parenthesis is the percentage of patients who suffered an event.
Discussion
The VWF-binding aptamer rondaptivon pegol doubled plasma levels of endogenous FVIII levels in nonsevere hemophilia and prolonged the half-life of different FVIII products severalfold in severe hemophilia A (Figure 7). The pre-treatment FVIII half-lives in patients were in the range of those published in pivotal trials (supplemental Table 3) and similar to real-world WAPPS data from adult patients,13 indicating that the patients in this study were representative of the hemophilia population. Half-life prolongation by rondaptivon pegol was observed for all tested FVIII products, including an extended half-life product, efmoroctocog. More important, the final half-life estimates for FVIII concentrates under rondaptivon pegol treatment invariably exceeded the maximal published half-lives in official regulatory documents of each of the FVIII products (supplemental Table 3). This provides a historical comparison to larger and independent populations and complements the intra-individual comparisons. In addition, it provides a context for current standards of FVIII replacement therapies. The half-life prolongation of FVIII products was similar to the 3-fold longer half-life of a new fusion protein (rFVIII-VWF-XTEN)31 that breaks the VWF-imposed half-life ceiling on FVIII. The consistent synergistic effect between rondaptivon pegol and several different FVIII concentrates demonstrated in our study suggests that these data may be generalizable to larger populations using other FVIII concentrates.
Rondaptivon pegol increases the half-lives of the substituted FVIII products. Patients with severe hemophilia (patients 1-8) followed the full rondaptivon pegol study protocol: 3 mg on days 1, 4, and 7 and weekly doses of 2 to 9 mg until day 28. FVIII activity was measured by 1-stage clotting assay, and half-lives were estimated by WAPPS population pharmacokinetics models. ∗Patient 9 followed an abbreviated regimen of only 3 doses of 3 mg rondaptivon pegol. Error bars depict 95% credible intervals. Patients are ordered by the FVIII half-life achieved during treatment with rondaptivon pegol.
Rondaptivon pegol increases the half-lives of the substituted FVIII products. Patients with severe hemophilia (patients 1-8) followed the full rondaptivon pegol study protocol: 3 mg on days 1, 4, and 7 and weekly doses of 2 to 9 mg until day 28. FVIII activity was measured by 1-stage clotting assay, and half-lives were estimated by WAPPS population pharmacokinetics models. ∗Patient 9 followed an abbreviated regimen of only 3 doses of 3 mg rondaptivon pegol. Error bars depict 95% credible intervals. Patients are ordered by the FVIII half-life achieved during treatment with rondaptivon pegol.
Rondaptivon pegol doses of about 6 mg increased VWF antigen and FVIII activity, but did not decrease VWF activity. This is in good agreement with the phase 1 trial in healthy volunteers,18 where VWF activity did not drop below 50% in the cohorts receiving doses up to 6 mg, while VWF antigen and FVIII activity increased. Median VWF:RCo levels of 83% on day 35 in the present trial indicate a sufficient safety head room, and healthy volunteers tolerated weekly doses of 12 mg rondaptivon pegol without relevant inhibition of VWF activity.18 Thus, while individual dose titration is possible, a fixed dose of 6 mg appears suitable for delivering the desired hemostatic effect without inhibition of primary hemostasis in the majority of patients with hemophilia.
Rondaptivon pegol appears to be a promising treatment for several patient groups. The improved half-life of VWF/FVIII could allow severe hemophilia patients, who often use prophylactic therapy every other day, to switch to a once-weekly regimen while maintaining trough levels above 1% (supplemental Table 4). While rebalancing agents, such as emicizumab,32 have been introduced to the market and others will become available, patients who prefer to remain on FVIII products or cannot afford frequent prophylaxis could benefit from rondaptivon pegol. This is of interest to caregivers, reimbursement agencies, and particularly patients in developing economies, where the life expectancy disadvantage of hemophilia A is even more pronounced.33
Gene therapy is nearly within reach for large-scale application to hemophilia A treatment.34,35 Despite this progress, some patients may not respond optimally and others may experience a substantial decline in FVIII levels over the ensuing years. One may expect that rondaptivon pegol could provide benefit to these patients similar to what we demonstrated in nonsevere hemophilia patients. Patients with nonsevere hemophilia also can have joint bleeding and hemophilic arthropathy.5 These patients include women whose risk of joint morbidity correlates with the degree of FVIII deficiency,36 a group that has been neglected in treatment. Only recently has a new nomenclature been proposed to distinguish between asymptomatic carriers and female hemophilia A carriers who have a bleeding phenotype.37 The incidence of joint hemorrhages appears to increase when FVIII levels drop below 15% in nonsevere hemophilia patients,38 making them potential candidates for long-term prophylaxis or use of rondaptivon pegol. All nonsevere hemophilia patients included in our trial reported late bleeds after surgery or trauma. Although desmopressin is used perioperatively in nonsevere hemophilia, repeated administration induces tachyphylaxis and may generate hyponatremia and other adverse effects.39 Thus, desmopressin cannot be used for long-term prophylaxis, whereas rondaptivon pegol appears to be suitable for long-term administration based on the long half-life and absence of tachyphylaxis. This notion is supported by the anecdotal hemostatic efficacy observed in a patient who underwent dental extraction 2 weeks after the last dose of rondaptivon pegol, with no additional procoagulant treatment. Interestingly, a decreased and less sustained desmopressin response has recently been reported in hemophilia A carriers,40 which may suggest an impaired ability to respond to hemostatic stress.
