Key Points
In relapsed CLL, 74% of deep responses to VenR are maintained for 5 years or more with either continuous or limited-duration venetoclax.
Achievement of CR/CRi or uMRD identifies patients who can discontinue venetoclax and maintain treatment-free remission of >3 years.
Abstract
We report long-term follow-up of the phase 1b study of venetoclax and rituximab (VenR) in patients with relapsed chronic lymphocytic leukemia (CLL), including outcomes with continuous or limited-duration therapy. Patients received venetoclax daily (200-600 mg) and rituximab over 6 months and then received venetoclax monotherapy. Patients achieving complete response (CR), CR with incomplete marrow recovery (CRi), or undetectable minimal residual disease (uMRD) assessed by flow cytometry (<10−4 cutoff) were allowed, but not required, to discontinue therapy, while remaining in the study and could be retreated with VenR upon progression. Median follow-up for all patients (N = 49) was 5.3 years. Five-year rates (95% CI) for overall survival, progression-free survival, and duration of response were 86% (72-94), 56% (40-70), and 58% (40-73), respectively. Of the 33 deep responders (CR/CRi or uMRD), 14 remained on venetoclax monotherapy (continuous therapy), and 19 stopped venetoclax therapy (limited-duration therapy) after a median of 1.4 years. Five-year estimates of ongoing response were similar between continuous (71%; 95% CI, 39-88) or limited-duration therapy (79% [49-93]). Six of 19 patients in the latter group had subsequent disease progression, all >2 years off venetoclax (range, 2.1-6.4). Four patients were retreated with VenR, with partial responses observed in the 3 evaluable to date. VenR induced deep responses that were highly durable with either continuous or limited-duration therapy. Retreatment with VenR induced responses in patients with CLL progression after discontinuing therapy. Continuous exposure to venetoclax in deep responders does not appear to provide incremental benefit.
Introduction
Patients with chronic lymphocytic leukemia (CLL) who achieve deep remission with undetectable minimal residual disease (uMRD) after initial treatment with chemoimmunotherapy (CIT) typically experience prolonged progression-free survival (PFS) without ongoing therapy.1,2 However, in the relapsed/refractory (R/R) setting, the proportion of patients who achieve deep remission with CIT is much reduced.3 The introduction of targeted therapies, such as the B-cell receptor (BCR) signaling inhibitors, has led to a shift in the treatment algorithm for CLL4 in both the first-line and R/R settings.5-9 Although most patients’ best response is only a partial response (PR) achieved with BCR inhibitors, these responses are often very durable, provided patients continue to receive long-term therapy.9-11 There is a need for new targeted therapy approaches that achieve and maintain deep remissions without requiring ongoing therapy for patients with treatment-naive or R/R CLL.
Inhibition of B-cell lymphoma 2 (BCL2) with venetoclax is another targeted approach,12 and emerging data suggest that it can address this need for many patients.13,14 BCL2 is uniformly overexpressed in CLL, resulting in the evasion of apoptosis, and consequently, enhanced cancer cell survival.15 Venetoclax kills CLL cells by inducing apoptosis.16 As a monotherapy, complete response (CR) rates of 16% to 20% and rates of uMRD in the peripheral blood of 30% have been reported in patients with R/R CLL, including those with the prognostically adverse chromosome 17p deletion (del[17p]).16-18 Higher rates for both CR and uMRD have been reported when venetoclax is combined with anti-CD20 monoclonal antibodies. In our initial account of a phase 1b study, we reported that the addition of rituximab achieved a 51% CR rate and that 57% of patients had uMRD in the bone marrow (BM), with a 2-year PFS rate of 82%.19 In addition, remission was sustained without ongoing therapy in select patients who had achieved deep responses. Subsequent randomized trials in both the R/R and frontline settings have demonstrated that time-limited therapy with venetoclax, in combination with the anti-CD20 monoclonal antibodies rituximab or obinutuzumab, respectively, is superior to CIT regimens.13,14
In the R/R setting, venetoclax is widely approved as both continuous monotherapy and in combination with rituximab for 2 years. Limited-duration therapy minimizes the potential toxicity and costs associated with continuous therapy. To be successful as limited-duration therapy, a regimen should decrease the number of CLL cells sufficiently to achieve a deep response that is durable after withdrawal of drug in most patients, and when progressive disease develops in patients who are off therapy, it may still be sensitive to retreatment.
An issue that has not been addressed with the venetoclax and rituximab regimen is whether indefinite continuous venetoclax is associated with more-durable responses than limited-duration venetoclax therapy. No randomized trials have been conducted; however, data for both approaches are available from long-term follow-up of the original phase 1b combination study, in which deep responders to venetoclax and rituximab could elect to discontinue venetoclax after achieving CR/CR with incomplete marrow recovery (CRi) or uMRD.
Herein, we report the long-term efficacy and safety analyses from this study with a median follow-up of ∼5 years, 3 years beyond the initial report.19 The additional post hoc objectives of this analysis are to assess and compare the durability of responses, with and without continuous therapy, and to report the responses of patients with progressive disease who have been retreated with venetoclax and rituximab.
Methods
Study design
This phase 1b open-label, multicenter, dose-escalation and cohort-expansion trial enrolled patients with R/R CLL from 6 August 2012 through 28 May 2014 (study M13-365; NCT01682616).
The study treatment schedule (supplemental Figure 1, available on the Blood Web site) and the primary outcomes assessing the safety profile, maximum tolerated dose, and recommended phase 2 dose of venetoclax when given in combination with rituximab have been published.19 After the initial treatment with combined venetoclax and rituximab, patients continued venetoclax alone. Those achieving a CR (irrespective of MRD status) or PR with uMRD were managed by either (1) continuing venetoclax monotherapy (continuous therapy) or (2) discontinuing venetoclax (limited-duration therapy) and remaining in the study according to the active protocol version (supplemental Figure 1). In protocol amendment 1 (May to September 2012), patients were required to stop therapy after achieving CR/CRi. In amendments 2 and 3 (September 2012 to May 2015), patients who achieved CR/CRi and uMRD had the option to stop therapy; this decision was made jointly between patients and their physicians. In amendment 4 and onward (May 2015 to present), patients who achieved uMRD (whether in CR/CRi or PR by 2008 International Workshop on CLL [iwCLL] criteria) had the option to discontinue therapy. The protocol allowed patients to reinitiate venetoclax and subsequent rituximab at the discretion of the principal investigator once there was evidence of disease progression and an indication for treatment, per iwCLL criteria.20
Patients
Full eligibility criteria for the study have been described.19 In summary, patients (aged ≥18 years) with CLL/small lymphocytic lymphoma, according to the 2008 iwCLL criteria,20 were eligible if they had R/R disease, Eastern Cooperative Oncology Group performance status ≤1, adequate marrow function (neutrophil count, ≥1 × 103/μL; platelets, ≥50 × 103/μL; hemoglobin, ≥9.0 g/dL [with transfusions performed to meet this criterion]), and adequate renal and hepatic function. Exclusion criteria included prior stem cell transplant, receipt of ≥3 prior myelosuppressive treatment regimens, uncontrolled autoimmune hemolytic anemia or thrombocytopenia, and infection with HIV or hepatitis B or hepatitis C virus. The study was approved by the institutional review board at each participating site and was conducted according to the Declaration of Helsinki and the International Council on Harmonization Good Clinical Practice guidelines. All patients provided written informed consent.
Assessments
Efficacy assessments included objective response, overall survival (OS), PFS, and duration of response (DOR). Tumor response assessment was mandatory at month 7, after completion of combination therapy. Responses were evaluated per 2008 iwCLL criteria,20 including the use of computed tomography (CT).
MRD was assessed in bone marrow using multicolor (4-8 colors) flow cytometry in local laboratories with 0.01% minimum sensitivity at month 7 and then as clinically indicated. uMRD was defined as <1 CLL cell present in 1 × 104/μL white blood cells.
Adverse events (AEs) were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.0. AEs within 30 days of stopping therapy were collected for all patients.
Statistical analysis
The data cutoff for this analysis was 4 June 2019. Descriptive statistics (medians, ranges, and standard deviations) were calculated. Objective response rate (ORR) was defined as CR+CRi+nodular PR+PR using 2008 iwCLL criteria.20 For ORR, 95% CIs based on the binomial distribution were calculated according to the Clopper-Pearson exact method. The distributions of OS, PFS, and DOR were estimated using the Kaplan-Meier method; median time and the corresponding 95% CIs were estimated. Data for PFS and DOR were censored for patients without an event at time of last assessment or at time of data cutoff for patients with tumor assessments showing ongoing response after data cutoff. Elective allograft in CR was handled by censoring the patient at the last disease assessment before allograft in time-to-event analyses. Best marrow MRD status is reported; 7-month marrow MRD status was used to evaluate outcomes in landmark analyses. For this report, we also calculated time to venetoclax treatment failure (TTVF), on the basis of the previously published criteria for time to rituximab treatment failure.21 TTVF is measured as time from first venetoclax dose to venetoclax-based therapy failure, where venetoclax-based therapy failure is defined as (1) no disease response to venetoclax-based treatment; (2) disease progression while actively receiving venetoclax-based treatment (at initial progression for patients on continuous venetoclax, or subsequent progression on venetoclax for patients who initially progressed after limited-duration therapy and who initiated retreatment with venetoclax-based therapy on trial); (3) disease progression after limited-duration venetoclax therapy in patients who would not be retreated with venetoclax (because they are ineligible for retreatment, elect not to receive retreatment with venetoclax, or are lost to follow-up); (4) death from any cause; and (5) start of an alternative treatment of CLL. Data for TTVF were censored for patients without an event at time of last assessment before data cutoff.
Analyses were performed with SAS software version 9.4 (Cary, NC).
Results
Patient demographics and disposition on treatment
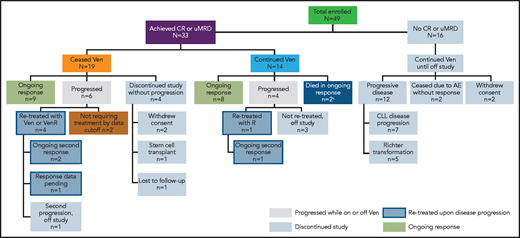
A total of 49 patients were enrolled in the study. Key patient demographics and clinical characteristics have been reported.19 The median age was 68 years (range, 50-88) and the patients had a median of 2 (range, 1-5) prior therapies. As of 4 June 2019, the median time in the study was 5.3 years (range, 0-6.6) and the median time on venetoclax was 2.5 years (range, 0-6.5). Overall, as of this writing, 24 patients had withdrawn from the study (Figure 1).
Patient disposition.aOne patient discontinued treatment because of cytopenias, subsequently proved to be caused by myelodysplasia after data cutoff for the publication. One patient died of an unrelated cause (ischemic heart disease) during ongoing response. Molecular characterization of samples at the point of CLL progression was not performed systematically as part of the trial, but was done in 6 patients treated in Melbourne. This revealed BCL2 mutations in 2 of 4 patients who had MRD+ PR as best response, as reported previously.22,23 No venetoclax resistance-associated mutations were detected in 2 patients who had CR as best response and who progressed after cessation of therapy. R, rituximab; Ven, venetoclax.
Patient disposition.aOne patient discontinued treatment because of cytopenias, subsequently proved to be caused by myelodysplasia after data cutoff for the publication. One patient died of an unrelated cause (ischemic heart disease) during ongoing response. Molecular characterization of samples at the point of CLL progression was not performed systematically as part of the trial, but was done in 6 patients treated in Melbourne. This revealed BCL2 mutations in 2 of 4 patients who had MRD+ PR as best response, as reported previously.22,23 No venetoclax resistance-associated mutations were detected in 2 patients who had CR as best response and who progressed after cessation of therapy. R, rituximab; Ven, venetoclax.
Efficacy results: overall study population
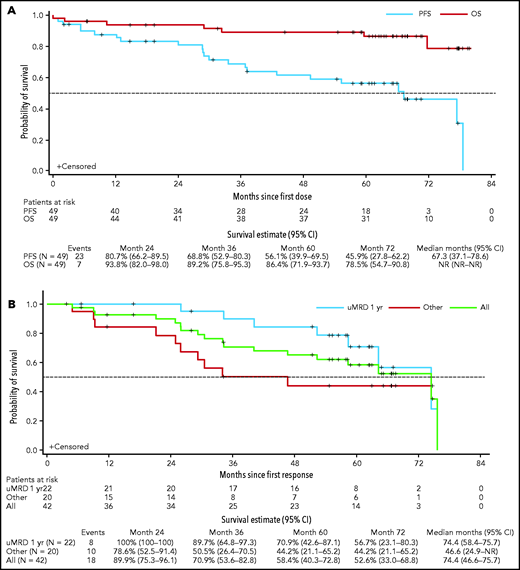
The ORR for all patients (n = 49) was 86% (95% CI, 73-94) with 53% (n = 26) achieving CR/CRi (95% CI, 38-68), and 14 (29%), 8 (16%), and 4 (8%) first achieving CR/CRi within 1 year, between 1 and 2 years, and after 2 years, respectively. One patient achieved CR relatively late, at ∼5.8 years, after additional infusions of rituximab and venetoclax dose escalation to 600 mg/day and so was not captured in the previous report. The median OS had not been reached, and the actuarial 5-year OS was 86% (95% CI, 72-94; Figure 2A). For PFS, the 5-year rate was 56% (95% CI, 40-70) and the estimated median was 5.6 years (95% CI, 3.1-6.6; Figure 2A). Among the 42 responders, the 5-year rate for ongoing response was 58% (95% CI, 40-73) with an estimated median DOR of 6.2 years (95% CI, 3.9-6.3; Figure 2B). Thirty (61%) patients achieved BM uMRD; median DOR was 6.2 years (95% CI, 5.4-6.3) for these patients vs 2.2 years (95% CI, 0.8-2.8) for patients who did not achieve uMRD (supplemental Figure 2). Of the patients who achieved uMRD, 22 (44.9%), 6 (12.2%), and 2 (4.1%) first achieved uMRD within 1 year, between 1 and 2 years, and after 2 years, respectively. Five-year probabilities of remaining in remission were 71% (95% CI, 43-87) for patients with uMRD by 12 months (n = 22), compared with 44% (95% CI, 21-65) for responders who did not achieve uMRD by 12 months or at any time in the study (n = 20; Figure 2B).
