In this issue of Blood, Ma et al1 report the long-term results of treatment with venetoclax plus rituximab, which lends strong support to the benefit of fixed duration rather than continuous therapy for patients with relapsed chronic lymphocytic leukemia (CLL).
Novel agents including the approved B-cell lymphoma 2 (BCL2) inhibitor venetoclax and the Bruton’s tyrosine kinase inhibitors (BTKi) ibrutinib, acalabrutinib, and zanubrutinib have revolutionized the treatment landscape in CLL.2-6 These agents have very largely replaced chemo-immunotherapy in both previously untreated and in relapsed disease. The treatment approach with BTKis is very different from that largely used with venetoclax. BTKis are highly effective agents to treat CLL, but deep remissions are rare, and continuous therapy is required for long-term disease control. On the other hand, venetoclax, particularly when given in combination with anti-CD20 monoclonal antibody (mAb), induces deep remission, with a high probability of achieving eradiation of measurable residual disease (MRD).5,6 It is this ability to induce deep responses that mean that this therapy can be discontinued. Whereas academic studies have often been designed with an MRD eradication end point, the registration studies of venetoclax and anti-CD20 mAbs have been performed as fixed duration therapy: 2 years when using venetoclax plus rituximab (VenR) in relapsed CLL as performed in the Murano trial (NCT02005471)5 or 1-year fixed duration therapy for venetoclax plus obinutuzumab for previously untreated CLL as documented in the CLL14 trial (NCT02242942).6 Notably, venetoclax monotherapy is also approved for continuous treatment.7 This approach raises a number of questions. How was this fixed duration term established? Is the approach correct indeed for all patients or might some patients benefit from more prolonged treatment?
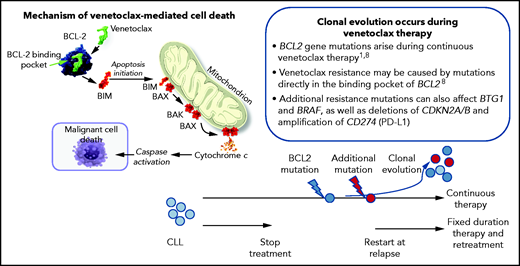
In this report, Ma et al report on the long-term follow-up of the phase 1b study of VenR in 49 patients with relapsed CLL, including outcomes with continuous or limited-duration therapy (NCT01682616).1 Patients received venetoclax daily (200-600 mg) and rituximab over 6 months and then venetoclax monotherapy. Patients achieving complete response (CR), CR with incomplete marrow recovery (CRi), or undetectable MRD (uMRD) assessed by flow cytometry (level of detection <10−4) were allowed, but not required, to discontinue therapy while remaining on study, and could then be re-treated with VenR on progression. At a median follow-up of 5.3 years, the 5-year rate for overall survival was 86%, progression-free survival was 56%, and duration of response was 58%. Of the 40 patients, 33 (67%) were deemed deep responders (CR/CRi or uMRD), of whom 19 stopped treatment after a median of 1.4 years, whereas 14 remained on venetoclax monotherapy as continuous therapy. Intriguingly, the 5-year estimates of ongoing response were similar between limited-duration therapy (79%) and continuous therapy (71%). Among the 19 patients who stopped therapy, 6 patients had subsequent disease progression, occurring at 2.1 to 6.4 years off therapy. Notably, 4 of these patients were retreated with VenR, with partial responses observed in the 3 evaluable to date. At least some of the mechanisms of resistance to venetoclax have been described,8 and in this present study, emergence of BCL2 mutations was seen in 2 patients who progressed in continuous therapy. This raises the issue of whether pressure from continued therapy can contribute to the emergence of resistance (see figure). The authors conclude that VenR induced deep responses that were highly durable with either limited-duration or continuous therapy. Although these patient numbers are small, they demonstrate that successful retreatment with VenR was possible, and the authors conclude that continuous exposure to venetoclax in deep responders does not seem to provide incremental benefit over the fixed duration approach, supporting the limited duration approach. This was the rationale for the design of the Murano trial, which is based on the premise of limited duration therapy.5
Veneteclax binds to the BCL2 binding pocket releasing proapoptotic factors, including BIM and BAX, leading to caspase activation and malignant cell death. Continuous therapy can lead to the development of BCL2 mutations in CLL cells and clonal evolution. This may be prevented by fixed duration theory and pretreatment at relapse and progression of disease.
Veneteclax binds to the BCL2 binding pocket releasing proapoptotic factors, including BIM and BAX, leading to caspase activation and malignant cell death. Continuous therapy can lead to the development of BCL2 mutations in CLL cells and clonal evolution. This may be prevented by fixed duration theory and pretreatment at relapse and progression of disease.
Of course, Murano is not just a limited duration approach but a fixed-duration approach. Although this is not discussed in Ma et al, it has been previously reported that in this M13-365 trial, the vast majority of cases achieved best response within 2 years, and this is true not only of clinical responses5 but also achievement of undetectable MRD.9 In this study, detailed MRD monitoring was performed and has been reported, demonstrating the peak of achievement of MRD eradication occurs within 2 years, and there is little evidence that any patients would benefit in terms of a deeper response by continuing beyond this time period.10
So, what is next in CLL? We have come a long way since the setup of this phase 1b study. However, the importance of this study is that it was the first to show the depth of response that could be achieved with venetoclax combination therapy, and it introduced the concept of limited-duration treatment with a novel agent. There is no doubt that venetoclax and the BTKis represent the best available treatment options for our patients with CLL. Ongoing clinical trials are now addressing the combination approach of venetoclax in combination with BTKi either alone (doublet therapy) or with anti-CD20 mAbs (triplet therapy). There are numerous trials underway, but as an example, the CAPTIVATE clinical trial (NCT02910583) has as its primary end point to assess the MRD-negative response rate and includes randomizations to continue with placebo (as a test of fixed duration therapy) or continuing therapy with either ibrutinib alone if the patient has achieved eradication of MRD or in those patients who remain MRD positive, to be randomized to continue either ibrutinib alone or ibrutinib in combination with venetoclax. The goal then is not simply to stop therapy based on fixed duration but to drive toward the deepest responses in our ultimate goal to achieve the elusive cure for CLL!
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal