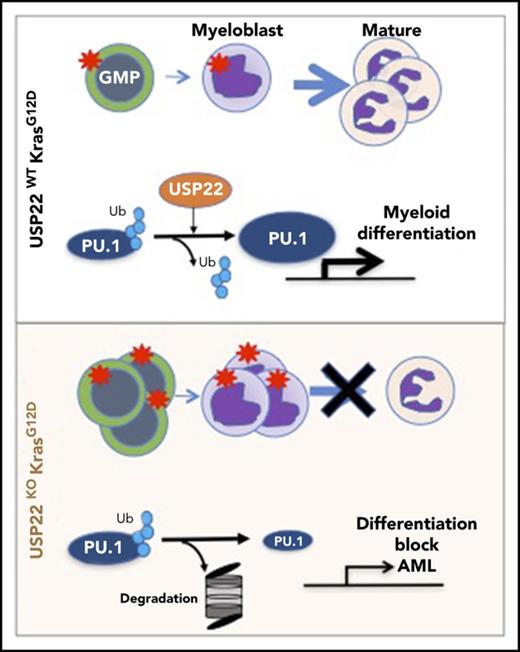
Key Points
USP22 deficiency in Ras-driven myeloproliferative neoplasm blocks myeloid differentiation promoting acute myeloid leukemia.
USP22 is a PU.1 deubiquitylase that positively regulates PU.1 stability and the expression of myeloid-differentiation genes.
Abstract
Ras mutations are commonly observed in juvenile myelomonocytic leukemia (JMML) and chronic myelomonocytic leukemia (CMML). JMML and CMML transform into acute myeloid leukemia (AML) in about 10% and 50% of patients, respectively. However, how additional events cooperate with Ras to promote this transformation are largely unknown. We show that absence of the ubiquitin-specific peptidase 22 (USP22), a component of the Spt-Ada-GCN5-acetyltransferase chromatin-remodeling complex that is linked to cancer progression, unexpectedly promotes AML transformation in mice expressing oncogenic KrasG12D/+. USP22 deficiency in KrasG12D/+ mice resulted in shorter survival compared with control mice. This was due to a block in myeloid cell differentiation leading to the generation of AML. This effect was cell autonomous because mice transplanted with USP22-deficient KrasG12D/+ cells developed an aggressive disease and died rapidly. The transcriptome profile of USP22-deficient KrasG12D/+ progenitors resembled leukemic stem cells and was highly correlated with genes associated with poor prognosis in AML. We show that USP22 functions as a PU.1 deubiquitylase by positively regulating its protein stability and promoting the expression of PU.1 target genes. Reconstitution of PU.1 overexpression in USP22-deficient KrasG12D/+ progenitors rescued their differentiation. Our findings uncovered an unexpected role for USP22 in Ras-induced leukemogenesis and provide further insights into the function of USP22 in carcinogenesis.
Introduction
Somatic mutations in Ras have been found recurrently in acute lymphophastic leukemia and myeloid leukemias.1 These mutations usually occur in the RAS genes, upstream activators such as FLT3, PTPN11, and NF1. Mutations in the Ras pathway have been associated with initiating events in which these mutations are necessary for early stages of leukemia development, as well as cooperative events necessary for continuous growth.2 Activation of the RAS signaling pathway leads to multiple intracellular changes, including the deregulation of transcriptional programs.3
NRAS or KRAS mutations are found in ∼20-40% of myeloid malignancies and in 10-15% of acute lymphoblastic leukemia.4,5 Expression of the most common KRAS mutation (G12D) in mouse hematopoietic cells causes a fatal myeloproliferative neoplasm (MPN).6,7 The disease observed in these mice resembles the human juvenile myelomonocytic leukemia (JMML) and chronic myelomonocytic leukemia characterized by leukocytosis, splenomegaly, and anemia.
The deubiquitylase ubiquitin-specific peptidase 22 (USP22) is part the epigenetic regulator complex Spt-Ada-GCN5-acetyltransferase. Most of the studies addressing USP22 function have been performed in yeast; few studies have been performed in mammalian cells. USP22 is important for the differentiation of embryonic stem cells in vitro,8 and its germ line deletion is lethal in mice.9 USP22-hypomorphic mice have defects in the brain and small intestine.10 USP22 was initially identified as part of a BMI1 signature. Elevated expression of this signature is associated with cancer development and chemotherapy resistance in different types of cancer, including hematological malignancies.11 Reports using cancer cell lines suggest that USP22 may behave as an oncogene. USP22 controls cell growth by activating transcription12,13 and inhibiting apoptotic pathways.9 USP22 functions involve the remodeling of chromatin by removing ubiquitin from histones (H2B and H2A) and activation/stabilization transcription factors.14,15 Moreover, analysis of human acute myeloid leukemia (AML) samples with an FLT3-ITD mutation showed higher expression of USP22 than did samples without FLT3 mutations.16 Despite the above-mentioned evidence regarding the role of USP22 in hematological malignancies, the function of USP22 in normal blood cell development or leukemogenesis is unknown.
To gain insight into the function of USP22 during hematopoiesis and Kras-induced myeloproliferation, we used a transgenic mouse model to genetically delete USP22 using the Cre-lox system. Our results show that USP22 was necessary for myeloid differentiation upon oncogenic Kras activation but not under normal conditions. Absence of USP22 in KrasG12D/+ mice induced the accumulation of myeloblasts in the bone marrow and infiltration of neoplastic cells in vital organs, which resulted in an early death of primary and recipient mice. We showed that USP22 is necessary for the differentiation of myeloid progenitors upon Kras activation by increasing PU.1 protein stability. The expression profile of USP22-deficient KrasG12D/+ myeloid progenitors resembled that of leukemic stem cells and was associated with a poor prognosis in pediatric cases of AML.
Materials and methods
Mice
Usp22F/F mice were generated using embryonic stem cells obtained from the Knockout Mouse Project Repository (clone D11). The neomycin selection cassette was removed by crossing these mice with FLIP recombinase–transgenic mice (supplemental Figure 1A, available on the Blood Web site). Usp22F/F mice were born at the expected Mendelian ratios and did not show any phenotypic abnormalities. Usp22F/F mice were crossed with Mx1-Cre and KrasG12D/+ mice. Mice with any genotypic combination of USP22 and Kras, but without Cre expression, were considered wild-type (WT). All mice were on a C57BL/6 genetic background and were maintained at the Northwestern University mouse facility under pathogen-free conditions. Animal studies were carried out according to institutional guidelines and Institutional Animal Care and Use Committee–approved animal protocols.
Histopathology
Organs were fixed in 10% neutral buffered formalin and processed at the Northwestern University Mouse Histology and Phenotyping Laboratory.
Flow cytometry
Bone marrow cells were isolated by crushing femur, tibiae, and hip bones. Splenocytes were isolated by dissociating tissue on a cell strainer. Cells were incubated with fixable viability dye (eBioscience) and blocked with CD16/32 antibody. Staining was performed with CD34 (RAM34), SCA1 (D7), CD16/32 (93), CD48 (HM48-1), CD45.2, CD117 (2B8), Lineage cocktail (BD Bioscience), CD150 (TC15-12F12.2), CD11b (M1/70), Gr1 (RB6-8C5), Ly6G (1A8), and Ly6C (HK1.4). 5-Bromo-2′-deoxyuridine (BrdU)-incorporation analysis was performed following the manufacturer’s instructions (BD Biosciences). Samples were acquired using a FACSCanto II flow cytometer (BD Biosciences) and analyzed using FlowJo software.
Statistics
Statistical analyses were performed with an unpaired 2-tailed Student t test using Prism (GraphPad). The P values <.05 were considered significant. The log-rank (Mantel-Cox) test was used for survival curve analysis.
Additional information can be found in supplemental Materials and methods.
Results
USP22 deletion does not affect myelopoiesis
To investigate the expression of USP22 in hematopoietic cells, we quantified Usp22 mRNA levels in sorted Lin−Sca+cKit+ (LSK) cells, common myeloid progenitors (CMPs), granulocyte-monocyte progenitors (GMPs), megakaryocyte-erythrocyte progenitors (MEPs), and common lymphoid progenitors (CLPs). Usp22 was highly expressed in all of these cell subsets, with the exception of MEPs (supplemental Figure 1B). We generated Usp22F/F mice and crossed them with Mx1-Cre mice to produce Usp22F/FMx1-Cre mice (UKO) to determine the effect of the absence of USP22 during hematopoiesis. Usp22 alleles were deleted and USP22 protein was not expressed in UKO mice after receiving polyinosinic–polycytidylic acid (pIpC) (supplemental Figure 1C-D). The absence of USP22 did not affect body weight or the weight of organs (supplemental Figure 1E). Blood cell counts and the frequency of different bone marrow populations in UKO mice were comparable to those in young and 1.5-year-old WT mice (supplemental Figures 1F-H and 2A-C). We also analyzed apoptosis of myeloid cells from WT and UKO mice and found no differences (supplemental Figure 2D). To further study whether USP22 deletion would affect myelopoiesis under stress conditions, we induced myelotoxic stress with 5-fluorouracil and measured blood cell counts at different time points. Our results showed similar kinetics of hematopoietic recovery of myeloid cells in UKO mice compared with WT mice (supplemental Figure 2E). Based on these results, we concluded that the absence of USP22 did not affect myelopoiesis.
USP22 deficiency accelerates demise of mice with Kras-induced MPNs
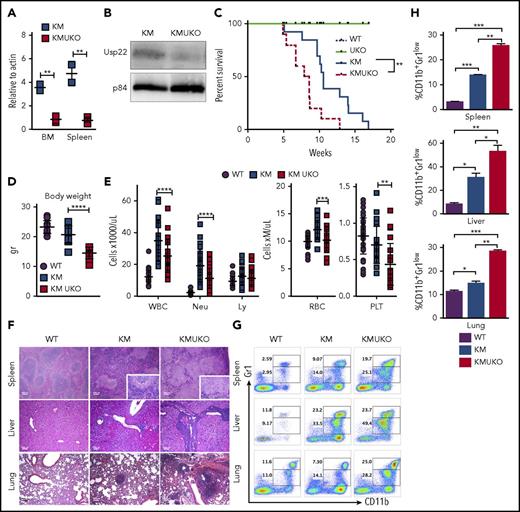
A previous report on myeloid leukemia showed high expression of USP22 in cells with an activated Ras pathway, and USP22 knockdown in an AML cell line inhibited cell growth.16 Thus, we sought to investigate the role of USP22 in an MPN mouse model induced by mutant Kras. Analysis of bone marrow cells from mice with a Kras-induced MPN (KrasG12D/+Mx1-Cre mice [KM]) showed a dramatic increase in USP22 protein levels but not mRNA levels (supplemental Figure 3A-B). Therefore, we hypothesized that USP22 deletion might inhibit Kras-induced MPNs. We generated USP22-deficient KM mice (KMUKO) and injected them with pIpC at 5-7 weeks of age. Unexpectedly, most KMUKO mice died 1-2 days after the first injection, whereas KM mice were still alive (supplemental Table 1). Analysis of bone marrow cells showed a complete deletion of USP22 in KMUKO mice, even without pIpC injection, unlike cells from UKO mice (Figure 1A-B; supplemental Figure 3C). Spontaneous activation of the Kras mutant allele in mice not injected with pIpC has been described previously.6 The fact that KMUKO cells undergoing spontaneous recombination (resulting in the deletion of USP22) became the majority of the cells present in the bone marrow suggests that KMUKO cells had a selective advantage over the nonrecombined cells. Because of the acute death of KMUKO mice after pIpC injection, we did not inject mice with pIpC for the remainder of the studies described in this article.
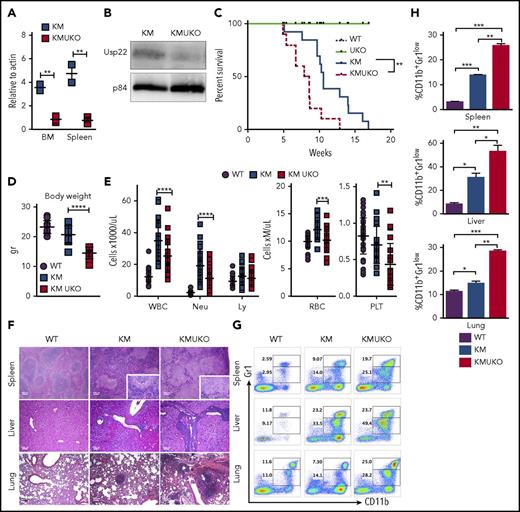
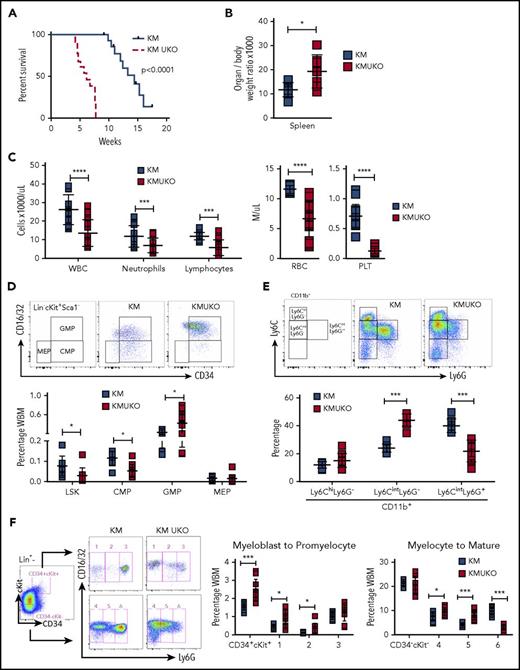
Usp22 deletion in Kras-driven MPN mice decreases mice survival.Usp22 mRNA (A) and USP22 protein (B) expression in bone marrow and spleen cells from KM and KMUKO mice. (C) Survival analysis of WT, UKO, KM, and KMUKO mice. (D) Body weight of 5-8-week-old mice of the indicated genotype. (E) Cell blood counts in WT, KM, and KMUKO mice at 4-7 weeks of age. (F) Hematoxylin and eosin staining of spleen, liver, and lung sections from the indicated mice. Spleen sections from KMUKO mice showed complete effacement of normal architecture by neoplastic cells and reduction of the white pulp. Liver sections from KMUKO mice were severely infiltrated by nonlymphoid cells that occurred with congestion of central veins. Lung sections from KMUKO mice presented areas of consolidation with accumulation of immature dysplastic cells and massive intra-alveolar involvement likely leading to impaired lung function. Scale bars, 100 μm. (G-H) Infiltration of immature myeloid cells in the spleen, liver, and lung in 3-4-week-old WT, KM, and KMUKO mice. *P < .05, **P < .01, ***P < .001, ****P < .0001, unpaired 2-tailed Student t test (A,D-E,H); log-rank (Mantel-Cox) test (C). BM, bone marrow; Ly, lymphocytes; Neu, neutrophils; PLT, platelets; RBC, red blood cells; WBC, white blood cells.
Usp22 deletion in Kras-driven MPN mice decreases mice survival.Usp22 mRNA (A) and USP22 protein (B) expression in bone marrow and spleen cells from KM and KMUKO mice. (C) Survival analysis of WT, UKO, KM, and KMUKO mice. (D) Body weight of 5-8-week-old mice of the indicated genotype. (E) Cell blood counts in WT, KM, and KMUKO mice at 4-7 weeks of age. (F) Hematoxylin and eosin staining of spleen, liver, and lung sections from the indicated mice. Spleen sections from KMUKO mice showed complete effacement of normal architecture by neoplastic cells and reduction of the white pulp. Liver sections from KMUKO mice were severely infiltrated by nonlymphoid cells that occurred with congestion of central veins. Lung sections from KMUKO mice presented areas of consolidation with accumulation of immature dysplastic cells and massive intra-alveolar involvement likely leading to impaired lung function. Scale bars, 100 μm. (G-H) Infiltration of immature myeloid cells in the spleen, liver, and lung in 3-4-week-old WT, KM, and KMUKO mice. *P < .05, **P < .01, ***P < .001, ****P < .0001, unpaired 2-tailed Student t test (A,D-E,H); log-rank (Mantel-Cox) test (C). BM, bone marrow; Ly, lymphocytes; Neu, neutrophils; PLT, platelets; RBC, red blood cells; WBC, white blood cells.
KMUKO mice had a shorter survival time and were smaller in size compared with the other groups (Figure 1C-D). USP22 deletion under oncogenic Kras resulted in a reduction in mature neutrophils, red blood cells, and platelets (Figure 1E). Histopathological analysis of the spleen showed distorted architecture and effacement by neoplastic cells in KMUKO mice. Liver and lung tissues presented mild to severe infiltration and accumulation of immature myeloid cells in KMUKO mice (Figure 1F). We also analyzed the accumulation of myeloid cells in liver, lung, and spleen tissues at 3-4 weeks of age when KM mice have not fully developed MPNs. KMUKO mice showed an early and severe accumulation of immature myeloid cells (CD11b+Gr1low), unlike KM or WT mice (Figure 1G-H). These results indicate that USP22 deletion accelerated the demise of KMUKO mice and that the decrease in blood cells, distorted spleen architecture, and the early accumulation of immature myeloid cells in vital organs were likely contributors to their early death.
USP22 deficiency blocks myeloid differentiation in Kras-induced MPNs
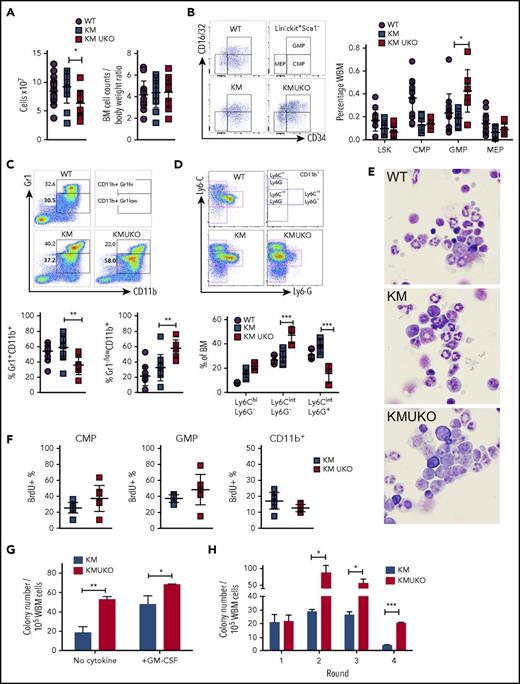
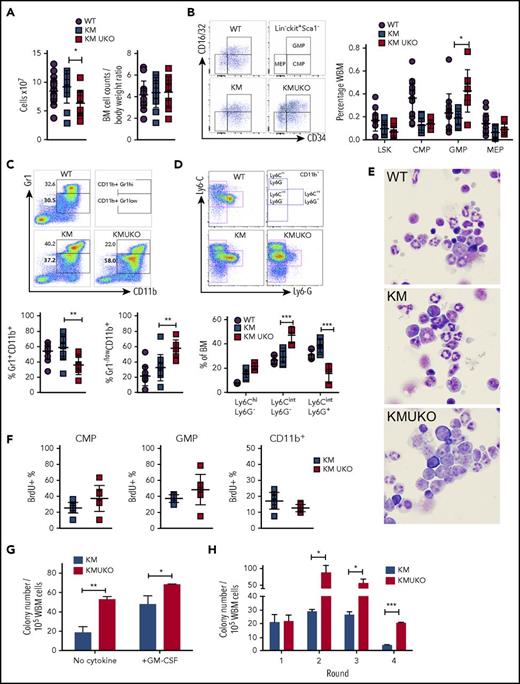
The accumulation of immature myeloid cells in vital organs and the reduction in mature neutrophils in the blood of KMUKO mice prompted us to analyze myeloid cell development in their bone marrow. Total bone marrow cell numbers were decreased in KMUKO mice compared with WT controls and KM mice (Figure 2A). This difference was due to the decreased body weight seen in these mice, because normalization of cell counts to body weight showed no differences among the groups (Figures 1D and 2A). KMUKO mice showed a higher frequency of GMPs, a dramatic decrease in mature myeloid cells (CD11b+Gr1hi), and accumulation of immature myeloid cells (CD11b+Gr1−/low) compared with KM mice (Figure 2B-C). Further analysis using Ly6G and Ly6C antibodies showed an increase in immature myelomonocytic cells and a decrease in Ly6G+ mature cells in KMUKO mice (Figure 2D). Accordingly, bone marrow cytospins confirmed the increased proportion of myeloblasts/immature myeloid cells and a decrease in mature forms in KMUKO mice (Figure 2E). The accumulation of GMPs and reduction in mature myeloid cells in KMUKO mice indicate that USP22 plays an important role in the differentiation of GMPs to mature cells under oncogenic Kras activation.
Usp22 deletion in KM mice blocks myeloid cell differentiation. (A) Bone marrow (BM) cell counts and their normalization to body weight. (B) Frequency of LSK cells, CMPs, GMPs, and MEPs from the indicated mice. (C) Frequency of myeloid cells: Gr1+CD11b+ primarily associated with mature myeloid cells and Gr1−/lowCD11b+ with immature cells. (D) Specific markers for detection of immature myelomonocytic cells (Ly6CintLy6G−), mature neutrophils (Ly6CintLy6G+), and monocytes (Ly6C+Ly6G−) after gating on CD11b+ cells. (E) Cytospin of BM cells isolated from the indicated mice. Wright-Giemsa stain, original magnification ×100. (F) BrdU incorporation analysis in CMPs, GMPs, and CD11b+ cells. (G) Colony-forming assay of whole BM (WBM) cells isolated from KM and KMUKO mice in the absence of cytokines or in the presence of GM-CSF (10 ng/mL). (H) Serial replating assay of BM cells isolated from the indicated mice in the presence of stem cell factor, interleukin-3 (IL-3), and IL-6. Representative data from 4 independent experiments. *P < .05, **P < .01, ***P < .001, unpaired 2-tailed Student t test.
Usp22 deletion in KM mice blocks myeloid cell differentiation. (A) Bone marrow (BM) cell counts and their normalization to body weight. (B) Frequency of LSK cells, CMPs, GMPs, and MEPs from the indicated mice. (C) Frequency of myeloid cells: Gr1+CD11b+ primarily associated with mature myeloid cells and Gr1−/lowCD11b+ with immature cells. (D) Specific markers for detection of immature myelomonocytic cells (Ly6CintLy6G−), mature neutrophils (Ly6CintLy6G+), and monocytes (Ly6C+Ly6G−) after gating on CD11b+ cells. (E) Cytospin of BM cells isolated from the indicated mice. Wright-Giemsa stain, original magnification ×100. (F) BrdU incorporation analysis in CMPs, GMPs, and CD11b+ cells. (G) Colony-forming assay of whole BM (WBM) cells isolated from KM and KMUKO mice in the absence of cytokines or in the presence of GM-CSF (10 ng/mL). (H) Serial replating assay of BM cells isolated from the indicated mice in the presence of stem cell factor, interleukin-3 (IL-3), and IL-6. Representative data from 4 independent experiments. *P < .05, **P < .01, ***P < .001, unpaired 2-tailed Student t test.
Previous reports have implicated a role for USP22 in regulating cell proliferation. In vitro knockdown of USP22 inhibits cell growth.14,17-19 Therefore, we performed proliferation analysis using BrdU. CMPs and GMPs from KMUKO mice were slightly more proliferative than cells from KM mice, showing that USP22 is dispensable for their proliferation. CD11b+ cells showed a mild decrease in the percentage of BrdU+ cells, although the differences were not statistically significant (Figure 2F). We also determined whether KMUKO immature myeloid cells were apoptotic, but we found no differences compared with KM mice (supplemental Figure 3D). This indicates that the reduction in mature myeloid cells upon USP22 deletion was not due to decreased proliferation of myeloid progenitors or apoptosis of myeloid cells. To further validate this notion, we next assessed the ability of KMUKO cells to form colonies in methylcellulose in the absence of cytokines and in the presence of granulocyte-macrophage colony-stimulating factor (GM-CSF) in vitro, as previously described for KM cells.20 Cells from KMUKO mice had increased ability to form colony forming unit-granulocyte/macrophage colonies in the absence of growth factors and in the presence of GM-CSF compared with KM cells (Figure 2G; supplemental Figure 3E). Bone marrow cells from WT and UKO mice did not show any differences (supplemental Figure 3F). Interestingly, a serial colony-forming assay showed that KMUKO cells acquired a leukemia stem cell phenotype by their enhanced ability to form colonies upon multiple rounds of replating (Figure 2H).
It has been shown previously that aberrant activation of Ras in myeloid progenitors results in increased activation of MEK/extracellular signal-regulated kinase (ERK) signaling. This causes hyperproliferation of myeloid progenitors upon stimulation with GM-CSF.21 Therefore, we tested whether MEK/ERK signaling was enhanced in KMUKO myeloid progenitors stimulated with GM-CSF. Our results show that KMUKO cells had slightly lower levels of phosphorylated ERK and S6 compared with KM cells (supplemental Figure 4), indicating that the absence of USP22 did not enhance MEK/ERK signaling.
Collectively, our data show that USP22 deficiency in KM mice blocked myeloid differentiation and was dispensable for the proliferation of myeloid progenitors. This effect led to the accumulation of myeloid progenitors and myeloblasts in the bone marrow.
USP22 deficiency in Kras-mutant myeloid progenitors results in AML
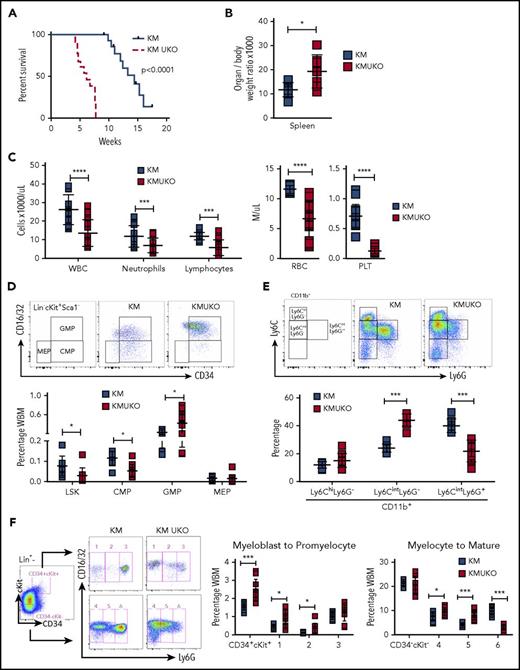
To evaluate whether the phenotype that we observed in KMUKO mice was cell intrinsic and could be recapitulated upon transplantation, we adoptively transferred bone marrow from KMUKO and KM mice into lethally irradiated WT mice. Mice receiving KMUKO cells died 4-7 weeks after transplant, whereas mice transplanted with KM cells survived for >10 weeks (Figure 3A). KMUKO-recipient mice had splenomegaly compared with KM-recipient mice (Figure 3B). In addition, mice receiving KMUKO cells had decreased neutrophil and lymphocyte counts and presented severe anemia and thrombocytopenia (Figure 3C). Control mice transplanted with UKO cells did not show changes in blood cell counts (supplemental Figure 2F-G).
Mice reconstituted with bone marrow cells from KMUKO mice have shorter survival and accumulation of myeloblasts in bone marrow. (A) Survival curve of mice transplanted with 5 × 106 bone marrow cells from KM or KMUKO mice. (B) Spleen weight 5-7 weeks after transplant. (C) Blood cell counts 4 weeks after transplant. Frequency of LSK cells, CMPs, GMPs, and MEPs (D) and Ly6C Ly6G CD11b+ cells (E) in KM- and KMUKO-recipient mice. (F) Analysis of bone marrow cells using Lin (Ter119, B220, CD3, Mac1), CD34, cKit, CD16/32 (FcγRII/III), and Ly6G antibodies. Lin− cells were separated into CD34+cKit+ and CD34−cKit− cells, and expression of CD16/32 and Ly6G was analyzed. KMUKO-recipient mice had increased numbers of myeloblasts (CD34+cKit+ fractions 1 and 2) and decreased numbers of segmented mature neutrophils (CD34−cKit− fraction 6), indicating a block at the myeloblast stage of maturation. *P < .05, **P < .01, ***P < .001, ****P < .0001, unpaired 2-tailed Student t test. PLT, platelets; RBC, red blood cells; WBC, white blood cells.
Mice reconstituted with bone marrow cells from KMUKO mice have shorter survival and accumulation of myeloblasts in bone marrow. (A) Survival curve of mice transplanted with 5 × 106 bone marrow cells from KM or KMUKO mice. (B) Spleen weight 5-7 weeks after transplant. (C) Blood cell counts 4 weeks after transplant. Frequency of LSK cells, CMPs, GMPs, and MEPs (D) and Ly6C Ly6G CD11b+ cells (E) in KM- and KMUKO-recipient mice. (F) Analysis of bone marrow cells using Lin (Ter119, B220, CD3, Mac1), CD34, cKit, CD16/32 (FcγRII/III), and Ly6G antibodies. Lin− cells were separated into CD34+cKit+ and CD34−cKit− cells, and expression of CD16/32 and Ly6G was analyzed. KMUKO-recipient mice had increased numbers of myeloblasts (CD34+cKit+ fractions 1 and 2) and decreased numbers of segmented mature neutrophils (CD34−cKit− fraction 6), indicating a block at the myeloblast stage of maturation. *P < .05, **P < .01, ***P < .001, ****P < .0001, unpaired 2-tailed Student t test. PLT, platelets; RBC, red blood cells; WBC, white blood cells.
KMUKO-recipient mice had increased numbers of GMPs and immature myeloid cells in the bone marrow (Figure 3D-E; supplemental Figure 5A-B). Myeloid differentiation analysis using cKit, CD34, and Ly6G markers22,23 detected a dramatic increase in myeloblasts in KMUKO mice compared with KM mice (Figure 3F; supplemental Figure 5C). These data show that KMUKO-recipient mice presented a similar phenotype to primary mice, and transplantation of these cells resulted in a rapid death of recipient mice. Taking together these results and the phenotype observed in primary KMUKO mice, we concluded that USP22 deficiency in Kras-induced MPNs resulted in the generation of myeloid leukemia.24
Previous reports showed that primary KM mice develop MPN with 100% penetrance, and ∼20% of them also develop T-acute lymphoblastic leukemia (T-ALL) and thymic lymphomas. Upon transplantation, KM recipient mice primarily develop T-ALL/lymphoma.6,25 Primary KM mice and KM-recipient mice used in this study also developed T-ALL/lymphomas with similar frequency. However, primary KMUKO mice, as well as KMUKO-recipient mice, did not develop lymphoid malignancies, as seen by the absence or reduced thymus size compared with KM mice (supplemental Figure 6). Therefore, our data show that, under Kras-oncogenic conditions, the absence of USP22 specifically resulted in an acute myeloid disease and reduced the generation of T-ALL/lymphoma.
The gene-expression profile of KMUKO myeloid progenitors resembles AML
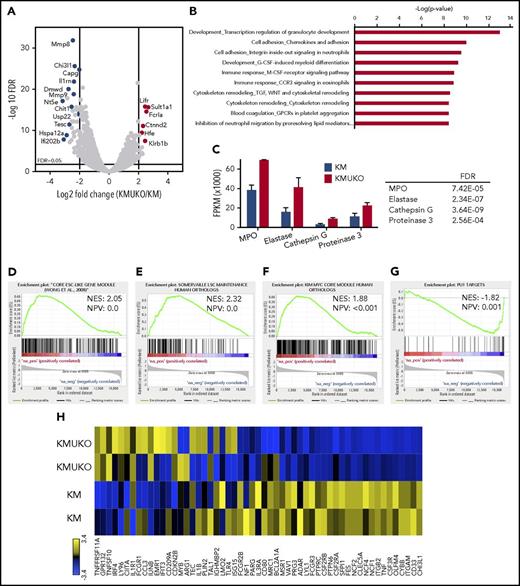
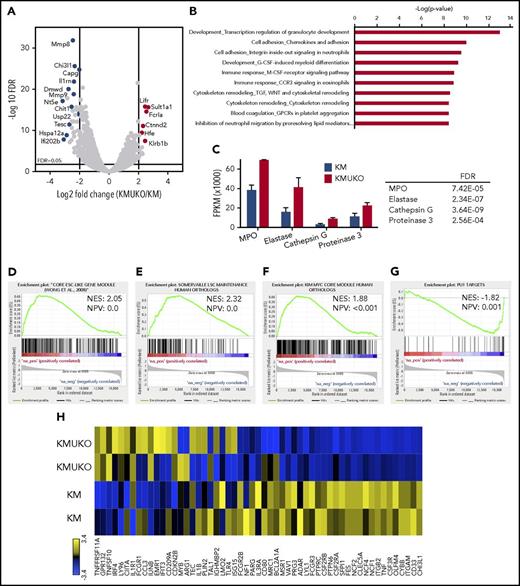
To determine the mechanisms by which the absence of USP22 leads to a myeloid differentiation block and AML in KM mice, we performed transcriptome analysis. We isolated myeloid progenitors (cKit+Lin−/low) from the bone marrow of KM and KMUKO mice and analyzed their gene-expression profile by RNA sequencing. Pathway analysis showed dysregulation of myeloid development and cell adhesion in KMUKO cells compared with KM cells (Figure 4A-B). KMUKO myeloid progenitors expressed high levels of myeloperoxidase, elastase, cathepsin G, and proteinase 3 (Figure 4C), which are highly expressed in myeloblasts, according to the cellular phenotype observed in KMUKO mice. Gene set enrichment analysis (GSEA) showed that KMUKO progenitors were highly enriched for genes expressed in embryonic stem cells and myeloid leukemia stem cells (Figure 4D-E).
Expression profile of KMUKO myeloid progenitors resembles AML. Bone marrow progenitors were isolated from 7-9-week-old KM and KMUKO mice and analyzed by RNA sequencing. (A) Volcano plot analysis of all transcripts in KMUKO vs KM myeloid progenitors. (B) Top 10 pathways affected in KMUKO cells compared with KM cells. (C) mRNA expression of myeloperoxidase (MPO), elastase, cathepsin G, and proteinase 3 in bone marrow progenitors from the indicated mice. GSEA showing that genes upregulated in KMUKO progenitors are significantly correlated with genes associated with embryonic stem cells (D) and leukemia stem cells (E). Targets of the Myc oncogene were highly enriched in KMUKO progenitors (F), whereas PU.1 target genes were inversely correlated (G). (H) Heat map showing PU.1 target genes from analysis in (G).
Expression profile of KMUKO myeloid progenitors resembles AML. Bone marrow progenitors were isolated from 7-9-week-old KM and KMUKO mice and analyzed by RNA sequencing. (A) Volcano plot analysis of all transcripts in KMUKO vs KM myeloid progenitors. (B) Top 10 pathways affected in KMUKO cells compared with KM cells. (C) mRNA expression of myeloperoxidase (MPO), elastase, cathepsin G, and proteinase 3 in bone marrow progenitors from the indicated mice. GSEA showing that genes upregulated in KMUKO progenitors are significantly correlated with genes associated with embryonic stem cells (D) and leukemia stem cells (E). Targets of the Myc oncogene were highly enriched in KMUKO progenitors (F), whereas PU.1 target genes were inversely correlated (G). (H) Heat map showing PU.1 target genes from analysis in (G).
USP22 regulates gene transcription by removing ubiquitin from specific transcription factors and ubiquitylated histone H2B (H2Bub).18 Therefore, we first analyzed the transcription factors and target genes that were affected in KMUKO cells. Targets of Myc and PU.1 showed the greatest changes (supplemental Table 2). Previous reports have shown that a Myc expression signature is required for leukemic transformation in AML.16,26,27 Myc maintains an oncogenic transcriptional program, leading to increased survival and proliferation of leukemia stem cells. PU.1 is an important determinant for the differentiation of myeloid cells. A reduction in PU.1 expression blocks myeloid differentiation and induces AML.28-30 GSEA showed that Myc target genes were overrepresented in KMUKO myeloid progenitors; in contrast, targets of PU.1 were underrepresented (Figure 4F-H; supplemental Figure 7). These results indicate that USP22 deficiency under Kras-oncogenic conditions led to the generation of myeloid leukemia by increasing the expression of Myc target genes and reducing the expression of PU.1 target genes.
USP22 regulates PU.1 and maintains its protein expression
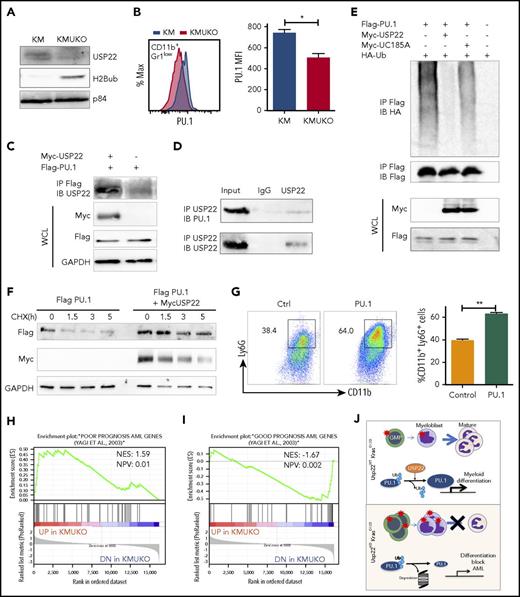
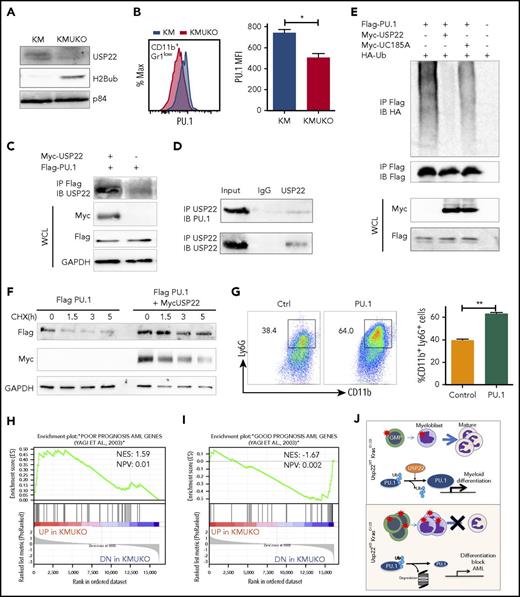
Our gene-expression profile revealed that PU.1 target genes were decreased in KMUKO myeloid progenitors. To further investigate how USP22 regulates gene expression, we measured H2Bub and PU.1 levels in myeloid progenitors. H2Bub levels were increased in KMUKO mice compared with KM mice (Figure 5A). Expression of PU.1 protein was modestly decreased in KMUKO cells compared with KM cells, but it was similar between WT and UKO cells (Figure 5B; supplemental Figure 8). This implied the possibility that USP22 protects PU.1 protein from ubiquitination-mediated degradation under Kras-oncogenic conditions. To support this speculation, we found that USP22 and PU.1 physically interacted, as shown by immunoprecipitation assays in transiently transfected HEK293 cells and endogenously in bone marrow cells from KM mice (Figure 5C-D). We then performed ubiquitylation assays and found that WT USP22, but not its catalytically inactive mutant (C185A), decreased PU.1 ubiquitylation (Figure 5E). As a consequence, USP22 expression significantly increased protein stability of PU.1 (Figure 5F). This indicated that USP22 protects PU.1 from ubiquitination-mediated degradation. If the myeloid-differentiation block that we observed in KMUKO mice was due to reduced PU.1 levels, we speculated that ectopic PU.1 expression could restore myeloid differentiation in KMUKO cells. We then isolated myeloid progenitors from KMUKO mice and transduced them with control or PU.1-expressing retrovirus. Indeed, PU.1 overexpression in KMUKO cells restored the differentiation of myeloid cells, unlike the control retrovirus (Figure 5G). Western blotting confirmed the ectopic PU.1 protein expression and, as a consequence, the expression of PU.1 target genes in KMUKO cells was restored (supplemental Figure 9A-B). Collectively, these results indicate that PU.1 overexpression rescued the differentiation of KMUKO progenitors.
USP22 protects PU.1 from proteasomal degradation. (A) Analysis of USP22 and H2Bub protein expression by western blot in myeloid progenitors. p84 was used as a loading control. (B) PU.1 expression in KM and KMUKO CD11b+Gr1low cells by flow cytometry. (C) USP22 and PU.1 interaction in HEK293 cells overexpressing myc-USP22 and Flag-PU.1. (D) USP22 and PU.1 endogenous interaction in cells isolated from KM mice. (E) PU.1 ubiquitylation in HEK293 cells expressing myc-USP22 WT or C185A mutant. (F) PU.1 protein stability in HEK293 cells overexpressing Flag-PU.1 and myc-USP22. (G) CD11b+Ly6G+ expression in cKit+ KMUKO cells transduced with murine stem cell virus retrovirus control or PU.1 and cultured in methylcellulose with GM-CSF (10 ng/mL) for 7 days. (H) GSEA showing that genes upregulated in KMUKO progenitors were highly correlated with genes associated with pediatric AML with poor prognosis. (I) Inverse correlation between genes downregulated in KMUKO progenitors and genes associated with pediatric AML with good prognosis. (J) Overview of USP22 function in Kras-induced MPNs: Kras activation leads to increased proliferation and differentiation of myeloid cells. Absence of USP22 blocks myeloid differentiation that results in the accumulation of GMPs and myeloblasts, leading to AML. At the molecular level, USP22 interacts and deubiquitylates PU.1, increasing its protein stability. This results in the expression of genes necessary for the differentiation of myeloid cells. In the absence of USP22, the levels of PU.1 protein and its target genes decrease, leading to a differentiation block and leukemic transformation.
USP22 protects PU.1 from proteasomal degradation. (A) Analysis of USP22 and H2Bub protein expression by western blot in myeloid progenitors. p84 was used as a loading control. (B) PU.1 expression in KM and KMUKO CD11b+Gr1low cells by flow cytometry. (C) USP22 and PU.1 interaction in HEK293 cells overexpressing myc-USP22 and Flag-PU.1. (D) USP22 and PU.1 endogenous interaction in cells isolated from KM mice. (E) PU.1 ubiquitylation in HEK293 cells expressing myc-USP22 WT or C185A mutant. (F) PU.1 protein stability in HEK293 cells overexpressing Flag-PU.1 and myc-USP22. (G) CD11b+Ly6G+ expression in cKit+ KMUKO cells transduced with murine stem cell virus retrovirus control or PU.1 and cultured in methylcellulose with GM-CSF (10 ng/mL) for 7 days. (H) GSEA showing that genes upregulated in KMUKO progenitors were highly correlated with genes associated with pediatric AML with poor prognosis. (I) Inverse correlation between genes downregulated in KMUKO progenitors and genes associated with pediatric AML with good prognosis. (J) Overview of USP22 function in Kras-induced MPNs: Kras activation leads to increased proliferation and differentiation of myeloid cells. Absence of USP22 blocks myeloid differentiation that results in the accumulation of GMPs and myeloblasts, leading to AML. At the molecular level, USP22 interacts and deubiquitylates PU.1, increasing its protein stability. This results in the expression of genes necessary for the differentiation of myeloid cells. In the absence of USP22, the levels of PU.1 protein and its target genes decrease, leading to a differentiation block and leukemic transformation.
KMUKO gene-expression profile correlates positively with poor prognosis in pediatric AML
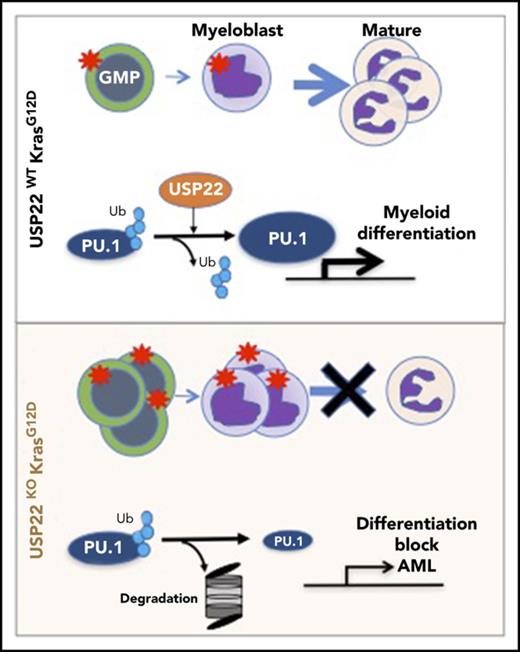
In an attempt to investigate whether our results in mice are relevant to humans, we analyzed publicly available databases of patients with JMML. To our knowledge, none of the data available includes samples from JMML patients before and after transformation into AML. Despite this, we analyzed the gene-expression profile of JMML patients reported by Bresolin et al31 and Helsmoortel et al32 but found no correlation or differences in patient survival with USP22 mRNA expression (supplemental Figure 10). However, in our mouse model, we found that oncogenic Ras activation led to increased USP22 expression only at the protein level without altering its mRNA level (supplemental Figure 3A-B), likely explaining why we do not see differences in human patients. Interestingly, we found a significant correlation between the gene-expression profile of KMUKO myeloid progenitors and the genes associated with poor prognosis in pediatric AML. In contrast, genes downregulated in KMUKO progenitors were inversely correlated with genes expressed in AML cases with good prognosis (Figure 5H-I). These results indicate that, although we did not see a direct correlation or survival difference when analyzing USP22 mRNA, the molecular signature observed in KMUKO progenitors resembles that of pediatric AML with poor prognosis. Overall, our results show that, under oncogenic Kras conditions, USP22 is necessary for the differentiation of myeloid cells by stabilizing PU.1 (Figure 5J).
Discussion
By using a genetic mouse model to study USP22 function under oncogenic conditions for the first time, we uncovered an unexpected role for USP22 in leukemogenesis. Previous studies in vitro have shown that USP22 behaves as an oncogene by promoting cell growth and proliferation.14,17,19 USP22 is highly expressed in different types of cancer and is induced upon Ras activation. Therefore, we hypothesized that USP22 deficiency would inhibit the development of MPNs driven by Ras. However, to our surprise, we found that the absence of USP22 in Kras-driven MPNs resulted in the generation of myeloid leukemia. We further demonstrated that USP22 was necessary for the differentiation of myeloid cells under oncogenic Kras conditions but not under normal conditions. These conclusions are supported by the following discoveries. First, USP22 deficiency in Kras-driven MPNs blocked myeloid differentiation, resulting in the generation of myeloid leukemia and rapid death in primary and transplanted mice. Second, the absence of USP22 reduced PU.1 protein levels and, as a consequence, PU.1 target genes were reduced. Third, ectopic expression of PU.1 in KMUKO progenitors rescued their differentiation. Fourth, the absence of USP22 in Kras-MPN mice resulted in a transcriptome profile that resembled AML with poor prognosis. Fifth, USP22 was dispensable for the differentiation of myeloid cells under steady-state or stress conditions.
Transformation to AML occurs in 10-50% of patients with JMML or chronic myelomonocytic leukemia.33,34 Previous reports have shown that acceleration of MPNs and transformation to AML occurs in KrasG12D/+ mice upon the acquisition of additional cooperative events. These include the loss of DNMT3A, p53, GFI1, RCE1, SPRY4, and Kras WT alleles.35-40 Our study reveals new insights into the mechanisms of transformation into AML. Using the Kras-driven MPN model, we showed that Ras activation induces the expression of USP22 at the protein level. USP22 was necessary for maintaining the expression of PU.1 and its target genes to drive myeloid differentiation. Upon USP22 deletion, PU.1 levels were decreased, leading to a myeloid-differentiation block and transformation into myeloid leukemia. A reduction in PU.1 expression and activity is commonly seen in mouse and human cases of AML.28-30 Likewise, irradiation-induced myeloid leukemias in mice had low PU.1 levels and mutations that affected its binding to the DNA.41,42 Moreover, downregulation of PU.1 was identified as a mechanism for the induction of leukemias generated by PML-RARα, FLT3-ITD, and AML1-ETO.43-45 In addition to PU.1, we observed an increase in H2B monoubiquitylation levels upon USP22 deficiency. H2B monoubiquitylation has been shown to stimulate the activity of DOT1L, a methyltransferase required for leukemia initiation and maintenance.46 Moreover, H2B monoubiquitylation induced by the ubiquitin ligase RNF20 was necessary for leukemic cell growth in MLL-AF9 leukemia.47 Therefore, it is possible that the effect of USP22 on H2Bub also played an important role in the transformation of Kras-MPN into AML.
Previous reports have shown that USP22 is necessary for the stability and transcriptional activation of Myc in fibroblasts and breast cancer cell lines.14 Paradoxically, we found that USP22 deficiency in Kras-MPN mice increased the expression of Myc and its targets genes. Studies in murine models of AML with decreased levels of PU.1 showed an elevated expression of c-Myc.28 Although we speculated that the increased expression of Myc in USP22-deficient Kras-MPN was due to reduced PU.1 levels, overexpression of PU.1 in KMUKO progenitors did not reverse Myc levels (supplemental Figure 9C). Therefore, the elevated Myc expression is unlikely a consequence of PU.1 reduction. Further studies are needed to understand the regulation of Myc by USP22 under oncogenic Ras conditions.
USP22 interacts with different cell cycle regulators, and its decrease impairs proliferation in vitro.14,17,19 However, in the Kras-induced MPN model, USP22 deletion did not have a major impact on cell proliferation. In fact, myeloid progenitors were slightly more proliferative. USP22 deletion also did not lead to apoptosis. It mainly affected the differentiation of myeloid cells. The transcription profile of USP22-knockout cells resembled the profile of embryonic and leukemic stem cells. Previous reports have shown that USP22 was necessary for the transition from self-renewal to differentiation in cultured embryonic stem cells. In this system, USP22 functioned as a transcriptional repressor of SOX2, an essential transcription factor for maintaining self-renewal.8 Moreover, USP22-hypomorphic mice showed differentiation defects in the small intestine and the brain.10 In our model, USP22 was dispensable for myeloid development under normal conditions; however, it was necessary for myeloid differentiation upon Kras activation. This suggests that the function of USP22 changes dramatically under oncogenic conditions. This change also seems to be dependent on the oncogenic drivers and the cell type involved.
The finding that USP22 was necessary for myeloid development under oncogenic conditions, but not under normal conditions, is interesting. We monitored USP22-deficient mice for 2 years and we did not find myeloid abnormalities; these mice did not develop hematopoietic malignancies, and their survival was comparable to WT mice. Therefore, how is it possible that USP22 was necessary for myeloid differentiation only under Kras activation? Oncogenic Ras activation induces aberrant and sustained signaling, leading to the activation of multiple pathways. We found that mutant Kras, by an unknown mechanism, led to a dramatic increase in USP22 protein levels (but not mRNA levels) in myeloid progenitors. Although the changes induced by Ras activation are highly complex, we speculate that increased levels of USP22 protein led to an aberrant interaction with PU.1, which does not occur under normal conditions. Moreover, the absence of USP22 decreased PU.1 protein levels only under Kras-oncogenic activation. Other mechanisms might involve the interaction of USP22 with other transcription factors or the effect of USP22 on H2B and H2A monoubiquitylation levels.
Taken together, our findings provide novel insights into the mechanisms that contribute to the transformation of MPNs into AML. In addition, our results highlight the importance of in vivo studies to understand the function of USP22 under normal and oncogenic conditions.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the Northwestern University Flow Cytometry Core Facility, which is supported by National Institutes of Health, National Cancer Institute Grant P30 CA060553 and National Institutes of Health, Office of the Director Grant 1S10OD011996-01; the Northwestern University Pathology Core Facility, which is supported by National Institutes of Health, National Cancer Institute Grant P30 CA060553; the Northwestern University NUSeq Core; and Matt Schipma for critical discussions and bioinformatics analyses.
D.F. is supported by National Institutes of Health, National Institute of Allergy and Infectious Diseases Grants R01 AI079056, R01 AI108634, and National Institute of Arthritis and Musculoskeletal and Skin Diseases R01 AR006634. J.M.-C. is supported by National Institutes of Health, National Heart, Lung, and Blood Institute Research Service Award Fellowship F31 HL136107-01.
Authorship
Contribution: J.M.-C., Y.X., C.T., S.K., J.W., E.M., and B.G. performed experiments and analyzed data; G.K., J.D.L., J.Y., J.D.C., and P.J. provided critical reagents and intellectual input and contributed to the experimental design and protocols; and J.M.-C. and D.F. designed the study and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Deyu Fang, Department of Pathology, Northwestern University Feinberg School of Medicine, 303 E Chicago Ave, Chicago IL 60611; e-mail: fangd@northwestern.edu