Kaplan-Meier analysis of OS, PFS, and DOR. (A) OS and PFS of all patients in the study; (B) DOR for all responders (green line), for responders who achieved uMRD by 12 months (blue line), and for responders who did not achieve uMRD by 12 months or at any time while in the study (red line). DOR includes only patients who achieved an objective response. Other, patients who did not achieve uMRD at any time during the study (n = 12), and those who did not achieve uMRD by 1 year but had uMRD after 12 months in the study (n = 8). NR, not reached.
Kaplan-Meier analysis of OS, PFS, and DOR. (A) OS and PFS of all patients in the study; (B) DOR for all responders (green line), for responders who achieved uMRD by 12 months (blue line), and for responders who did not achieve uMRD by 12 months or at any time while in the study (red line). DOR includes only patients who achieved an objective response. Other, patients who did not achieve uMRD at any time during the study (n = 12), and those who did not achieve uMRD by 1 year but had uMRD after 12 months in the study (n = 8). NR, not reached.
All 16 (33%) patients who did not achieve CR and/or uMRD withdrew from the study (at time points ranging from 0.0 months to 58.3 months): 12 because of disease progression (7 for progression of CLL after initial PR, and 5 for Richter’s transformation; all instances described previously19 ), 2 due to AEs (tumor lysis syndrome and neuropathy), and 2 who withdrew consent.
From the overall study population, data for 11 patients who reconsented to survival follow-up and have data on poststudy therapies are provided in supplemental Table 1. Nearly all patients received ibrutinib-based therapy after progression in the study, with most achieving best response of PR. Molecular characterization of CLL at relapse was performed in samples from 6 patients with CLL progression (Figure 1). BCL2 mutations associated with venetoclax resistance were identified in 2 patients who received continuous venetoclax and had MRD+ PR as their best response, as described previously.22,23
Patients with deep response
A total of 33 (67%) patients achieved CR and/or uMRD and opted to either continue receiving venetoclax monotherapy (continuous therapy; n = 14) or stopped therapy with venetoclax (limited-duration therapy) per patient/investigator decision (n = 19) and remain in the study (Figure 1). Bulky adenopathy (≥5 cm) was less frequently present in deep responders than in patients who did not achieve deep response (39% vs 56%), but frequency of baseline absolute lymphocyte count (ALC) ≥100 × 109/L (21% vs 19%), and TP53 mutation and/or del(17p) (21% vs 37%) did not differ significantly (supplemental Table 2). Additional characteristics for deep responders are presented in supplemental Table 2. The DOR and PFS in the 33 deep responders are presented in Table 1. With a median follow-up of ∼5.5 years (range, 0.8-6.7), the 5-year PFS rate was 79% (95% CI, 59.1-90.0), with an estimated median of 6.5 years (95% CI, 5.5-6.6; Table 1).
Continuous venetoclax in deep responders: CR and/or uMRD
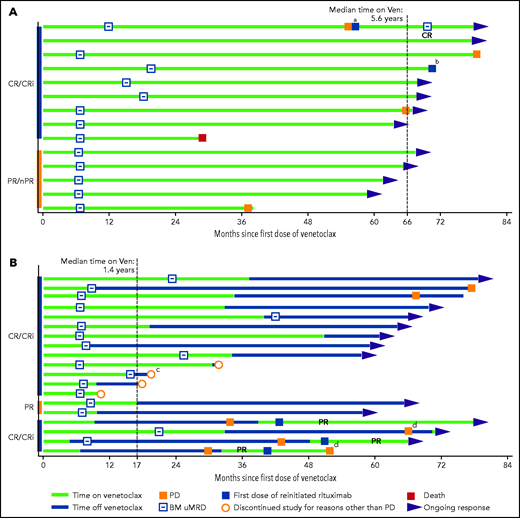
Of the 14 deep responders on continuous venetoclax therapy, 8 remained in continuous ongoing remission on treatment, and 2 discontinued in ongoing remission (1 patient died of ischemic heart disease and 1 developed myelodysplasia). Four had disease progression (Figure 3A), of whom 3 were off study and 1 remained in the study with disease controlled after modified therapy (increasing venetoclax dose to 600 mg daily and adding a second course of rituximab). The median time on venetoclax was 5.6 years (range, 2.4-6.6) with a median time in the study of 5.6 years (range, 2.4-6.7) to date. The 5-year rate for PFS for these patients was 79% (95% CI, 47-93) with an estimated median of 6.6 years (95% CI, 4.6-6.6) (Table 1; Figure 4A). Five-year rates for remaining in remission and estimated median DOR were 71% (95% CI, 39-88) and 6.3 years (95% CI, 4.4-6.3), respectively (Table 1; Figure 4B).
Disease response and treatment status timelines for deep responders. Patients who received continuous venetoclax (n = 14) (A) or limited-duration venetoclax (n = 19) (B). In each panel, patients are grouped by their best iwCLL response category. In panel B, the 4 patients receiving retreatment with venetoclax or venetoclax plus rituximab after progression are grouped together for ease of reference. aThe first patient received a second course of rituximab and increased dose of venetoclax to 600 mg/day upon progression, and then achieved CR with uMRD. bPatient was retreated with rituximab for thrombocytopenia unrelated to CLL progression; the patient withdrew because of myelodysplasia 6 months after data cutoff for the publication. cThe patient proceeded to elective allograft in CR. dPD assessment data were not included in the clinical database at the time of the data cutoff. nPR, nodular PR; PD, progressive disease; Ven, venetoclax.
Disease response and treatment status timelines for deep responders. Patients who received continuous venetoclax (n = 14) (A) or limited-duration venetoclax (n = 19) (B). In each panel, patients are grouped by their best iwCLL response category. In panel B, the 4 patients receiving retreatment with venetoclax or venetoclax plus rituximab after progression are grouped together for ease of reference. aThe first patient received a second course of rituximab and increased dose of venetoclax to 600 mg/day upon progression, and then achieved CR with uMRD. bPatient was retreated with rituximab for thrombocytopenia unrelated to CLL progression; the patient withdrew because of myelodysplasia 6 months after data cutoff for the publication. cThe patient proceeded to elective allograft in CR. dPD assessment data were not included in the clinical database at the time of the data cutoff. nPR, nodular PR; PD, progressive disease; Ven, venetoclax.
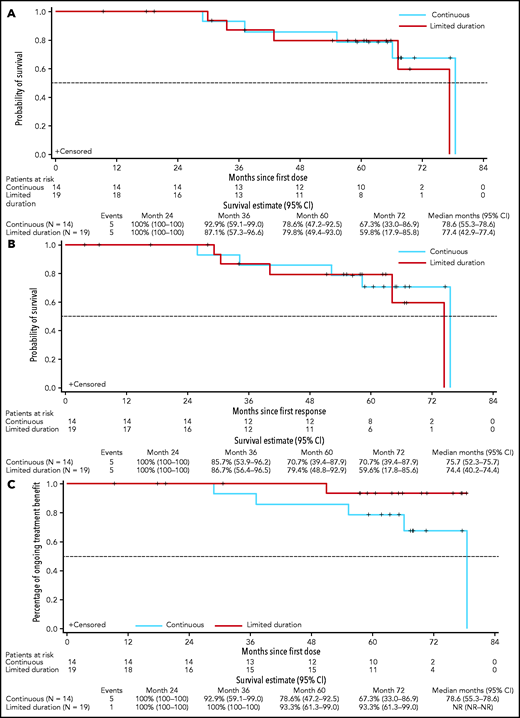
Continuous vs limited-duration therapy in patients who achieved deep response (uMRD or CR). PFS (A); DOR (B); duration of benefit as measured by TTVF (C).
Continuous vs limited-duration therapy in patients who achieved deep response (uMRD or CR). PFS (A); DOR (B); duration of benefit as measured by TTVF (C).
Limited-duration therapy in deep responders: CR and/or uMRD
Of the 19 deep responders who elected to stop treatment, 9 remained in the study in continuing response, and 4 withdrew while in ongoing response (2 withdrew consent, 1 elected to proceed with stem cell transplant, and 1 was lost to follow-up). Of the 19 patients, 15 had achieved uMRD CR, 2 uMRD PR with residual adenopathy precluding assignment of CR (18 mm in 1 patient and 2 lesions of 16 and 17 mm in another patient), and 2 MRD+ CR as their best response (Figure 3B). The median time of initial venetoclax therapy was 1.4 years (range, 0.4-4.2) and the median time of total venetoclax therapy including retreatment was 2.1 years (range, 0.4-4.2). After a median time in the study of 5.4 years (range, 0.8-6.5) to date, the median time off therapy is 3.2 years (range, 0.1-5.7). The 5-year rate for PFS for these patients was 80% (95% CI, 49.4-93.0), with an estimated median of 6.5 years (95% CI, 3.6-6.5) (Table 1; Figure 4A). Five-year rates for remaining in remission and estimated median DOR were 79% (95% CI, 49-93) and 6.2 years (95% CI, 3.4-6.2), respectively (Table 1; Figure 4B).
Six of the 19 deep responders who stopped treatment had subsequent disease progression at a median of 3.4 years (range, 2.1-6.4) off therapy. Of these, 2 had not required further therapy by the data cutoff date, and 4 were retreated with venetoclax and rituximab (supplemental Table 3; Figure 3B). Two of the patients who progressed had an initial response of MRD+ CR and subsequently developed asymptomatic disease progression with increasing ALC at 2.1 and 2.5 years off venetoclax, respectively. Upon retreatment with venetoclax and rituximab, 1 patient achieved a best response of PR (normalization of blood counts and CT scan, but no marrow study performed) and remains on therapy. The second patient achieved a PR with 10% residual marrow disease after retreatment with venetoclax monotherapy and then in combination with rituximab, but later discontinued the study after a second CLL progression after 1.6 years of venetoclax retreatment. The third patient had an initial response of uMRD CR but developed asymptomatic progression with increasing ALC at 3.6 years after discontinuing venetoclax. Upon retreatment with venetoclax and rituximab, the patient achieved a PR (normalization of blood counts and CT scan, with no marrow study performed) and remains on therapy. The fourth patient had an initial response of uMRD CR and had disease progression with an increase in ALC and thrombocytopenia, lymphadenopathy, and ≥50% CLL cells in BM at 3.1 years after cessation of venetoclax. As this patient had recently been retreated with venetoclax monotherapy, formal response data were pending as of data cutoff. After data cutoff, this patient achieved PR with normalization of blood counts and CT scan but with 2% residual marrow disease.
Time to venetoclax treatment failure
To account for the clinical benefit of retreatment after progression while off venetoclax, TTVF was assessed for patients who achieved uMRD or CR (Figure 4C). For the entire trial population, the 5-year estimates for freedom from venetoclax failure were 63% (95% CI, 47-75; supplemental Figure 3). Among deep responders, the 5-year estimates for freedom from venetoclax failure were 93% (95% CI, 61-99) for limited-duration therapy and 79% (95% CI, 47-93) for continuous therapy (Figure 4C).
Safety
Treatment-emergent AEs (TEAEs) were summarized for all patients in the study and also for events occurring after 2 years of therapy in patients with ongoing treatment beyond 2 years. For patients who discontinued therapy because of a good response and had received >2 years of therapy, data on TEAEs within 30 days of stopping therapy were collected. TEAEs occurred in all patients (N = 49; 100%) during the study, and in 95% (20 of 21) of patients beyond 2 years of ongoing venetoclax treatment (summarized in Table 2). The most common TEAEs of any grade reported in all patients in the study or reported after 2 years of treatment were upper respiratory tract infection (63% and 48%), diarrhea (59% and 33%), and neutropenia (55% and 29%). Grade 3/4 TEAEs were reported in 40 (82%) of all treated patients and in 11 (52%) patients beyond 2 years of treatment. The most common event was neutropenia (53% and 19%, respectively). Serious AEs (SAEs) were reported in 28 (57%) of all treated patients and in 9 (43%) patients beyond 2 years of treatment. The most common SAE in all treated patients was pyrexia (n = 5; 10%), and in patients beyond 2 years of treatment was pneumonia (n = 2; 10%). One fatal AE of myocardial ischemia occurred after 2 years of treatment. Infections of any grade occurred in 71% of patients after 2 years of treatment and principally comprised events of upper respiratory tract infection (48%), lower respiratory tract infection (19%), pneumonia, and urinary tract infection (14% each). Grade 3/4 infections, or those causing an SAE were uncommon (5 episodes in 4 patients). Of those with a pathogen isolated, the causative organisms were Haemophilus, mycoplasma, and parainfluenza. No invasive fungal, Pneumocystis jiroveci, or cytomegalovirus infections were reported. Five patients reported secondary malignancies occurring 2 or more years after starting treatment and before treatment discontinuation. One patient had both melanoma and basal cell carcinoma of the skin, 2 had squamous cell carcinomas of the skin, and 2 had prostate cancer (supplemental Table 4).
Autoimmune diseases have not been observed among study patients during this follow-up period, after 2 years of therapy. Long-term analysis of immunoglobulins showed no significant change over time (supplemental Figure 4).
Discussion
Long-term follow-up efficacy and safety analysis of the phase 1b study of venetoclax and rituximab in relapsed CLL, with a median follow-up of more than 5 years, demonstrated durable clinical benefit in patients receiving either continuous or limited-duration therapy, with a 5-year PFS of 56% for all patients and no additional adverse safety findings in patients receiving venetoclax beyond 2 years. Overall, a high proportion of patients with relapsed CLL who were treated with combination venetoclax+rituximab achieved durable remissions, with most of the patients achieving deep response (CR or uMRD) by 12 months of therapy.
In light of the need for durable, fixed-duration therapy in R/R CLL, the current analyses suggest that patients who receive limited-duration therapy after achieving deep response may derive a duration of clinical benefit similar to that of those who remain on continuous therapy. We acknowledge that the study was not formally designed to address this question rigorously. After achieving deep response, patients were not assigned randomly to a continuous or limited-duration approach, and the number of patients was modest. With a starting population of 49 patients, analysis becomes difficult because of the small number of patients in subgroups. Nevertheless, in this post hoc analysis incorporating >5 years of follow-up, no difference in DOR or PFS could be discerned between patients who achieved a deep response and opted to receive continuous therapy with venetoclax and those who ceased treatment. Very durable remissions of between 2 and 5 years have been observed after drug withdrawal. Among the 19 patients who elected to discontinue venetoclax treatment per protocol after achieving CR or uMRD (median time receiving venetoclax, 1.4 years), 9 patients remain in response after discontinuing treatment. To date, 6 patients have shown disease progression after a median of >3 years of treatment-free response.
The current dosing recommendation for venetoclax+rituximab for R/R CLL on the venetoclax label is 24 months of fixed-duration therapy.24 This recommendation is based on the phase 3 study of venetoclax+rituximab in R/R CLL (MURANO trial), where durable responses after 2 years of fixed-duration therapy were demonstrated.25,26 The MURANO trial showed continued benefit with durable uMRD rates and longer PFS for patients receiving fixed-duration venetoclax+rituximab compared with bendamustine+rituximab, demonstrating the survival benefit of this combination therapy and the feasibility of fixed-duration therapy.13,25,26 The data reported herein suggest, but do not prove, that long-term continuous venetoclax therapy is unnecessary for most patients with R/R CLL and support the current standard fixed-duration approach for the venetoclax+rituximab regimen. However, it will require a randomized trial to definitively answer the question of continuous vs limited-duration treatment. In our study, the median time on venetoclax before cessation was 17 months, with 8 patients receiving >24 months of therapy. Most patients who achieved uMRD (28/30; 93%) did so by 24 months, supporting other observations.27 As detailed earlier, improvement in DOR or PFS was not observed in those patients continuing therapy (median time on venetoclax, 5.5 years) compared with limited-duration therapy. uMRD is now well established as correlating with durability of response with venetoclax-based therapies,13,24,28 a point further exemplified in this study, with the most mature follow-up of any venetoclax CLL trial.
Importantly, data from this phase 1b study are the first to demonstrate that retreatment with venetoclax after progression can be effective in reattaining responses that can be durable. The current data show response durations after retreatment in 3 patients ranging from 18.7 to 40.3 months, 2 of which are ongoing.
On the basis of these observations, we have developed the concept of TTVF, which is effectively the sum of the periods of disease control while on therapy, durability of ongoing response after drug withdrawal, and the duration of subsequent response(s) after retreatment. This total duration of treatment benefit (continuous vs intermittent) may be a more meaningful method of comparing treatment approaches, given the option for retreatment, rather than the more traditional PFS. Such an approach was used in the evaluation of rituximab benefit (maintenance vs retreatment) in the RESORT study, in the setting of follicular lymphoma.21
Finally, this analysis of venetoclax and rituximab demonstrated a tolerable long-term safety profile. No new toxicities were reported beyond 2 years of treatment. The only any-grade TEAEs reported in ≥20% of patients were upper respiratory infection, neutropenia, diarrhea, and nausea. The only grade 3/4 TEAEs reported in ≥10% of the patients were neutropenia and leukopenia. Overall, the incidence of neutropenia decreased with time, and notably, there were no instances of febrile neutropenia reported after the first 2 years of treatment.
The lack of additional immune-profile data was a limitation of the study. Our ability to assess the number of immune cells (T, B, and NK cells) was limited by the potential “cross-reactivity” of coexpressed antigens on CLL cells and T cells, as well as difficulties in combining data generated by local laboratories using different methodologies. Given these caveats, we were not able to draw any substantial conclusion from the data.
In summary, with long-term follow-up beyond 5 years, limited-duration therapy with venetoclax in combination with rituximab has durability of effectiveness similar to that of continuous therapy in patients who achieve an initial deep response. The 5-year rate for PFS for both groups was ≥79%, and was achieved with a median of only 1.4 years of venetoclax exposure in the limited-duration therapy group. Venetoclax plus rituximab can be effectively reintroduced upon subsequent off-therapy disease progression in patients who have achieved an initial deep response with the possibility of reattaining disease control. Thus, intermittent time-limited venetoclax treatment is a feasible paradigm with TTVF as a more practical measurement of long-term outcome for venetoclax-based therapy.
Acknowledgments
AbbVie and the authors thank the patients, their families and caregivers, as well as the study investigators, research, and supporting staff. No honoraria or payments were made for authorship. Venetoclax (ABT-199/GDC-0199) is being developed in a collaboration between AbbVie and Genentech.
AbbVie and Genentech funded this study (NCT01682616) and participated in the study design, research, analysis, data collection, interpretation of data, reviewing, and approval of the publication. Medical writing support was provided by Mary L. Smith, CMPP (Aptitude Health, Atlanta, GA) and was funded by AbbVie.
Authorship
Contribution: All authors, but especially S.M., J.F.S., and A.W.R., had access to the data and participated in data collection and interpretation; A.A., A.J., and S.Y.K. performed initial data analysis, and all authors contributed thereafter; and all authors participated in the revision and final approval of the manuscript.
Conflict-of-interest disclosure: S.M. has received institutional research funding from AbbVie, Acerta, BeiGene, Gilead, Incyte, Juno, NCCN, Novartis, Pharmacyclics, and TG Therapeutics and has served on advisory boards and delivered lectures for AbbVie, AstraZeneca, Genentech, Gilead, Kite, Janssen, and Pharmacyclics. J.F.S. has received research funding from AbbVie, Celgene, Janssen, and Genentech and has been a consultant and advisory board member for AbbVie, AstraZeneca, Celgene, Gilead, Janssen, MEI Pharmaceuticals, Nurix, Roche, and Genentech. D.M.B. has been a consultant for Teva, Pharmacyclics, Novartis, and Genentech and an advisory board member and consultant for AbbVie, Genentech, Pharmacyclics, Pfizer, and TG Therapeutics, Verastem; has obtained institutional grants for clinical trials for AbbVie, ArQule, Ascentage, AstraZeneca, BeiGene, DTRM, Genentech, Juno/Celgene/BMS, LOXO, MEI Pharma, Novartis, Pharmacyclics, and TG Therapeutics; and was a panel member for NCCN. T.J.K. has been a consultant for and receives research funding from AbbVie. M.Y.C. has received research funding from AbbVie, has served on the advisory board and has been a consultant for AbbVie and Gilead; has received institutional research funding from AbbVie; has served on the speakers’ bureau for Gilead, Pharmacyclics, and Genentech; and has been a consultant for Pharmacyclics. M.A.A. received research funding from AbbVie and Genentech; is an employee of Walter and Eliza Hall Institute of Medical Research, which receives milestone and royalty payments related to venetoclax for which she receives a financial benefit as a result of previous research related to venetoclax. K.H. is an employee of Roche. A.J. is an employee of Pfizer and a former employee of AbbVie and may hold AbbVie stock or options. S.Y.K. is a former employee of AbbVie and may hold AbbVie stock or options. B.C., A.H.S., J.A., A.A., J.P., and R.N. are employees of AbbVie and may hold stock or stock options. A.W.R. has received research funding from AbbVie, Genentech, BeiGene, Janssen, and Servier; has been an unremunerated advisor to AbbVie Australia; and is an employee of Walter and Eliza Hall Institute of Medical Research, which receives milestone and royalty payments related to venetoclax, for which he receives a financial benefit as a result of previous research related to venetoclax.
Correspondence: John F. Seymour, Department of Haematology, Peter MacCallum Cancer Centre & Royal Melbourne Hospital, Locked Bag 1, A’Beckett St, Melbourne, VIC 3002 Australia; e-mail: john.seymour@petermac.org.
Presented in part at the 60th annual meeting of the American Society of Hematology (ASH), San Diego, CA, 1-4 December 2018, and at the 61st annual meeting of ASH, Orlando, FL, 7-10 December 2019.
AbbVie is committed to responsible data sharing regarding the clinical trials they sponsor. This includes access to anonymized, individual, and trial-level data (analysis data sets), as well as other information (eg, protocols and Clinical Study Reports), as long as the trials are not part of an ongoing or planned regulatory submission. This includes requests for clinical trial data for unlicensed products and indications. This clinical trial data can be requested by any qualified researchers who engage in rigorous, independent scientific research, and will be provided following review and approval of a research proposal and Statistical Analysis Plan (SAP) and execution of a Data Sharing Agreement (DSA). Data requests can be submitted at any time, and the data will be accessible for 12 months, with possible extensions considered. For more information on the process, or to submit a request, visit the following link: https://www.abbvie.com/our-science/clinical-trials/clinical-trials-data-and-information-sharing/data-and-information-sharing-with-qualified-researchers.html.
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
REFERENCES
Author notes
S.M. and J.F.S. contributed equally to this study.