Rondaptivon pegol had good tolerability and safety, with no clinically relevant adverse events. This was not unexpected, because aptamers have a large molecular size and tend to cause few nonspecific adverse effects.41 Whereas healthy volunteers who received single doses of up to 48 mg rondaptivon pegol had some gingival bleeding,18 the highest dose in the current trial (9 mg) did not cause these exaggerated pharmacologic effects. Although some patients experienced bleeding, this was attributed to hemophilia and not considered to have been related to rondaptivon pegol. Rondaptivon pegol increased VWF antigen and FVIII levels and did not inhibit VWF activity in this study, which makes it unlikely that these bleeding events were due to rondaptivon-induced inhibition of VWF-dependent platelet function, including for a female patient whose platelet function was already compromised at baseline and who presented gum bleeding.
Limitations
The number of patients was small, as expected for an orphan disease. Larger patient numbers in a multicenter trial are needed to fully characterize the therapeutic potential of rondaptivon pegol. The 28-day treatment period in the study together with the dose escalation was too short to achieve a steady state. However, we estimate that we reached about 90% of the plateau effect, based on data from the phase 1 study and results from individual patient data in the present trial. The sparse-sampling population pharmacokinetics estimation was performed with the use of specific population models derived for each one of the concentrates used by the patients. This approach assumes that the pharmacokinetics profile (including the decay-curve shape and compartmentalization) of the FVIII under study remains the same. This is a conservative assumption that may have limited appraisal of the full potential of rondaptivon pegol. Future trials may allow us to develop a dedicated population pharmacokinetics model for patients treated with rondaptivon pegol to explore the effects on model parametrization and consequently increase the precision of the estimates.
In conclusion, rondaptivon pegol is a first-in-class prohemostatic molecule that extended the half-life of substituted FVIII approximately 3-fold and increased endogenous FVIII levels approximately 2-fold in hemophilia patients.
Acknowledgment
This investigator-initiated trial was sponsored by the Medical University of Vienna with funding from Band Therapeutics, a Guardian Therapeutics company.
Authorship
Contribution: C.A., I.P., and D.K. informed and included patients; C.S., G.G., C.F., and U.D. (the principal investigator) were responsible for conducting the trial; K.D.K., P.Q., and P.J.-S. were responsible for laboratory analyses and quality-checking methods; J.C.G., B.J., U.D., and I.P. designed the trial and wrote the protocol; S.Z. developed the analytical methods; M.B. performed the pharmacokinetics analysis; F.K. planned and performed the statistical analysis; D.K. and A.I. performed the WAPPS analysis; and all authors contributed to writing and critically reviewing the manuscript.
Conflict-of-interest disclosure: C.A. received honoraria for lectures or participation in advisory board meetings from Bayer, CSL Behring, NovoNordisk, Pfizer, Roche, Sobi, and Takeda. J.C.G. and S.Z. are employees of Guardian Therapeutics. M.B. and B.J. are consultants to Guardian Therapeutics. I.P. received honoraria for lectures or advisory board meetings from Bayer, Biomarin, CSL Behring, NovoNordisk, Pfizer, Roche, Sobi, and Takeda. The remaining authors declare no competing financial interests.
Correspondence: Ulla Derhaschnig, Department of Clinical Pharmacology, Medical University of Vienna, Währinger Gürtel 18-20, 1090 Vienna, Austria; e-mail: ulla.derhaschnig@meduniwien.ac.at; and Ingrid Pabinger, Clinical Division of Hematology and Hemastaseology, Department of Medicine I, Medical University of Vienna, Währinger Gürtel 18-20, 1090 Vienna, Austria; e-mail: ingrid.pabinger@meduniwien.ac.at.
References
Author notes
∗C.A. and K.D.K. contributed equally to this study.
Presented as a late-breaking abstract at the International Society on Thrombosis and Haemostasis Congress, Philadelphia, Pennsylvania, 17-21 July 2021.
Data will be shared with qualified investigators in justified cases. For original data, please contact bernd.jilma@meduniwien.ac.at. Deidentified individual patient data will be shared on reasonable request according to the General Data Protection Regulation of the European Union.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal