In this issue of Blood, Maas-Bauer and colleagues have defined, in unprecedented detail, the genotypic, phenotypic, and functional characteristics of invariant natural killer T-cell (iNKT) subsets, both at steady state and after allogeneic hematopoietic stem cell transplantation (HCT).1 iNKTs are members of the unconventional T-cell family and are defined by their invariant T-cell receptor-α (TCR-α) chain usage (Vα14-Jα18 in mice and Vα24-Jα18 in humans) and a limited repertoire of paired Vβ chains. Although classically differentiated from polyclonal conventional T cells (Tcons) by their reactivity specifically to phospholipid antigens presented by the class 1–like molecule CD1d, iNKTs can also be activated in an antigen-independent fashion (eg, by interleukin 12 [IL-12]).2 Upon activation, they can rapidly gain effector function and acquire an iNKT1, iNKT2, or iNKT17 phenotype. Although the iNKTs have been of significant interest to the transplant field for several years, the specific contribution of each subset to antitumor effects and immunoregulatory effects in allogeneic HCT have remained unclear.3
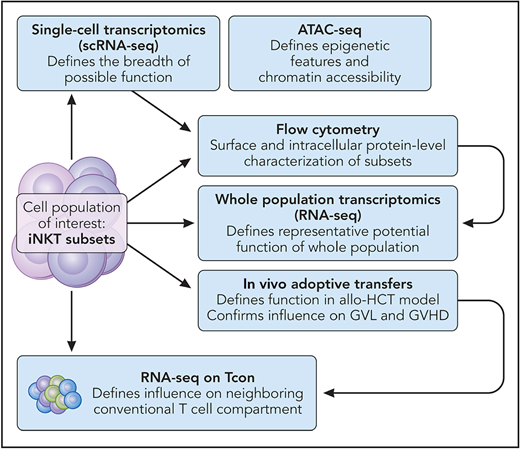
Maas-Bauer, Lohmeyer, et al addressed this knowledge gap by carefully characterizing steady-state mouse iNKT subsets by using state-of-the-art methods (see figure). After starting with single-cell transcriptomics (scRNA-seq) of thymic iNKT subsets, thus defining the breadth of possible gene expression, they next used the scRNA-seq data to establish subset-specific surface markers. To close the loop and confirm that the surface staining strategy faithfully captured the subsets defined by scRNA-seq, the authors next performed bulk transcriptomics (RNA-seq) of sort-purified subpopulations. Subset-specific chromatin accessibility was explored by using ATAC-seq,4 demonstrating that, among other findings, the cytolytic molecules granzyme and perforin (Gzmb and Prf1) are likely to be transcribed most actively by the iNKT1 subset. To further establish the function of each iNKT lineage in mouse models of HCT, iNKT subsets were adoptively transferred alongside Tcons. These experiments demonstrate that the iNKT2 and iNKT17 subtypes have immunoregulatory effects (ie, less graft-versus-host disease [GVHD] develops in their presence) and that iNKT1s have the strongest cytolytic effect against CD1d-expressing tumors in vitro and in vivo, consistent with the ATAC-seq data.
A key challenge in studying any single unique cell type; small-molecule, metabolic pathway; or signaling cascade in models of allogeneic HCT and GVHD, is that GVHD occurs as the net result of an interwoven network of donor- and host-derived circulating factors and cellular interactions.5 This begs the question: in such a complex network, how does our cell type of interest influence the rest of the inflammatory milieu and thus change overall outcome? To investigate, the polyclonal Tcons, which were cotransferred with each iNKT subset were analyzed with RNA-seq. The data suggest that early environmental changes induced by the adoptively transferred iNKTs alter the transcriptional programming of the polyclonal pool. The iNKT2 and iNKT17 subsets appear to drive differential expression of several functionally relevant genes in both the CD4+ and CD8+ Tcon populations (eg, IL27ra and Stat1). These transcriptomic changes were functionally associated with reduced GVHD lethality and less intestinal damage, as measured by traditional histopathologic analysis.
The methodological approach taken by Maas-Bauer et al, which can be broadly applied to any cell subset of interest. Professional illustration by Patrick Lane, ScEYEnce Studios.
The methodological approach taken by Maas-Bauer et al, which can be broadly applied to any cell subset of interest. Professional illustration by Patrick Lane, ScEYEnce Studios.
Unconventional T cells (the iNKT population studied here, as well as mucosa-associated invariant T cells [MAITs] and γδ T cells) are increasingly recognized as contributors to HCT outcome,6 and the adoptive transfer of iNKTs in particular is the focus of several clinical trials of cancer-directed immunotherapy because of their antitumor properties.7 Importantly, it is necessary that these cells undergo an in vitro expansion step before therapeutic transfer, and this study suggests that care should be taken to assess the subset composition of the product to be transferred, to maximize either regulatory or antitumor potential. As unconventional T cells are not donor-HLA restricted, but instead recognize antigens in the context of their particular HLA-like molecules (CD1d for NKT cells and MR1 for MAIT cells) and butyrophilins in the case of γδ T cells, they are likely to be more suitable for development into “off-the-shelf” cellular therapy products in the allogeneic HCT setting than therapies derived from Tcons. With respect to iNKT therapies, they are likely to be most successful if the subset composition is optimized for the desired clinical outcome in accordance with the subset-specific functions defined by Maas-Bauer and colleagues in their study.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal