Key Points
Combined oral azacytidine and romidepsin induced high response rates and prolonged remissions in PTCL patients, particularly those with tTFH.
Mutations of genes involved in DNA methylation and histone deacetylation appear more frequently in patients responding to epigenetic therapy.
Abstract
Peripheral T-cell lymphomas (PTCLs) are uniquely vulnerable to epigenetic modifiers. We demonstrated in vitro synergism between histone deacetylase inhibitors and DNA methyltransferase inhibitors in preclinical models of T-cell lymphoma. In a phase 1 trial, we found oral 5-azacytidine and romidepsin to be safe and effective, with lineage-selective activity among patients with relapsed/refractory (R/R) PTCL. Patients who were treatment naïve or who had R/R PTCL received azacytidine 300 mg once per day on days 1 to 14, and romidepsin 14 mg/m2 on days 8, 15, and 22 every 35 days. The primary objective was overall response rate (ORR). Targeted next-generation sequencing was performed on tumor samples to correlate mutational profiles and response. Among 25 enrolled patients, the ORR and complete response rates were 61% and 48%, respectively. However, patients with T-follicular helper cell (tTFH) phenotype exhibited higher ORR (80%) and complete remission rate (67%). The most frequent grade 3 to 4 adverse events were thrombocytopenia (48%), neutropenia (40%), lymphopenia (32%), and anemia (16%). At a median follow-up of 13.5 months, the median progression-free survival, duration of response, and overall survival were 8.0 months, 20.3 months, and not reached, respectively. The median progression-free survival and overall survival were 8.0 months and 20.6 months, respectively, in patients with R/R disease. Patients with tTFH enjoyed a particularly long median survival (median not reached). Responders harbored a higher average number of mutations in genes involved in DNA methylation and histone deacetylation. Combined azacytidine and romidepsin are highly active in PTCL patients and could serve as a platform for novel regimens in this disease. This trial was registered at www.clinicaltrials.gov as #NCT01998035.
Introduction
The biology of peripheral T-cell lymphoma (PTCL) is driven by widespread epigenetic dysregulation. This notion is supported by the frequent finding of mutations in epigenetic regulators such as ten-eleven translocation-2 (TET2), DNA methyl transferase-3A (DNMT3A), and isocitrate dehydrogenase-2 (IDH2), particularly in patients with the angioimmunoblastic T-cell lymphoma (AITL) or PTCL-not otherwise specified subtypes.1,2 These mutations cause aberrant DNA methylation and transcriptional silencing of tumor suppressor genes.3 Furthermore, a transgenic mouse model expressing a mutation (Gly17Val) in Ras homolog family member A (RHOA), which encodes a small GTPase4,5 in cooperation with a TET2 mutation, was shown to produce spontaneous AITL-like tumors.6-8
A central role of epigenetic disruption in the pathogenesis of PTCL is also indirectly supported by the marked single-agent activity of epigenetic modifiers, including the histone deacetylase (HDAC) inhibitors vorinostat, romidepsin, belinostat, and chidamide.9 These agents produced overall response rates (ORRs) of ∼25%, and are associated with a highly favorable duration of response (DOR).10-13
Our group was the first to report that combinations of epigenetic drugs, including HDAC inhibitors and DNA methyl transferase (DNMT) inhibitors exhibited marked synergy in preclinical models of PTCL across a variety of cell lines and xenograft models.14,15 Furthermore, a unique pattern of gene expression was induced by the combination, which was dramatically distinct from that seen with the single agents.14,15 These data formed the rationale for combining an HDAC inhibitor and a DNMT inhibitor as a novel, chemotherapy-free approach for treating patients with PTCL. In a recently published phase 1 trial, O’Connor et al16 reported that oral 5-azacytidine and romidepsin were significantly more active in patients with PTCL compared with patients with B-cell lymphoma (ORRs, 73% and 10%, respectively). In that experience, a preliminary analysis of mutations in epigenetic regulators suggested that the specific mutational landscape did not correlate with response, although the sample size was small. Herein, we report on the efficacy and safety of oral azacytidine plus romidepsin in a multicenter phase 2 study of molecularly annotated PTCL patients.
Patients and methods
Study design and patients
Patients were required to be age 18 years or older, have an Eastern Cooperative Oncology Group performance status of ≤2, and histologically confirmed treatment naïve or relapsed or refractory (R/R) PTCL. Previous autologous stem cell transplantation (ASCT) and/or allogeneic SCT (allo-SCT) were allowed. Other inclusion criteria were aspartate aminotransferase or alanine aminotransferase levels less than 2× the institutional upper limit of normal; total bilirubin ≤1.5× upper limit of normal; creatinine clearance ≥50 mL/min; absolute neutrophil count ≥1000 cells per μL, and platelet count ≥75 × 109/L. Patients were excluded if they had received chemotherapy or radiotherapy within 2 weeks of study entry; had unresolved adverse events (AEs) caused by recent antineoplastic therapy; were taking >10 mg/day of prednisone or equivalent; had active malignancy; had HIV; had hepatitis A, B, or C infection; were pregnant or nursing; or had an uncontrolled concurrent illness.
The study was approved by all institutional review boards and was conducted according to the provisions of the Declaration of Helsinki and the International Conference on Harmonization Guidelines for Good Practice. All patients enrolled provided informed consent. All authors had full access to all the data in the study.
Treatment
Study drugs were given at the previously established recommended phase 2 doseAzacytidine was administered at a flat dose of 300 mg orally on days 1 through 14.16 Romidepsin was given intravenously (IV) at a dose of 14 mg/m2 on days 8, 15, and 22, on a 35-day cycle. Treatment was given until disease progression, unacceptable toxicity, or withdrawal of consent. Prophylactic granulocyte colony-stimulating factor was allowed at the investigator’s discretion. Additional supportive treatments were allowed as clinically indicated, including antiemetics, antipyretics, antihistamines, analgesics, antibiotics, antivirals, and blood products. The antiemetic regimen used for all patients consisted of transdermal granisetron 3.1 mg once every 24 hours plus metoclopramide 5 mg orally 30 minutes before dosing with azacytidine, along with dexamethasone 12 mg IV plus ondansetron 16 mg IV 30 minutes before each dose of romidepsin. AEs were evaluated by using the Common Toxicity Criteria for Adverse events, version 4.0. The dose of the study drugs was reduced if severe or recurrent nonhematologic or severe, long-lasting, or recurrent hematologic toxicity was recorded. Dose deescalation followed the dose cohorts identified in the phase 1 portion of the trial in reverse order: first to cohort 5 (azacytidine 300 mg on days 1 to 14 and romidepsin 14 mg/m2 on days 8 and 15 every 28 days), then to cohort 4 (azacytidine 300 mg on days 1 to 14 and romidepsin 10 mg/m2 on days 8 and 15 every 28 days), and then to cohort 3 (azacytidine 200 mg on days 1 to 14 and romidepsin 10 mg/m2 on days 8 and 15 every 28 days).16 Further dose reductions were not allowed and prompted permanent treatment discontinuation.
Outcome measures
The primary objective was investigator-assessed ORR (ie, the sum of complete response [CR] and partial response [PR]). Secondary objectives included progression-free survival (PFS), defined as the time from enrollment until disease progression or death from any cause, DOR, overall survival (OS), and identification of potential biomarkers of response. Response assessment was based on the International Harmonization Project Group 2007 Revised Response Criteria.17 Computed tomography or positron emission tomography/computed tomography scans were performed after cycles 2 and 6 and repeated every 3 to 6 months thereafter until disease progression or start of a new therapy. Patients who withdrew from study for reasons other than disease progression were censored at that time.
Next-generation sequencing
Next-generation sequencing (NGS) was performed locally and used 1 of 3 platforms. The first was a custom panel of 465 cancer-associated genes (Columbia Comprehensive Cancer Panel [CCCP]), details of which have been previously reported.16 Briefly, genomic DNA was extracted using the Qiamp Mini Kit or the Qiamp FFPE Kit (Qiagen, Germantown, MD) and fragmented. Sequencing was performed on Illumina HiSeq2500 using Illumina TruSeq v3 paired-end sequencing chemistry (San Diego, CA). Mapping and alignment were performed with NextGene Software (Softgenetics, State College, PA). Germline polymorphisms were excluded after cross-referencing to the population databases (ExAC Browser, 1000 Genomes Project, and Exome Variant Server). The second platform was a custom panel used at the University of Washington School of Medicine (UWSM). In that assay, extracted DNA from samples were amplified with polymerase chain reaction using a custom-developed Illumina TruSeq assay, and validated in a Clinical Laboratory Improvement Amendments (CLIA)-certified laboratory. Targeted or full gene sequencing was performed with subsequent analysis using bioinformatics developed in-house, as previously described.18 Variants were identified after filtering against common reference and local databases, and variants were called with limit of detection set at a variant allele fraction of 0.05. The third was the FoundationOne Heme platform, for which methodologic details were previously reported.19
Statistical analysis
In this open-label, single-arm phase 2 trial, patients were enrolled following a Simon 2-stage design. Patients receiving at least 1 dose of study drug were evaluable for toxicity, and all patients completing at least 2 cycles of therapy were evaluable for response. The data set was locked on 30 May 2020. PFS, DOR, and OS were estimated using the product-limit method of Kaplan-Meier, and the estimated hazard ratios (HRs) and 95% confidence intervals (CIs) were calculated using Cox regression. The average numbers of mutations between responders and nonresponders were compared using a Student t test. All analyses were performed using SAS software, version 9.4.
Results
Patients
Between April 2017 and March 2019, 25 patients were enrolled. Five patients were treated in the dose expansion cohort of the phase 1 study16 and are reported here in aggregate. Baseline patient characteristics are summarized in Table 1. Seventeen patients (68%) had AITL or PTCL of T-follicular helper (TFH) cell phenotype, hereafter collectively referred to as T-cell lymphoma of TFH origin (tTFH). Thirteen patients with R/R disease had received a median of 2 previous therapies (range, 1-6 therapies), and the remaining 10 were treatment naïve.
Efficacy
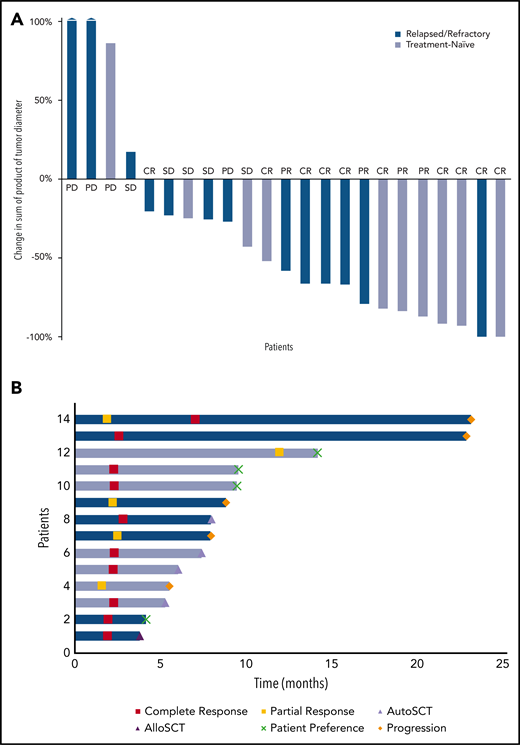
Two patients were not evaluable for response: 1 because of a concurrent malignancy (rectal carcinoma) after an episode of rectal bleeding and 1 for fatal sepsis from autoimmune neutropenia, both before completing 2 cycles of therapy. Among evaluable patients, the ORR was 61% (14 of 23) and the CR was 43% (10 of 23). The 10 treatment-naïve patients exhibited slightly higher response rates, with an ORR of 70% and CR of 50% compared with the 13 patients who had R/R disease (ORR and CR of 54% and 38%, respectively). Patients with tTFH (15 of 23) also seemed to exhibit a slightly higher response rate compared with the aggregate study population, achieving an ORR of 80% and CR of 60% (Table 2). No obvious differences in response rates were noted among the small subset of patients who did not have tTFH histology. Of note, tumor burden decreased by ≥50% in the majority of patients (61%) irrespective of treatment setting (treatment naïve or R/R) (Figure 1A). The dynamics of response are illustrated in Figure 1B. As observed in the phase 1 experience, the depth of response improved over time in some cases. Specifically, 1 patient achieved a PR after 2 cycles and a CR after 6 cycles. One patient had stable disease after 2 cycles and achieved PR after 6 cycles with an ongoing decrease in tumor volume at 1 year. Five patients successfully underwent ASCT (n = 4) or allo-SCT (n = 1), and 1 patient withdrew from the trial to receive consolidative radiation for his localized disease.
Responses to combined oral azacytidine and romidepsin. (A) Waterfall plot demonstrating best response during the study period. (B) PFS (colored bars) and outcome (dashed lines) of responding patients. AlloSCT, allogeneic stem cell transplantation; AutoSCT, autologous stem cell transplantation; PD, progressive disease; SD, stable disease.
Responses to combined oral azacytidine and romidepsin. (A) Waterfall plot demonstrating best response during the study period. (B) PFS (colored bars) and outcome (dashed lines) of responding patients. AlloSCT, allogeneic stem cell transplantation; AutoSCT, autologous stem cell transplantation; PD, progressive disease; SD, stable disease.
Safety
The toxicity profile of azacytidine-romidepsin largely recapitulated that observed during the phase1 study. Seven patients had 1 or more dose reductions of 1 or both study drugs, and 1 patient had to permanently discontinue treatment because of Epstein-Barr virus reactivation (that person was later found to have disease progression). An 83-year-old patient was admitted for neutropenic fever at the end of cycle 2. His course was complicated by aspiration pneumonia, causing supraventricular tachycardia and hypotension, and warm autoimmune hemolytic anemia. During hospitalization, he was found to have biopsy-proven disease progression in the nasopharynx and was discharged to hospice.
Treatment-emergent AEs are described in Table 3. The most commonly reported grade ≥3 AEs were cytopenias, particularly thrombocytopenia (48%), neutropenia (40%), and lymphopenia (32%). Other common toxicities were mild, transient, and readily manageable. Notably, nausea and/or vomiting, common problems encountered during the phase 1 trial, were effectively managed with the aforementioned antinausea regimen.
Time-dependent outcomes
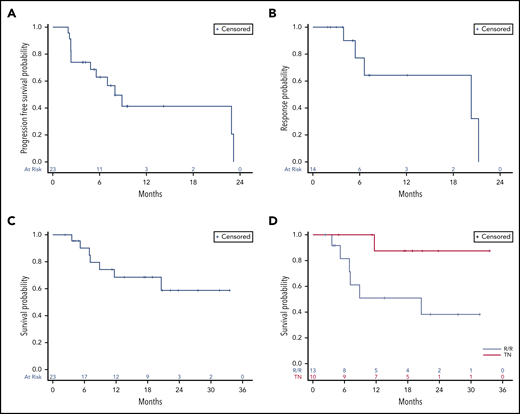
At the time of data cutoff, after a follow-up of 13.5 months (range, 2.3-33.5 months), the median PFS for all patients was 8.0 months (Figure 2A). The median PFS was 8.9 months for patients with tTFH and 2.3 months for those with other PTCL subtypes (HR, 0.3; 95% CI, 0.09-1.06; P = .05). No other baseline characteristic was found to be associated with longer PFS (data not shown). treatment-naïve patients also exhibited a longer PFS compared with patients with R/R disease (not reached vs 8 months), although this difference was not statistically significant (HR, 0.7; 95% CI, 0.21-2.45; P = .58). Responses were generally durable, with a median DOR of 20.3 months (Figure 2B), generally lasting longer in treatment-naïve patients compared with those with R/R disease (not reached vs 13.5 months), although this difference was not statistically significant (HR, 0.5; 95% CI, 0.05-6.08; P = .62). The median OS for the entire population was not reached (Figure 2C) and was significantly longer for patients with tTFH compared with those with other subtypes (not reached vs 9.4 months; HR, 0.2; 95% CI, 0.03-1.0; P = .03). The median OS in treatment-naïve patients and those with R/R disease were not reached and 20.6 months, respectively, as shown in Figure 2D.
Time-dependent outcomes. (A) PFS, (B) DOR, and (C) OS for all patients. (D) OS for treatment-naïve (TN) patients and for those with R/R disease.
Time-dependent outcomes. (A) PFS, (B) DOR, and (C) OS for all patients. (D) OS for treatment-naïve (TN) patients and for those with R/R disease.
Mutations and response
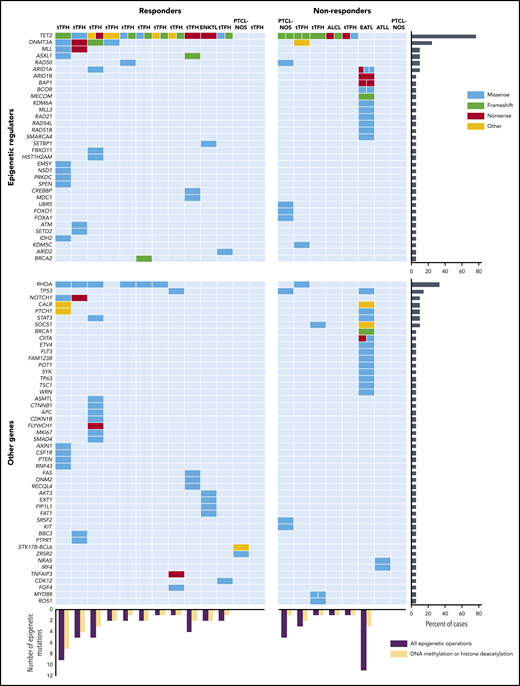
To explore the potential relationship between mutational landscape and response to therapy, we performed multigene NGS on pretreatment samples in 22 patients (Figure 3). NGS data were missing in 3 patients because of unavailability of tumor tissue. The CCCP panel was used in 14 patients, the UWSM custom panel in 5, and the FoundationOne Heme panel in 3. Lists of genes included in each panel are reported in the supplemental Appendix, available on the Blood Web site.
Relationship between somatic mutations and response to oral azacytidine and romidepsin. The chart compares and contrasts responders and nonresponders and epigenetic vs nonepigenetic genes. Each row represents a gene, and each column represents a patient. The number of separate colored boxes in a given gene/patient box denotes the number of mutations in that gene. ALK-ALCL, ALK-negative anaplastic large cell lymphoma; ATLL, adult T-cell leukemia/lymphoma; EATL, enteropathy-associated T-cell lymphoma; ENKTCL, extranodal natural killer (NK)/T-cell lymphoma; NOS, not otherwise specified.
Relationship between somatic mutations and response to oral azacytidine and romidepsin. The chart compares and contrasts responders and nonresponders and epigenetic vs nonepigenetic genes. Each row represents a gene, and each column represents a patient. The number of separate colored boxes in a given gene/patient box denotes the number of mutations in that gene. ALK-ALCL, ALK-negative anaplastic large cell lymphoma; ATLL, adult T-cell leukemia/lymphoma; EATL, enteropathy-associated T-cell lymphoma; ENKTCL, extranodal natural killer (NK)/T-cell lymphoma; NOS, not otherwise specified.
All but 4 patients had 1 or more mutations in epigenetic genes. Seventeen patients (77%) had 1 or more mutations of TET2. Among 15 evaluable patients with tTFH, the prevalence of mutations of TET2, DNMT3A, IDH2, and RHOA was 93%, 33%, 7%, and 47%, respectively. Because the presence of TET2 abnormalities was proposed as a potential biomarker of response to hypomethylating agents,20 we analyzed response rates according to the presence of these mutations in our population. Objective and complete responses were seen in 11 (69%) and 9 (53%) of the 16 TET2-mutated patients, respectively, and 2 (40%) and 1 (20%) of the 5 patients with wild-type mutations, with no statistically significant differences between the results in this small sample.
Both azacytidine and romidepsin are expected to affect the epigenome globally rather than target selective epigenetic pathways. Therefore, we sought to correlate responses with the number of somatic mutations in general, mutations in any epigenetic regulator (ie, genes involved in DNA methylation, chromatin remodeling, histone methylation, histone acetylation, histone readers, histone genes, and those coding for certain metabolic enzymes, such as IDH2), and mutations in genes specifically involved in DNA methylation, histone methylation, or histone acetylation (predictably targeted by azacytidine and romidepsin, respectively). The average numbers of mutations per patient in each of these 3 categories were 5.5, 2.7, and 2.0, respectively, among responders and 5.8, 2.8, and 1.1, respectively, in nonresponders (Figure 3). There were no statistically significant differences between these findings. A nonresponding patient with enteropathy-associated T-cell lymphoma exhibited a much higher number of somatic mutations than any other patient on study and was, therefore, considered a bona fide outlier (Figure 3). Upon removal of this patient from the analysis, the average number of mutations in nonresponders for each of the abovementioned categories was 2.9, 1.6, and 0.9, respectively. In this specific scenario, the difference in average number of mutations per patient in genes involved in DNA methylation, histone methylation, or histone acetylation between responders and nonresponders approached statistical significance (2.0 vs 0.9; P = .06).
Discussion
In this study, we found that the combination of azacytidine and romidepsin is a safe and effective regimen for treatment-naïve patients and R/R PTCL, with response rates being slightly higher in the former.16 This trial was conceived on the basis of collective evidence suggesting that the PTCL may have a unique vulnerability to epigenetic modulators. Indeed, patients with tTFH, a subtype with especially high frequency of mutations in epigenetic regulators, seemed to exhibit slightly higher ORR and CR compared with other PTCL subtypes. Our data support the notion that targeting the PTCL epigenome with 2 distinct classes of epigenetic drugs can produce frequent and durable responses, suggesting that chemotherapy may not be a requisite to achieve clinically meaningful benefit. Our results also support the use of oral azacytidine and romidepsin in both the first-line and R/R settings, including as a bridging therapy. Patients who were treatment naïve exhibited a higher response rate and trended toward having more durable responses. The most common AEs included thrombocytopenia, neutropenia, and anemia, all of which were readily managed with dose delays and, rarely, with granulocyte colony-stimulating factor. Similarly, nonhematologic AEs consisted mostly of nausea and diarrhea, which were mitigated by supportive medications. Among responders, 4 patients elected to withdraw their consent. Three of them remained in remission after a median follow-up of 17.6 months (range, 4.2-18.9 months), and 1 patient in PR started chemotherapy with cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP). Four patients proceeded to ASCT. Three are in remission after 20.8 months (range, 11.2-33.5 months), and 1 died of complications from the transplant. One patient proceeded to allo-SCT but relapsed 3 months later.
Recognizing the small numbers of patients, the potentially higher response rate, and longer median PFS among patients with tTFH relative to those with other PTCL subtypes suggests that the tTFH histology may be more vulnerable to epigenetic modifiers. Larger numbers of patients who do not have tTFH histology will need to be treated with azacytidine and romidepsin to enable further commenting on the performance of this combination outside the tTFH setting. Another notable finding in our trial is that the PFS of patients with R/R disease compared favorably (∼8 months) with the historical benchmark of around 3 months reported in most other studies.21 Importantly, the median survival of 20.6 months observed in this subset of patients seems substantially longer than the previously reported median OS of 5.5 months.21 Compared with other epigenetic therapy-based combinations, oral azacytidine and romidepsin are both efficacious and well tolerated. The experience with the combination of romidepsin and duvelisib demonstrated that 65% of 22 patients with PTCL developed grade 3 or 4 AEs, had an ORR of 55% (CR, 27%), and a median PFS of 8.8 months.22 Conversely, romidepsin and lenalidomide resulted in grade 3 or higher AEs in 71% of 21 PTCL and cutaneous T-cell lymphoma patients, an ORR of 50%, with no CR in 10 PTCL patients, and a median event-free survival of 13.5 weeks.23 Finally, the combination of panobinostat and bortezomib led to grade 3 or 4 thrombocytopenia in 68% and neutropenia in 36% of 25 PTCL patients, an ORR of 43%, and a CR of 22%.24 Delarue and colleagues20 had previously reported, in abstract form, that a retrospective analysis of single-agent IV azacytidine produced an ORR of 53% in 19 patients with R/R PTCL, 10 of whom had concurrent myeloid neoplasms. In a subsequent report focused only on patients with AITL (half of whom were diagnosed with a concurrent myeloid neoplasm) from the same series, the ORR was 75% with a CR of 50%. Although azacytidine was active as a single agent in that experience, a direct comparison of results is unjustified, given the small numbers in both studies, the presence of previously untreated patients in our study, the co-existence of a myeloid neoplasm in many patients and, importantly, the selection bias inherent to retrospective studies.25
To our knowledge, this is the largest report of molecularly annotated PTCL patients treated with epigenetic modifiers and the first to address the question of whether mutations in the epigenome predict sensitivity to epigenetically predicated therapies. In a study by Lemonnier et al,25 a correlation between TET2 mutation status and the probability of response to azacytidine could not be established, even though all patients with AITL had mutations in TET2. Similarly, a correlation between TET2 mutation status and response could not be demonstrated in either our previously published phase 1 data16 or in this phase 2 experience, largely because of the small sample size and the limited number of patients with wild-type TET2.
We speculate that both azacytidine and romidepsin are likely to counter multiple epigenetic abnormalities rather than merely the effects of a single gene mutation. In line with this hypothesis, we found that responding patients seemed to exhibit a higher average load of mutations in genes coding for DNA methyltransferases, histone methyltransferases, or HDACs compared with nonresponders, although this difference was not statistically significant. Our results suggest that a broader array of mutations in epigenetic regulators, rather than a single genetic alteration, may serve as a more sensitive biomarker predictive of response to epigenetically predicated therapies in patients with PTCL. Studies that explore novel epigenetic therapies in PTCL should prospectively incorporate unbiased epigenetic mutation analyses to better understand their association with response. In this sense, we would caution against broad generalizations regarding the vulnerability of one subtype over another to these or other therapies, at least until they can be compared directly in controlled studies. Finally, although responders seemed enriched in patients with RHOA mutations, the absence of mutations in patients without the tTFH phenotype and the very small number of nonresponders within the tTFH group preclude conclusions regarding a correlation between RHOA mutations and the likelihood of response to azacytidine-romidepsin
This study, like many others in this disease, carries the usual limitations including small sample size, the heterogeneity of patients in terms of both histology and treatment history, the relatively short duration of follow-up, and the use of different NGS platforms to analyze mutational landscapes in a small group of patients.
Results from our preclinical work have demonstrated that the combination of azacytidine and romidepsin induces expression of numerous cancer-testis antigens which, in theory, may enhance tumor immunogenicity, thereby creating a potential rationale to build on this doublet by integrating programmed cell death protein 1/programmed death-ligand 1 (PD-1/PD-L1)–targeted drugs into this regimen.14,26 To this end, 2 studies led by Marchi et al27 are evaluating the addition of immune checkpoint inhibitors to various epigenetically predicated combinations (NCT03240211 and NCT03161223). Preliminary results from these trials seem promising.
In summary, the results of this study suggest that the combination of oral azacytidine and romidepsin are highly active in patients with PTCL, especially those with a tTFH phenotype or who are treatment naïve. No clear molecular biomarker has emerged as being predictive of response, although clearly more research is needed to validate gene panels of interest. There is a strong rationale with supportive preclinical evidence for the next generation of studies exploring integration of immune checkpoint inhibitors to develop novel immunoepigenetic platforms for patients with PTCL.
Presented in part in abstract form at the 60th annual meeting of the American Society of Hematology, San Diego, CA, 1-4 December 2018, and at the 15th International Conference on Malignant Lymphoma, Lugano, Switzerland, 18-22 June 2019.
Deidentified individual participant data that underlie the results reported in this article will be made available after deidentification and for up to 3 years after the publication. Interested individuals should submit a written request to the corresponding author Owen A. O’Connor at owenaoconnor27@gmail.com. Only requests that contain a methodologically sound, clearly stated proposal will be considered. To gain access, interested requestors will need to sign a data access agreement.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Gina Petroni for help with the statistical analyses. O.A.O. thanks the American Cancer Society for an American Cancer Society Research Professorship. Celgene Corporation provided oral azacytidine and romidepsin free of charge.
This work was supported by the Leukemia & Lymphoma Society Translational Research Project (LLS 6091-09) and the Lymphoma Research Fund at Columbia University.
The funders of the study had no role in designing the clinical study, collecting or analyzing data, or writing the report.
Authorship
Contribution: O.A.O. conceived and designed the study; L.F., H.M., S.K., J.K.L., F.M., E.M., C.D., H.A.K., A.R., A.T.J., C.K., M.M.F., C.R.S., D.C.P., G.B., R.N., D.M., L. Scotto, L. Sokol, A.R.S., and O.A.O. acquired the data; L.F., H.M., J.K.L., F.M., R.N., C.R.S., D.C.P., A.R., A.T.J., D.M., A.R.S., L. Sokol, and O.A.O. analyzed and interpreted the data; M.F.F. provided radiological interpretation; R.N., C.R.S., D.C.P., and G.B. provided correlative analyses; and L.F., H.M., J.K.L., A.R., D.M., C.R.S., and O.A.O. wrote the paper.
Conflict-of-interest disclosure: L.F. received research funding from Hoffmann-La Roche and Genmab (outside of this work); J.K.L. received research funding from Kymera Therapeutics; served on the scientific advisory board of Kymera Therapeutics, Astex Pharmaceuticals, and Kura Oncology; received consulting fees from Daiichi Sankyo; and served on the speakers bureau for AstraZeneca. F.M. received research funding from Seattle Genetics. E.M. received research funding from Merck, Celgene/Bristol Myers Squibb, Astex Pharmaceuticals, and Acrotech; served on the scientific advisory board for Acrotech, Mundipharma, and Myeloid Therapeutics; and served on the data safety monitoring committee for Everest Clinical Research. D.M. is an employee of and owns equity, patents, and royalties at Bristol Myers Squibb. L. Sokol served on the advisory board for Kyowa-Kirin and Kymera Therapeutics. A.R.S. received consulting fees from Portola Pharmaceuticals and Kyowa-Hakko-Kirin, and travel support and research funding from Celgene/Bristol Myers Squibb, Verastem, and Spectrum. O.A.O. received a salary from TG Therapeutics with an equity stake; received consulting fees and has an equity stake in Kymera Therapeutics, Nomocan, and Myeloid Therapeutics; served as consultant scientific adviser for Mundipharma; and received research funding from Kymera Therapeutics, Merck, Astex Pharmaceuticals, Celgene/Bristol Myers Squibb, Trillium Therapeutics, and Affimed. The remaining authors declare no competing financial interests.
The current affiliation for L.F. is Lymphoma Service, Memorial Sloan Kettering Cancer Center, New York, NY.
The current affiliation for H.M. is Long Beach VA Health Care System, Long Beach, CA.
Correspondence: Owen A. O’Connor, University of Virginia Cancer Center, University of Virginia, 1300 Jefferson Park Ave, MSB, Room 6002, Charlottesville, VA 22908-0716; e-mail: owenaoconnor27@gmail.com.
REFERENCES
Author notes
L.F. and H.M. contributed equally to this study.