Key Points
Transgenic expression of ASXL1aa1-587 truncating protein in the hematopoietic system leads to diverse myeloid malignancies in mice.
ASXL1aa1-587 gains an interaction with BRD4 and Asxl1Y588XTg hematopoietic stem/progenitor cells are hypersensitive to BET bromodomain inhibitors.
Abstract
Additional Sex Combs-Like 1 (ASXL1) is mutated at a high frequency in all forms of myeloid malignancies associated with poor prognosis. We generated a Vav1 promoter-driven Flag-Asxl1Y588X transgenic mouse model, Asxl1Y588XTg, to express a truncated FLAG-ASXL1aa1-587 protein in the hematopoietic system. The Asxl1Y588XTg mice had an enlarged hematopoietic stem cell (HSC) pool, shortened survival, and predisposition to a spectrum of myeloid malignancies, thereby recapitulating the characteristics of myeloid malignancy patients with ASXL1 mutations. ATAC- and RNA-sequencing analyses revealed that the ASXL1aa1-587 truncating protein expression results in more open chromatin in cKit+ cells compared with wild-type cells, accompanied by dysregulated expression of genes critical for HSC self-renewal and differentiation. Liquid chromatography–tandem mass spectrometry and coimmunoprecipitation experiments showed that ASXL1aa1-587 acquired an interaction with BRD4. An epigenetic drug screening demonstrated a hypersensitivity of Asxl1Y588XTg bone marrow cells to BET bromodomain inhibitors. This study demonstrates that ASXL1aa1-587 plays a gain-of-function role in promoting myeloid malignancies. Our model provides a powerful platform to test therapeutic approaches of targeting the ASXL1 truncation mutations in myeloid malignancies.
Introduction
Next-generation sequencing of whole exomes or whole genomes has provided a comprehensive view of the recurring somatic mutations in myeloid malignancies. Additional Sex Combs-Like 1 (ASXL1) mutations occur at high frequencies in diverse myeloid malignancies, happening in ∼48% of chronic myelomonocytic leukemia, ∼20% of myelodysplastic syndromes (MDSs), ∼10% of myeloproliferative neoplasms (MPNs), and ∼20% of acute myeloid leukemia (AML).1-9 Notably, ASXL1 mutations confer a poor prognosis in patients with these conditions.10,11 Despite the clinical importance of ASXL1 mutations in these diseases, a mechanistic understanding of how these mutations drive leukemogenesis and strategies for tailored therapeutics remain lacking.
We, and others, have reported that the deletion of Asxl1 constitutively or conditionally in the hematopoietic system in mice leads to the development of classic features of MDS, including dysplastic neutrophils and cytopenias.12,13 The ASXL1 gene maps the human chromosome 20q11, a region commonly altered in cancer.14 ASXL1 contains an N-terminal ASX homology (ASXH) domain and a C-terminal plant homeodomain (PHD).15,16 Recent studies have highlighted a critical role of PHD domains in leukemia.17 The majority of patient-derived ASXL1 mutations are nonsense or frameshift causing truncation of downstream of the ASXH domain with consequent loss of the PHD domain.6,7,9,18 Inoue et al reported that truncated forms of the ASXL1 protein were detectable in leukemic samples from patients with ASXL1 mutations.19 However, it remains controversial whether truncating mutations in ASXL1 result in gain or loss of function, or whether they confer dominant-negative activity in vivo.
Inoue and colleagues reported that transplantation of bone marrow (BM) cells transduced with mutated Asxl1 can induce MDS-like disease in recipient mice.20 Balasubramani et al showed that viral-mediated ASXL1-truncation expression in a cell line resulted in a biased mast cell differentiation, suggesting a gain of function of ASXL1 truncations.21 ASXL1 truncation aberrantly enhances the deubiquitinase activity of the ASXL1–BAP1 complex, leading to global erasure of H2AK119Ub and selective upregulation of a subset of genes marked by both H2AK119Ub and H3K4me3.21 In addition to the PHD domain, ASXL1 contains other functional domains, including ASXH, ASXM1, and ASXM2 domains.22 However, whether ASXL1 truncation affects its protein interactome remains unknown.
In this study, we generated a Vav1 promoter-driven Flag-Asxl1Y588X transgenic mouse model, Asxl1Y588XTg, to express a truncated FLAG-ASXL1aa1-587 protein in the hematopoietic system. The Asxl1Y588XTg mice have a shortened survival rate and develop a spectrum of myeloid malignancies, closely recapitulating the characteristics of myeloid malignancy patients with ASXL1-truncation mutations. ASXL1aa1-587, but not full-length ASXL1, interacts with BET bromodomain-containing protein 4 (BRD4). Assay for transposase-accessible chromatin with high throughput sequencing (ATAC-seq) and RNA sequencing (RNA-seq) analyses revealed that hematopoietic stem/progenitor cells (HSC/HPCs) from Asxl1Y588XTg mice had more open chromatin, especially around the transcription state site (TSS) of genes, accompanied by a dysregulated gene expression that alters the balance between HSC self-renewal and differentiation. Epigenetic drug screening assays demonstrated that Asxl1Y588XTg BM cells were sensitive to inhibitors targeting BET bromodomains. The BET bromodomain inhibitors significantly reduced the colony forming capacity of Asxl1Y588XTg HSC/HPCs. This study demonstrates that the truncated ASXL1 protein acquires a gain of function that mediates myeloid malignancies and Asxl1Y588XTg HSC/HPCS are hypersensitive to BET bromodomain inhibitors. This model provides us a powerful platform for testing therapeutic compounds for targeting ASXL1-truncating mutations in myeloid malignancies.
Materials and methods
Generation of Asxl1Y588XTg mice
The entire coding region of mouse ASXL1 full-length with a stop codon mutation on the Y588 site (Asxl1Y588X) was cloned into the HS321/45-vav vector. The plasmid DNA was digested with SacII to remove the pBSIISK backbone and was used for injection into pronuclei of eggs from C57BL/6 mice. Successful expression of Asxl1Y588X (TAC→TGA) was confirmed by RNA-seq analysis (supplemental Figure 1J, available on the Blood Web site).
Drug screening assays
Drug sensitivity screening was performed using whole BM cells of Asxl1Y588XTg and wild-type (WT) mice with an array of 165 epigenetic compounds, covering inhibitors for HDAC, DNA methyltransferase, BET bromodomain, and some other targets. Positive hits were further evaluated for their dose response. Dose response curves were generated for each compound. Modified drug sensitivity score (DSSmod) was calculated as described previously.23
Data availability
All relevant data are available from the authors.
Results
Generation of Asxl1Y588XTg mice
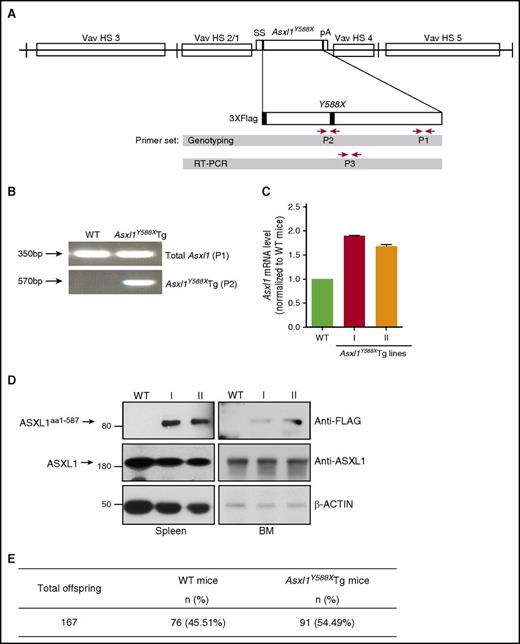
To delineate the role of truncated forms of ASXL1 resulting from patient-derived ASXL1 mutations in hematopoiesis, we generated a transgenic mouse model that mimicked the most frequent nonsense ASXL1 mutation in patients24 using HS321/45-vav vector that expresses the Asxl1Y588X complementary DNA (cDNA) under the control of the Vav1 promoter.25,26 The transgene contains the entire coding region of mouse Asxl1 (full length) with a stop codon mutation at the Y588 site (Asxl1Y588X; Figure 1A). Five Asxl1Y588XTg founder mice were generated and designated as lines I through V. All the founder transgenic mice had a C57BL/6 background. Line I and II mice were viable and fertile; however, line III and V mice were weak, infertile, and died by 8 months of age. Line I and II mice were used for the present study. Genotyping polymerase chain reaction (PCR) analysis using primer pairs P1 (both Tg and endogenous Asxl1) and P2 (Asxl1Y588XTg specific) showed that the Asxl1Y588X cDNA was inserted into the mouse genome (Figure 1A-B). Asxl1Y588X transgene was expressed in the BM cells and the messenger RNA expression of Asxl1Y588XTg was ∼80% and ∼60% of the endogenous AsxL1 in lines I and II, respectively (Figure 1C). ASXL1aa1-587 protein was also detected in the BM and spleen cells of Asxl1Y588XTg mice by western blot (Figure 1D). The endogenous ASXL1 protein level was not affected by the transgene expression. The mean litter size of the Asxl1Y588XTg mice crossing with WT mice was similar to that of WT mice intercrossing (10 ± 2 pups/litter vs 9 ± 2 pups/litter). Genotyping of the pups revealed that the genetic trait of Asxl1Y588XTg mice followed the Mendelian inheritance, similar to WT mice (Figure 1E). Asxl1Y588XTg-negative littermates were used as controls throughout the study.
Generation of Vav1 promoter-driven Asxl1Y588XTg mice. (A) Schematic depiction of the Flag-Asxl1Y588X transgene. P1, P2, and P3 showed the location of the primer pairs used for genotyping and quantitative PCR (qPCR). HS, hypersensitive to DNase-I; pA, polyadenylation region; ss, splice sites. (B) Genotyping PCR using genomic DNA from WT and transgenic mice with 2 sets of primers: P1 (for both transgenic and endogenous Asxl1) and P2 (Asxl1Y588XTg specific). (C) RT-PCR showing the expression level of both endogenous ASXL1 and transgenic mutant Asxl1Y588X using primer set P3. I and II indicate mice lines. Glyceraldehyde-3-phosphate dehydrogenase was used as a control. Error bars represent mean ± standard error of the mean (SEM). mRNA, messenger RNA. (D) Western blots showing ASXL1aa1-587 expression and endogenous ASXL1 expression levels in the spleen (left) and BM (right) cells of WT and Asxl1Y588XTg mice. β-action was used as a loading control. (E) Total offspring number for WT and transgenic mice.
Generation of Vav1 promoter-driven Asxl1Y588XTg mice. (A) Schematic depiction of the Flag-Asxl1Y588X transgene. P1, P2, and P3 showed the location of the primer pairs used for genotyping and quantitative PCR (qPCR). HS, hypersensitive to DNase-I; pA, polyadenylation region; ss, splice sites. (B) Genotyping PCR using genomic DNA from WT and transgenic mice with 2 sets of primers: P1 (for both transgenic and endogenous Asxl1) and P2 (Asxl1Y588XTg specific). (C) RT-PCR showing the expression level of both endogenous ASXL1 and transgenic mutant Asxl1Y588X using primer set P3. I and II indicate mice lines. Glyceraldehyde-3-phosphate dehydrogenase was used as a control. Error bars represent mean ± standard error of the mean (SEM). mRNA, messenger RNA. (D) Western blots showing ASXL1aa1-587 expression and endogenous ASXL1 expression levels in the spleen (left) and BM (right) cells of WT and Asxl1Y588XTg mice. β-action was used as a loading control. (E) Total offspring number for WT and transgenic mice.
Asxl1Y588XTg mice develop myeloid malignancies
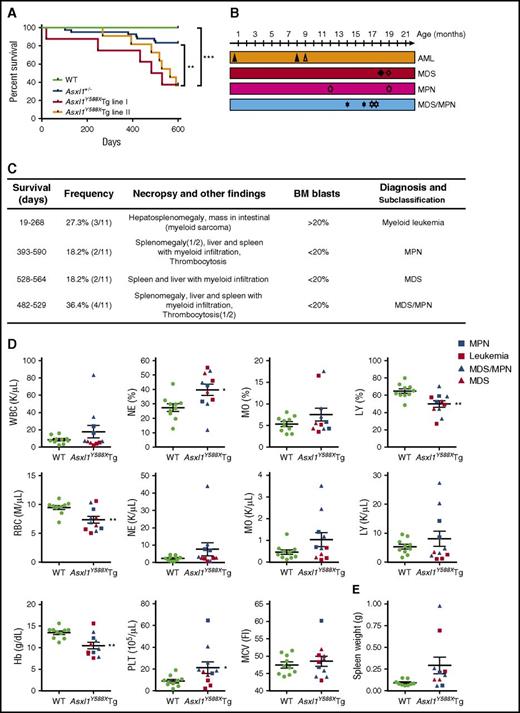
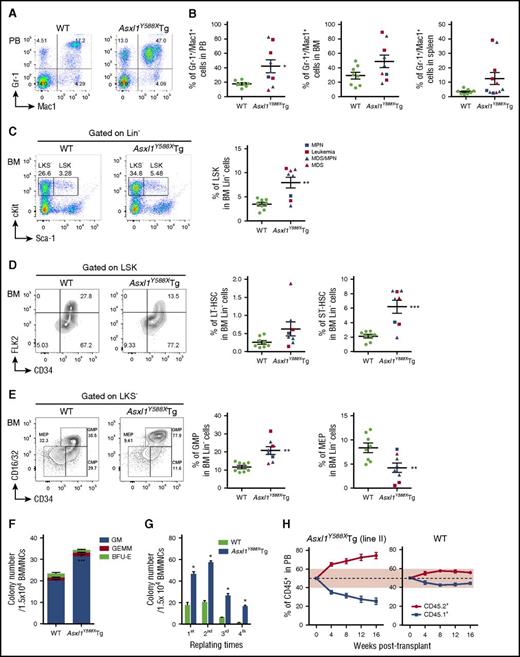
To investigate the impact of transgenic expression of ASXL1aa1-587 on hematopoiesis in vivo, we compared hematopoietic parameters of Asxl1Y588XTg mice with littermate controls and Asxl1+/− mice. Asxl1Y588XTg mice had a significantly shorter mean survival than WT littermates and Asxl1+/− mice (***P < .0001, **P < .01) (Figure 2A). No significant difference was detected for the survival curves between lines I and II. To classify the hematopoietic phenotypes in Asxl1Y588XTg mice, we performed a series of analyses including necropsy, histology, and flow cytometry on peripheral blood (PB), BM, and spleen of the moribund/deceased Asxl1Y588XTg mice. The moribund mice had lower body weight than WT littermates (32.9 ± 1.87 g vs 40.1 ± 2.39 g, *P = .032, n = 9/genotype for each group) (supplemental Figure 1A). Interestingly, Asxl1Y588XTg mice, but not littermate controls, developed progressive myeloid malignancies mostly after 8 months of age, including AML, MPN, MDS, and MDS/MPN (Figure 2B-C). Both lines of Asxl1Y588XTg mice had similar disease phenotypes.
Transgenic ASXL1aa1-587expression leads to myeloid malignancies in mice. (A) Kaplan-Meier survival curve of the Asxl1Y588XTg line I (n = 8), Asxl1Y588XTg line II (n = 11), Asxl1+/− (n = 42), and WT mice (n = 20). ***P < .0001, **P < .01. Log-rank (Mantel-Cox) test was used to assess statistical significance. (B) Timeline of mice developing myeloid malignancies. Solid shapes are mice from line I; the hollow shapes are from line II. (C) Diagnosis and subclassification of the myeloid malignancies developed in 11 aged Asxl1Y588XTg mice. (D) Peripheral blood counts of white blood cell (WBC; P = .242), percent of neutrophils (*P = .017), percent of monocytes (P = .1959), percent of lymphocytes (**P = .005), RBC (**P = .007), neutrophils (P = .2055), monocytes (P = .1174), lymphocytes (P = .3606), hemoglobin (**P = .004), platelets (*P = .048), and mean corpuscular volume (P = .5452) in Asxl1Y588XTg (n = 11) and WT mice (n = 10). (E) Spleen weight of Asxl1Y588XTg and WT mice (WT, n = 8; Asxl1Y588X, n = 9; P = .0643). Blue square, MPN; red square, leukemia; blue triangle, MDS/MPN; red triangle, MDS. *P < .05, **P < .01. Error bars represent mean ± SEM; unpaired t test was used to assess statistical significance.
Transgenic ASXL1aa1-587expression leads to myeloid malignancies in mice. (A) Kaplan-Meier survival curve of the Asxl1Y588XTg line I (n = 8), Asxl1Y588XTg line II (n = 11), Asxl1+/− (n = 42), and WT mice (n = 20). ***P < .0001, **P < .01. Log-rank (Mantel-Cox) test was used to assess statistical significance. (B) Timeline of mice developing myeloid malignancies. Solid shapes are mice from line I; the hollow shapes are from line II. (C) Diagnosis and subclassification of the myeloid malignancies developed in 11 aged Asxl1Y588XTg mice. (D) Peripheral blood counts of white blood cell (WBC; P = .242), percent of neutrophils (*P = .017), percent of monocytes (P = .1959), percent of lymphocytes (**P = .005), RBC (**P = .007), neutrophils (P = .2055), monocytes (P = .1174), lymphocytes (P = .3606), hemoglobin (**P = .004), platelets (*P = .048), and mean corpuscular volume (P = .5452) in Asxl1Y588XTg (n = 11) and WT mice (n = 10). (E) Spleen weight of Asxl1Y588XTg and WT mice (WT, n = 8; Asxl1Y588X, n = 9; P = .0643). Blue square, MPN; red square, leukemia; blue triangle, MDS/MPN; red triangle, MDS. *P < .05, **P < .01. Error bars represent mean ± SEM; unpaired t test was used to assess statistical significance.
Blood counts revealed leukocytosis in 3 of the 11 Asxl1Y588XTg mice. The percentage of neutrophils was significantly increased in most of these mice, whereas monocytosis was observed in 2 of the 11 mice (Figure 2D). The absolute numbers of neutrophils, monocytes, and lymphocytes were also higher in a fraction of these Asxl1Y588XTg mice (Figure 2D). Interestingly, thrombocytosis was seen in 7 of the 11 Asxl1Y588XTg mice, but in none of the mice with AML (Figure 2D). In contrast, a significant decrease in the proportion of lymphocytes, red blood cells (RBCs), and hemoglobin were observed in these Asxl1Y588XTg mice compared with WT controls (Figure 2D), whereas mean corpuscular volume was similar between the 2 genotypes of mice (Figure 2D). Furthermore, most of these Asxl1Y588XTg mice exhibited splenomegaly with higher cellularity compared with WT spleen (Figure 2E; supplemental Figure 1B-C). The BM cellularity of Asxl1Y588XTg mice was comparable to that of WT mice (supplemental Figure 1C). Of note, when the PB parameters between Asxl1Y588XTg and Asxl1+/− mice were compared, transgenic expression of ASXL1aa1-587 caused markedly increased numbers of monocytes, platelets, and lymphocytes in the PB of older mice (supplemental Figure 1E-I). Neutrophil counts were higher in a subset of Asxl1Y588XTg mice compared with WT or Asxl1+/− mice, although the P value did not reach significance.
A fraction of Asxl1Y588XTg mice develop myeloid leukemia
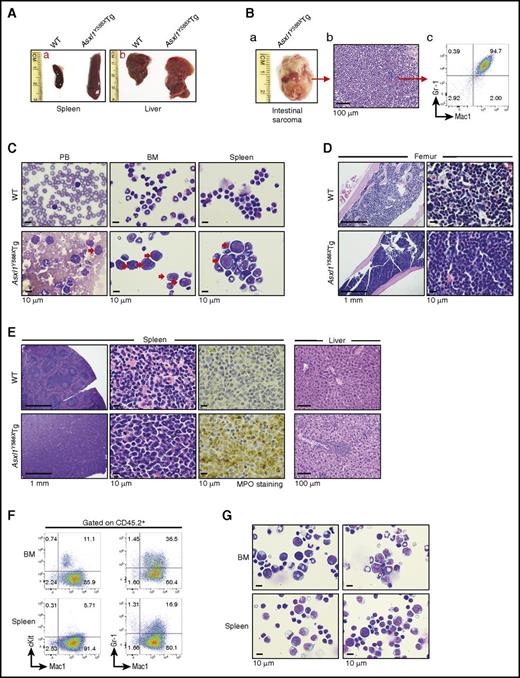
Three moribund Asxl1Y588XTg mice had enlarged spleen and liver (Figure 3A). One had an intestinal myeloid sarcoma, which was verified by histology and flow cytometric analyses (Figure 3B). The PB smear of this mouse showed blast cells; the cytospin preparation of BM and spleen cells confirmed the blast cell count being >20% (Figure 3C; supplemental Figure 2A). Analyses of BM histologic sections revealed an increase in the proportion of myeloid cells and a decrease in erythroid islands in these Asxl1Y588XTg mice (Figure 3D). The histologic analyses of the Asxl1Y588XTg spleen sections showed a disrupted splenic architecture with an increased proportion of myeloid cells (confirmed by myeloperoxidase [MPO] staining) (Figure 3E). In addition, histologic analyses of the liver of these mice revealed infiltration of myeloid cells (Figure 3E). When we transferred 1 × 106 spleen cells from a moribund Asxl1Y588XTg mouse into sublethally irradiated WT recipient mice (supplemental Figure 2B), similar to the donor mouse, these WT recipient mice developed splenomegaly and myeloid blast cell infiltration in their BM and spleen (Figure 3F-G), verifying the malignant nature of the cells from the moribund Asxl1Y588XTg mouse. Given the long latencies of the myeloid leukemia, we reasoned it might be possible that additional mutations occur after ASXL1 mutation. To test this hypothesis, we performed exome sequencing using spleens and tails from the same mice; however, we did not find important mutations in the Asxl1Y588XTg mouse (supplemental Table 3).
A fraction of Asxl1Y588XTg mice developed myeloid leukemia. (A) The gross appearance of spleen and liver of a WT mouse and a representative Asxl1Y588XTg mouse with leukemia. (B) Photographs of a sarcoma from a leukemic Asxl1Y588XTg mouse (left), histological (middle), and flow cytometric (right) analyses showed the predominant granulocytic/monocytic (Gr-1+/Mac1+) cells in the sarcoma. Bar represents 100 µm. (C) May-Giemsa–stained PB smears, BM, and spleen cell cytospins prepared from representative WT (top) and Asxl1Y588XTg mice (bottom). Red arrow, blast cells. Bars represent 10 µm. (D,E) H&E-stained sections of femurs (D), H&E- and MPO-stained spleens (E, left), and H&E-stained livers (E, right) from representative WT (top) and Asxl1Y588XTg mice (bottom). Bars represent 1 mm, 10 µm, 100 μm. (F) Flow cytometric analysis of cKit+/Mac1+ or Gr-1+/Mac1+ cells in the BM and spleen cells of representative recipient mice transplanted with spleen cells from a leukemic Asxl1Y588XTg mice. Cells were gated on CD45.2+. (G) May-Grünwald-Giemsa–stained cytospins of the BM and spleen cells from representative recipient mice transplanted with spleen cells from a leukemic Asxl1Y588XTg mice. Bars represent 10 µm.
A fraction of Asxl1Y588XTg mice developed myeloid leukemia. (A) The gross appearance of spleen and liver of a WT mouse and a representative Asxl1Y588XTg mouse with leukemia. (B) Photographs of a sarcoma from a leukemic Asxl1Y588XTg mouse (left), histological (middle), and flow cytometric (right) analyses showed the predominant granulocytic/monocytic (Gr-1+/Mac1+) cells in the sarcoma. Bar represents 100 µm. (C) May-Giemsa–stained PB smears, BM, and spleen cell cytospins prepared from representative WT (top) and Asxl1Y588XTg mice (bottom). Red arrow, blast cells. Bars represent 10 µm. (D,E) H&E-stained sections of femurs (D), H&E- and MPO-stained spleens (E, left), and H&E-stained livers (E, right) from representative WT (top) and Asxl1Y588XTg mice (bottom). Bars represent 1 mm, 10 µm, 100 μm. (F) Flow cytometric analysis of cKit+/Mac1+ or Gr-1+/Mac1+ cells in the BM and spleen cells of representative recipient mice transplanted with spleen cells from a leukemic Asxl1Y588XTg mice. Cells were gated on CD45.2+. (G) May-Grünwald-Giemsa–stained cytospins of the BM and spleen cells from representative recipient mice transplanted with spleen cells from a leukemic Asxl1Y588XTg mice. Bars represent 10 µm.
Expression of ASXL1aa1-587 can lead to MPN and MDS-like disease in mice
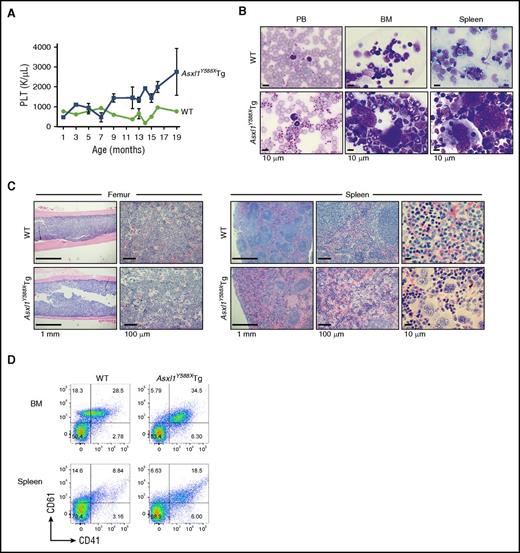
Dynamic PB counts revealed an age-dependent increase of platelets in a significant portion of Asxl1Y588XTg mice, compared with WT mice (Figure 4A), which was confirmed by PB smears (Figure 4B). BM and spleen cell cytospins showed increased numbers of multinucleated megakaryocytes (Figure 4B). Histological sections of BM and spleen of a moribund Asxl1Y588XTg mouse demonstrated disorganized spleen architecture and higher frequencies of large/multinucleated megakaryocytes (Figure 4C). Flow cytometric analyses of BM and spleen cells further confirmed that Asxl1Y588XTg mice contained a higher percentage of CD41+/CD61+ megakaryocytic cells in these organs (Figure 4D). These data indicate that these Asxl1Y588XTg mice developed a MPN-like disease.
A fraction of Asxl1Y588XTg developed MPN. (A) Assessment of platelet (PLT) levels in PB from Asxl1Y588XTg mice (n = 39) and WT littermates (n = 16) at various ages. (B) May-Grünwald-Giemsa–stained PB smears, cytospins of BM and spleens from representative WT (top), and Asxl1Y588XTg mice with MPN (bottom) are shown. Bar represents 10 µm. (C) H&E–stained sections of femurs and spleens from representative WT (top) and Asxl1Y588XTg mice with MPN (bottom) are shown. Bars represent 1 mm, 10 μm, or 100 μm. (D) Representative flow cytometric analysis of CD41+/CD61+ cells in BM and spleen cells of WT littermate (left) and Asxl1Y588XTg mice with MPN (right).
A fraction of Asxl1Y588XTg developed MPN. (A) Assessment of platelet (PLT) levels in PB from Asxl1Y588XTg mice (n = 39) and WT littermates (n = 16) at various ages. (B) May-Grünwald-Giemsa–stained PB smears, cytospins of BM and spleens from representative WT (top), and Asxl1Y588XTg mice with MPN (bottom) are shown. Bar represents 10 µm. (C) H&E–stained sections of femurs and spleens from representative WT (top) and Asxl1Y588XTg mice with MPN (bottom) are shown. Bars represent 1 mm, 10 μm, or 100 μm. (D) Representative flow cytometric analysis of CD41+/CD61+ cells in BM and spleen cells of WT littermate (left) and Asxl1Y588XTg mice with MPN (right).
Besides AML and MPN-like diseases, some Asxl1Y588XTg mice had dysplastic features in PB, BM, and spleen cells, including hyposegmented neutrophils with fine nuclear bridging, aberrant nuclear structure, and Howell-Jolly bodies in the erythrocytes (supplemental Figure 3A). Histologic sections of the femur showed an increased proportion of myeloid cells in Asxl1Y588XTg compared with WT mice. Histological analyses of the spleen, using hematoxylin and eosin (H&E) and MPO staining, showed disrupted spleen architecture with myeloid infiltration (supplemental Figure 3B). These data suggest that transgenic expression of ASXL1aa1-587 in mice leads to hematological characteristics resembling a variety of myeloid malignancies.
Transgenic ASXL1aa1-587 expression in mice increases HSC/HPC pool and skews differentiation toward myeloid lineage
To determine whether ASXL1aa1-587 transgenic expression affects myeloid differentiation in vivo, we performed flow cytometric analyses on PB, BM, and spleen cells of WT and Asxl1Y588XTg mice. An increased proportion of granulocytic/monocytic cells (Gr-1+/Mac1+) was observed in PB, BM, and spleens of Asxl1Y588XTg mice (Figure 5A-B). There is a decreased percentage of B220+ cells in PB and BM of Asxl1Y588XTg mice (supplemental Figure 4A). The proportion of CD4+ and CD8+ cells was comparable in the 2 mouse genotypes (supplemental Figure 4A).
Transgenic expression of ASXL1aa1-587affects HSC pool in vivo. (A) Flow cytometric analysis of Gr-1+/Mac1+ cell population in PB of representative WT (left) and Asxl1Y588XTg mice (right, 8 months old). (B) Quantitation of the percent of Gr-1+/Mac1+ cell populations in PB (*P = .024), BM (P = .074), and spleen (P = .080) of WT and Asxl1Y588XTg mice. Data are presented as mean ± SEM from 8 to 11 WT and Asxl1Y588XTg mice (8-19 months old). Unpaired Student t test was used to assess statistical significance. (C) Flow cytometric analysis of LSK compartments in BM of representative WT and Asxl1Y588XTg mice (8-19 months old) (left). Cells are gated on Lin− cells. Quantitation of the percentage of LSK compartments in BM Lin− of WT and Asxl1Y588XTg mice (right), n = 8 per group; **P = .002. (D) Flow cytometric analysis of the percent LT-HSC, ST-HSC, and multipotent progenitor cell populations (LSK/CD34+/FLK2+) in the BM LSK cells of representative WT and Asxl1Y588XTg mice (8-19 months old) (left). Cells are gated on LSK cells. Quantitation of the percent LT-HSC (P = .089) and ST-HSC (***P = .0006) populations in the BM Lin− cells of each genotype of mice (right). Unpaired Student t test was used to assess statistical significance. (E) Flow cytometric analysis of the GMP, common myeloid progenitor, and MEP populations in the BM LKS− cells of representative WT and Asxl1Y588XTg mice (8-19 months old) (left). Cells are gated on LKS− cells. Quantitation of the percent GMP (**P = .002) and MEP (**P = .0098) populations in the BM Lin− cells of each genotype of mice (right). Blue square, MPN; red square, leukemia; blue triangle, MDS/MPN; red triangle, MDS. (F) Colonies from 15 000 bone marrow mononuclear cells of WT and Asxl1Y588XTg mice. Blue bars, CFU-granulocytes/macrophages (GM); green bars, burst forming unit-erythrocyte (BFU-E); red bars, granulocyte, erythroid, macrophage, megakaryocyte (GEMM). Three mice per genotype were analyzed. ***P = .000424. (G) Serial cell replating assays using whole BM cells (3 mice per genotype) were performed to determine HSC self-renewal capability. A total of 15 000 bone marrow mononuclear cells were used for each methylcellulose cultures. The cells were replated weekly for 4 weeks. *P < .05. (H) Monthly assessment of donor chimerism (line II) in the peripheral blood of recipient animals is shown up to 16 weeks after transplant (n = 5 recipient mice were used for each genotype).
Transgenic expression of ASXL1aa1-587affects HSC pool in vivo. (A) Flow cytometric analysis of Gr-1+/Mac1+ cell population in PB of representative WT (left) and Asxl1Y588XTg mice (right, 8 months old). (B) Quantitation of the percent of Gr-1+/Mac1+ cell populations in PB (*P = .024), BM (P = .074), and spleen (P = .080) of WT and Asxl1Y588XTg mice. Data are presented as mean ± SEM from 8 to 11 WT and Asxl1Y588XTg mice (8-19 months old). Unpaired Student t test was used to assess statistical significance. (C) Flow cytometric analysis of LSK compartments in BM of representative WT and Asxl1Y588XTg mice (8-19 months old) (left). Cells are gated on Lin− cells. Quantitation of the percentage of LSK compartments in BM Lin− of WT and Asxl1Y588XTg mice (right), n = 8 per group; **P = .002. (D) Flow cytometric analysis of the percent LT-HSC, ST-HSC, and multipotent progenitor cell populations (LSK/CD34+/FLK2+) in the BM LSK cells of representative WT and Asxl1Y588XTg mice (8-19 months old) (left). Cells are gated on LSK cells. Quantitation of the percent LT-HSC (P = .089) and ST-HSC (***P = .0006) populations in the BM Lin− cells of each genotype of mice (right). Unpaired Student t test was used to assess statistical significance. (E) Flow cytometric analysis of the GMP, common myeloid progenitor, and MEP populations in the BM LKS− cells of representative WT and Asxl1Y588XTg mice (8-19 months old) (left). Cells are gated on LKS− cells. Quantitation of the percent GMP (**P = .002) and MEP (**P = .0098) populations in the BM Lin− cells of each genotype of mice (right). Blue square, MPN; red square, leukemia; blue triangle, MDS/MPN; red triangle, MDS. (F) Colonies from 15 000 bone marrow mononuclear cells of WT and Asxl1Y588XTg mice. Blue bars, CFU-granulocytes/macrophages (GM); green bars, burst forming unit-erythrocyte (BFU-E); red bars, granulocyte, erythroid, macrophage, megakaryocyte (GEMM). Three mice per genotype were analyzed. ***P = .000424. (G) Serial cell replating assays using whole BM cells (3 mice per genotype) were performed to determine HSC self-renewal capability. A total of 15 000 bone marrow mononuclear cells were used for each methylcellulose cultures. The cells were replated weekly for 4 weeks. *P < .05. (H) Monthly assessment of donor chimerism (line II) in the peripheral blood of recipient animals is shown up to 16 weeks after transplant (n = 5 recipient mice were used for each genotype).
To assess whether transgenic expression of ASXL1aa1-587 affects the HSC/HPC pool in vivo, we analyzed subpopulations of HSC/HPCs in the BM cells of WT and Asxl1Y588XTg mice by flow cytometry. Significantly higher percentages of Lin−Sca-1+cKit+ (LSK) cells, long-term (LT)-HSCs (LSK/CD34−/FLK2−), short-term (ST)-HSCs (LSK/CD34+/FLK2−), and granulocyte-macrophage progenitor cells (GMPs) were observed in the BM of Asxl1Y588XTg mice, compared with WT mice, whereas the percent of megakaryocyte-erythroid progenitor cells (MEPs) were much lower in Asxl1Y588XTg compared with WT mice (Figure 5C-E). In addition, the absolute cell numbers of LSK, LT-HSC, ST-HSC, and GMP were higher in a fraction of Asxl1Y588XTg mice compared with WT controls (supplemental Figure 4B). Collectively, these data indicate that expression of ASXL1aa1-587 dysregulates HSC/HPC pool in vivo.
ASXL1aa1-587 expression increases HSC/HPC functions
To verify the effect of ASXL1aa1-587 expression on HSC/HPC functions, we next performed colony-forming unit cell (CFU-C) and serial replating assays using BM cells from WT and Asxl1Y588XTg mice. The frequency of CFU-C was significantly higher in the BM of Asxl1Y588XTg mice (Figure 5F). We also observed a significantly higher replating capacity in the Asxl1Y588XTg BM cells compared with WT cells (Figure 5G). To examine whether ASXL1aa1-587 expression affected the survival of HSC/HPCs, we performed flow cytometry analysis and found that the Asxl1Y588XTg Lin−cKit+ (LK) cells had fewer Annexin V+/7-AAD− cells than WT LK cells (supplemental Figure 4C-D), indicating less apoptosis. Competitive transplantation of WT and Asxl1Y588XTg BM cells revealed that the recipient mice transplanted with cells of both lines of Asxl1Y588XTg mice had a higher percentage of CD45.2+ cells in their PB compared with the recipients transplanted with WT BM cells (supplemental Figure 2C; Figure 5H; supplemental Figure 4E). We also observed increased LSK cell frequencies and a higher proportion of Gr-1+/Mac1+ populations in the CD45.2+ BM of recipient mice transplanted with Asxl1Y588XTg BM cells compared with mice receiving WT BM cells (supplemental Figure 4F). These data confirm that expression of ASXL1aa1-587 increases HSC self-renewal capacity and skews differentiation toward myeloid lineage in vivo.
ASXL1aa1-587 expression alters gene expression profiling in HSC/HPCs
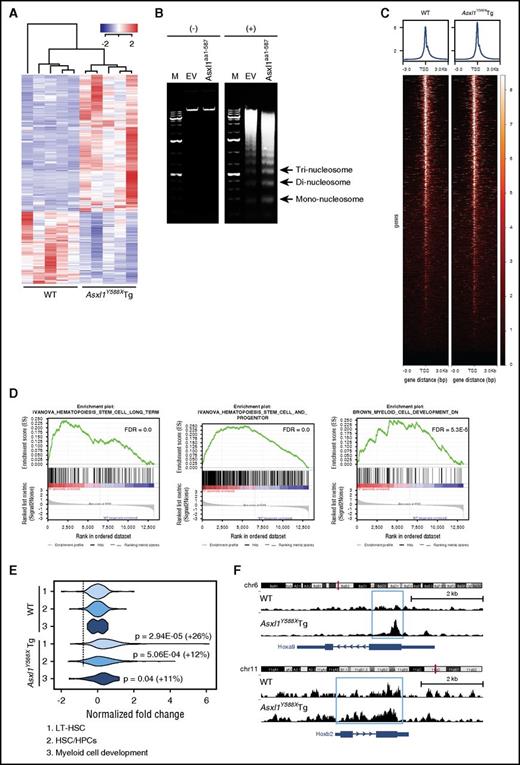
The phenotype and cell fate of a given cell relies on the precise control of gene expression by complex transcriptional and epigenetic networks. To determine the impact of ASXL1aa1-587 on the maintenance of HSC/HPC functions, we performed RNA-seq on BM cKit+ cells from Asxl1Y588XTg and WT mice (n = 5 mice/genotype). The genes expressed in WT and Asxl1Y588XTg cKit+ cells clearly clustered into 2 distinct groups with 1385 differentially expressed genes in Asxl1Y588XTg cKit+ cells (supplemental Figure 5A) compared with WT cells (false discovery rate [FDR] < 0.05). Among the top 500 dysregulated genes, 327 (65.4%) genes were upregulated and 173 (34.6%) were downregulated (Figure 6A).
Increased chromatin accessibility in Asxl1Y588XTg cKit+cells. (A) RNA-seq heat map depicting the 500 most significantly dysregulated genes (FDR) in BM cKit+ cells isolated from Asxl1Y588XTg and WT mice (n = 5 each genotype). (B) Gel electrophoretic analyses of DNA recovered from MNase-digested nuclei of 32D cells (+) with either ASXL1aa1-587 or empty vector (EV) nontreated cells were used as control (−). (C) Heat maps of the genome-wide ATAC-seq signal around TSS (±3000 bp). (D) GSEA plots depicting HSC and myeloid pathway gene enrichment. (E) Violin plots displaying chromatin accessibility around TSS for the genes in the 3 pathways indicated (also see panel C). Data were normalized to WT, percentage of increase in normalized reads and P values (unpaired Student t test) are indicated in the right of the plots. (F) ATAC-seq signal tracks around the Hoxa9 and Hoxb2 genes. Increased accessibility was present in Asxl1Y588XTg cKit+ cells.
Increased chromatin accessibility in Asxl1Y588XTg cKit+cells. (A) RNA-seq heat map depicting the 500 most significantly dysregulated genes (FDR) in BM cKit+ cells isolated from Asxl1Y588XTg and WT mice (n = 5 each genotype). (B) Gel electrophoretic analyses of DNA recovered from MNase-digested nuclei of 32D cells (+) with either ASXL1aa1-587 or empty vector (EV) nontreated cells were used as control (−). (C) Heat maps of the genome-wide ATAC-seq signal around TSS (±3000 bp). (D) GSEA plots depicting HSC and myeloid pathway gene enrichment. (E) Violin plots displaying chromatin accessibility around TSS for the genes in the 3 pathways indicated (also see panel C). Data were normalized to WT, percentage of increase in normalized reads and P values (unpaired Student t test) are indicated in the right of the plots. (F) ATAC-seq signal tracks around the Hoxa9 and Hoxb2 genes. Increased accessibility was present in Asxl1Y588XTg cKit+ cells.
ASXL1aa1-587 expression increases chromatin accessibility in cKit+ cells
That a large population of differently expressed genes (DEGs) in Asxl1Y588XTg cKit+ cells are being upregulated led us to hypothesize that the chromatin structure might be affected in the Asxl1Y588XTg cKit+ cells. To test this, we performed micrococcal nuclease (MNase) digestion assays, using 32D cells (a myeloblast-like cell line)25 stably expressing either empty vector or ASXL1aa1-587. Notably, ASXL1aa1-587 expressing 32D cells have a lower percentage of Mac1+/F4/80+ cells compared with control cells (supplemental Figure 5C), consistent with the previous report that mutant ASXL1 inhibited 32D cell differentiation.20 ASXL1aa1-587 expressing 32D cells showed increased MNase sensitivity compared with control 32D cells, suggesting that ASXL1aa1-587 expression increases chromatin accessibility (Figure 6B).
ATAC-seq is the most current method for probing open chromatin.27 We performed ATAC-seq on Asxl1Y588XTg and WT cKit+ cells (n = 2/genotype) followed by correlation with transcriptomes to determine potential novel ASXL1aa1-587 target genes. A heat map for ATAC-seq signal around all mouse TSS shows a slight increase in overall accessibility (Figure 6C), consistent with the RNA-seq data that DEG are predominantly upregulated in Asxl1Y588XTg cKit+ cells. GSEA enrichment analysis of the RNA-seq data further revealed specific gene pathways to be upregulated in the Asxl1Y588XTg cells, including LT-HSC, HPC, and myeloid cell development pathways (Figure 6D), alongside gene sets for MYC targets, NUP98-HOXA9 fusion targets, and megakaryocytes (supplemental Figure 5B). qPCR confirmed higher expression of 2 selected genes, Prdm16 and Fos (supplemental Figure 5D), which have been shown to be important for HSC.28,29 To assess the effect of Prdm16 overexpression on the cellular phenotypes of Asxl1Y588XTg cKit+ cells, Prdm16 was knocked down in Asxl1Y588XTg cKit+ cells (supplemental Figure 5E) and the frequencies of CFU-C and LSK cells were examined after 7 days in culture. Knocked down Prdm16 significantly decreased the frequency of CFU-C and reduced the percentage of LSK cells in Asxl1Y588XTg cKit+ cells compared with WT cells (supplemental Figure 5E-F).
We next correlated the ATAC-seq data with the DEG in these pathways and found a significant increase in chromatin accessibility in the TSS of genes in the HSC and myeloid pathways (Figure 6E). The violin plot reveals a positive correlation between ATAC-seq signal and LT-HSC, HPC, and the myeloid cell development pathways (Figure 6E). Figure 6F shows the chromatin accessibility around the Hoxa9 and Hoxb2 genes, key players in stem cell development that are upregulated in the Asxl1Y588XTg cKit+ cells (log fold change = 0.69, FDR = 0.02). Collectively, the ATAC-seq data demonstrate an increased chromatin accessibility, especially for LT-HSC, HPC, and the myeloid cell development pathways in ASXL1aa1-587 HSC/HPCs.
ASXL1aa1-587 interacts with BRD4
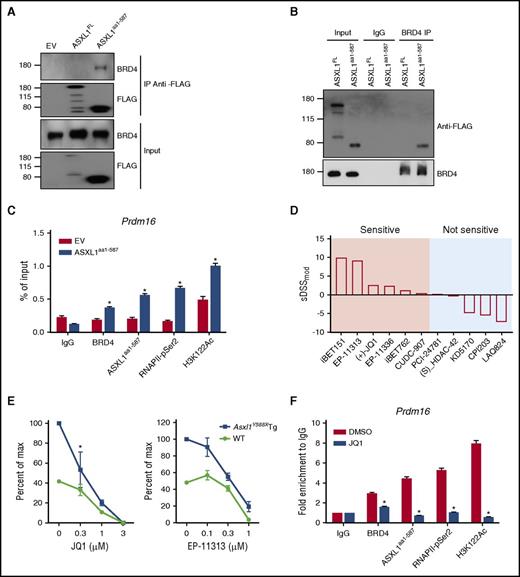
We and others have reported that Asxl1 loss impairs the recruitment of PRC2 complex and reduces the levels of histone H3 lysine 27 trimethylation (H3K27me3).13,18 Balasubramani and colleagues reported that ASXL1 truncations confer enhanced activity of the ASXL1-BAP1 complex.21 However, no dramatic changes were observed at the levels of H2AK119Ub and H3K27me3 in ASXL1aa1-587 cKit+ cells using western blots (supplemental Figure 6A). Most of the truncating mutations of ASXL1 result in deletion of the PHD domain of ASXL1 at the C terminus. To determine if the truncated ASXL1 alters the interactions of ASXL1 with other nuclear proteins, we performed protein affinity purification using an anti-FLAG antibody and nuclear extracts prepared from HEK293T cells engineered to express FLAG-tagged ASXL1FL or ASXL1aa1-587. Their interacting proteins were identified by the liquid chromatography-tandem mass spectrometry analysis (supplemental Table 4).30 Among the identified candidate interacting proteins, 1 ASXL1aa1-587 binding partner, BRD4, drew our attention (supplemental Figure 6B).
BRD4 is a member of the BET family of bromodomain-containing proteins that binds to acetylated histones to influence transcription.31 The interaction of ASXL1aa1-587 with BRD4 was confirmed by reciprocal immunoprecipitation (IP; Figure 7A-B). To map the region of ASXL1aa1-587 that mediates with BRD4 interaction, we overexpressed multiple constructs that encode various FLAG-tagged fragments of the ASXL1 truncations in HEK293T cells and examined their ability to bind BRD4. ASXL1 full-length, aa1-587, aa1-500, and aa1-420 successfully pulled down BRD4, but aa201-587 and aa401-587 failed to pull down BRD4 (supplemental Figure 6C). These data suggest that the region spanning aa1-201 is likely important for BRD4 binding.
ASXL1aa1-587interacts with BRD4 to regulate gene expression. (A,B) Reciprocal IP and western blotting confirmed interaction of ASXL1aa1-587 with BRD4 in the nuclear fraction of HEK293T cells transfected with EV, FLAG-tagged ASXL1FL or FLAG-tagged ASXL1aa1-587. Nuclear extractions were subjected to IP using indicated antibodies against FLAG or BRD4. (C) ChIP-qPCR on 32D cells expressing Flag-ASXL1aa1-587 or an EV control. The antibodies used for ChIP are indicated. Normal immunoglobulin G (IgG) was used as a control. PCR was performed with primers specific for the Prdm16 promoter regions. (D) Drug screenings were performed on BM cells of Asxl1Y588XTg or WT mice. Whole BM cells from WT mice were used as a control. The tests were done in triplicates using a 10-point 1:3 dilution series starting at a nominal test concentration of 10 μM (20 000-fold concentration range). DSSmod was calculated for all samples and the selective DSSmod (sDSSmod) for each drug was calculated according to the formula sDSSmod = DSSmod (Asxl1Y588XTg BM cells) − DSSmod (WT BM cells). Positive values represent drugs that show higher specificity in affecting the survival of the Asxl1Y588XTg BM cells; negative values represent drugs that show higher specificity in affecting WT BM cells. (E) CFU-C assay using whole BM cells with or without BET bromodomain inhibitors treatment (EP-11313 and JQ1). Whole BM cells from WT mice were used as a control. The experiment was performed in triplicate. The concentration of the drug used in colony assay is indicated on the x-axis. *P = 0.0407. Unpaired Student t test was used to assess statistical significance. (F) ChIP-qPCR was performed on 32D cells expressing ASXL1aa1-587 with or without 24 hours of treatment with JQ1. The concentration of the drug was 0.1 μM. The antibodies used for ChIP are indicated. Normal IgG was used as a control. PCR was done using primers specific for the Prdm16 promoter region. *P < .05. Unpaired Student t test was used to assess statistical significance.
ASXL1aa1-587interacts with BRD4 to regulate gene expression. (A,B) Reciprocal IP and western blotting confirmed interaction of ASXL1aa1-587 with BRD4 in the nuclear fraction of HEK293T cells transfected with EV, FLAG-tagged ASXL1FL or FLAG-tagged ASXL1aa1-587. Nuclear extractions were subjected to IP using indicated antibodies against FLAG or BRD4. (C) ChIP-qPCR on 32D cells expressing Flag-ASXL1aa1-587 or an EV control. The antibodies used for ChIP are indicated. Normal immunoglobulin G (IgG) was used as a control. PCR was performed with primers specific for the Prdm16 promoter regions. (D) Drug screenings were performed on BM cells of Asxl1Y588XTg or WT mice. Whole BM cells from WT mice were used as a control. The tests were done in triplicates using a 10-point 1:3 dilution series starting at a nominal test concentration of 10 μM (20 000-fold concentration range). DSSmod was calculated for all samples and the selective DSSmod (sDSSmod) for each drug was calculated according to the formula sDSSmod = DSSmod (Asxl1Y588XTg BM cells) − DSSmod (WT BM cells). Positive values represent drugs that show higher specificity in affecting the survival of the Asxl1Y588XTg BM cells; negative values represent drugs that show higher specificity in affecting WT BM cells. (E) CFU-C assay using whole BM cells with or without BET bromodomain inhibitors treatment (EP-11313 and JQ1). Whole BM cells from WT mice were used as a control. The experiment was performed in triplicate. The concentration of the drug used in colony assay is indicated on the x-axis. *P = 0.0407. Unpaired Student t test was used to assess statistical significance. (F) ChIP-qPCR was performed on 32D cells expressing ASXL1aa1-587 with or without 24 hours of treatment with JQ1. The concentration of the drug was 0.1 μM. The antibodies used for ChIP are indicated. Normal IgG was used as a control. PCR was done using primers specific for the Prdm16 promoter region. *P < .05. Unpaired Student t test was used to assess statistical significance.
BRD4 is shown to acetylates H3K122 (a residue critical for nucleosome stability32 ) and activate P-TEFb for RNA polymerase II CTD phosphorylation.33 Interestingly, increased levels of H3K122Ac and H3K27Ac were observed in Asxl1Y588XTg cKit+ BM cells compared with WT cells (supplemental Figure 6D). To examine whether the regulated genes are dependent on the gained BRD4 interaction, we performed chromatin IP qPCR (ChIP-qPCR) using 32D cells with or without overexpression of ASXL1aa1-587 to quantify the BRD4, ASXL1aa1-587, RNAPII-pSer2, and H3K122Ac occupancies in the promoter regions of selected upregulated genes. Increased enrichment of BRD4, RNAPII-pSer2, and H3K122Ac was observed at the promoter region of Prdm16 and was accompanied with ASXL1aa1-587 binding at the same loci (Figure 7C), suggesting the increased level of Prdm16 expression is associated with gained BRD4 binding.
Asxl1Y588XTg BM cells are sensitive to BET bromodomain inhibitors
To identify the potential therapeutic targets of Asxl1Y588XTg BM cells, we performed drug screening assays by testing an array of 165 epigenetic compounds, covering BET bromodomain inhibitors, HDAC inhibitors, and DNA methyltransferase inhibitors. Cell survival was used as readout. Asxl1Y588XTg BM cells showed sensitivity to 26 of the 165 compounds, including BET bromodomain inhibitors, HDAC inhibitor, and some of other targets (supplemental Table 5). To further verify these results, 5 BET bromodomain inhibitors and 5 HDAC inhibitors were selected for a cell survival assay in a dose-dependent manner. Consistent with the results of a single dose screen, Asxl1Y588XTg BM cells displayed high sensitivity toward all 5 BET bromodomain inhibitors (Figure 7D; supplemental Figure 6E). In contrast, ASXL1aa1-587 BM cells did not respond to the 5 HDAC inhibitors compared with WT cells (Figure 7D). Consistently, western blot revealed that EP-11313 dramatically reduced the levels of H3K122Ac and H3K27Ac in 32D cells expressing ASXL1aa1-587 (supplemental Figure 6F).
We performed CFU-C assays using BM cells of Asxl1Y588XTg and WT mice to extend our drug screen results to HSC/HPCs of Asxl1Y588XTg mice. The addition of EP-11313 and JQ1 reduced the numbers of CFU-C of Asxl1Y588XTg BM cells compared with WT BM cells (Figure 7E). To further validate the BET bromodomain inhibitors in targeting BRD4 in Asxl1Y588X cells, we performed ChIP-qPCR using ASXL1aa1-587 expressing 32D cells treated with or without JQ1. JQ1 significantly reduced the occupancy of ASXL1aa1-587, BRD4, H3K122Ac, and RNAPII-pSer2 on Prdm16 promoter region in ASXL1aa1-587 expressing 32D cells (Figure 7F). Collectively, these results support a role of the ASXL1aa1-587–BRD4 axis in the hematopoietic phenotypes observed in Asxl1Y588XTg mice.
Discussion
Truncation mutations of the ASXL1 genes occur in many diseases, including hematological malignancies, autism, Bohring-Opitz and related syndromes, and solid tumors, such as prostate cancer, breast cancer, and high-grade glioma. In the current study, we generated an Asxl1Y588XTg mouse model and showed that transgenic expression of a truncated form of ASXL1 in the hematopoietic compartment in mice leads to a shortened lifespan because of the development of myeloid malignancies, including MDS, MPN, and AML. These in vivo studies suggest that truncating ASXL1 confers an oncogenic role in the pathogenesis of myeloid malignancies.
Consistent with Asxl1-deficient mice, Asxl1Y588XTg mice also had higher percent neutrophils and lower RBC counts compared with WT littermates. Three of 11 Asxl1Y588XTg mice had a higher percentage of blast cells in their PB, BM, and spleens, indicating the development of leukemia. Distinct from Asxl1-deficient mice, ASXL1aa1-587 expression significantly increased the LSK and GMP populations with skewed differentiation favoring monocytic/granulocytic lineage, suggesting a gain of function of ASXL1aa1-587. Furthermore, a higher replating capacity by Asxl1Y588XTg BM cells suggests that ASXL1aa1-587 expression confers an increased HSC self-renewal and a biased myeloid differentiation. Skewed myeloid differentiation is a prerequisite for leukemic stem cell formation and AML development,34 and skewed expansion of GMP population is associated with higher risks of leukemic transformation in MDS.35,36 The increased HSC self-renewal and a higher percentage of GMP in Asxl1Y588XTg mice likely facilitate leukemogenesis.
That expression of ASXL1aa1-587 alone, without altering WT ASXL1 expression, is sufficient to induce leukemogenesis supports the notion that ASXL1aa1-587 expression results in a gain-of-function role in leukemogenesis. RNA-seq analyses revealed that Asxl1Y588XTg cKit+ cells had an increased expression of genes related to myeloid cell development and leukemia signatures in Asxl1Y588XTg HSC/HPCs.
ASXL1 contains multiple domains to exert its regulatory effects in the nuclear through interaction/corporation with other protein complexes, such as DUB,21 SRC-1,37 PRC2,13,18 and cohesin.30 Our study revealed that ASXL1aa1-587 acquired an interaction with BRD4, involving multiple biologic functions, including transcription, DNA replication, epigenetic regulation,31,38 and transcription elongation.39,40 Devaiah et al shows that BRD4 mediates chromatin decompaction by acetylating and evicting nucleosomes, and the decreased nucleosomal occupancy allows access to the transcriptional machinery and recruitment of transcription factors, leading to transcription activation.32 In fact, our RNA-seq and ATAC-seq analyses showed that in Asxl1Y588XTg cKit+ cells, an increase in the opening of chromatin around genes regulating stem cell, hematopoiesis, and myeloid pathways leads to an increase in the expression of those genes, shifting cell identities toward malignant phenotypes. Asxl1Y588XTg cKit+ cells had a significantly higher expression of several genes, critical for leukemogenesis, such as Prmd16. ChIP-qPCR confirmed the increased BRD4 occupancy at the promoter region of Prmd16, suggesting an association between the gained BRD4 occupancy and increased Prmd16 expression.
Recent development of potent, specific, and reversible BET bromodomain inhibitors, such as the first-in-class thienodiazepine small-molecule JQ1, has accelerated mechanistic discovery and translation in cancer.41 In fact, EP-11313, a BET bromodomain inhibitor, reduced the frequency of CFU-C by Asxl1Y588XTg BM cells, further highlighting the significance of the gained ASXL1aa1-587–BRD4 interaction in leukemogenesis. Consistently, JQ1 significantly reduced the occupancy of ASXL1aa1-587, BRD4, H3K122Ac, and RNAPII-pSer2 on Prdm16 promoter region in ASXL1aa1-587 expressing 32D cells. Further study to determine the genome-wide occupancy of ASXL1aa1-587 is warranted in Asxl1Y588XTg HSC/HPCs. Our current study sheds light on the impact of ASXL1aa1-587 on increasing BRD4 genomic binding affinity and/or activity, which in turn shows that altering the transcription of genes is important for HSC functions and preferential myeloid differentiation. A caveat of the current model is the enforced overexpression of a truncated form of mouse ASXL1; thus, future studies establishing a patient relevant ASXL1 truncation mutation knock-in mouse model are warranted.
Collectively, our study indicates that truncated forms of ASXL1 functions in a gain-of-function fashion to promote the pathogenesis of myeloid malignancies. The Asxl1Y588XTg mouse model presents a novel platform for testing novel therapeutic agents to treat myeloid malignancies.
Raw data generated from RNA sequencing and assay for transposase-accessible chromatin with high throughput sequencing have been deposited in the Genome Sequence Archive (https://submit.ncbi.nlm.nih.gov/subs/bioproject/) at the National Center for Biotechnology Information; the BioProject accession number is PRJNA388673.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the Drug Discovery Core, Satellite Histological Core, Flow Cytometry Core, Oncogenomics Core, and Biostatistics and Bioinformatics Core Facilities of Sylvester Comprehensive Cancer Center, University of Miami Miller School of Medicine.
This work was supported by grants from the National Institutes of Health, National Cancer Institute (CA172408 and CA185751) (F.-C.Y. and M.X.) and the National Heart, Lung, and Blood Institute (HL112294) (M.X.), Chinese Academy of Medical Sciences (202016-I2M-2-001) (Y.Z.), the Alcon Research Institute (J.W.H.), the Sylvester Comprehensive Cancer Center (PG006470 [F.-C.Y., J.W.H.]), the University of Miami Sheila and David Fuente Graduate Program in Cancer Biology (M.G.F. and M.A.D.), and the Center for Computational Science Fellowship (M.G.F. and M.A.D), and by a generous gift from Dr. Mark J. Daily (J.W.H.). Epigenetic drug discovery work in the Wahlestedt laboratory is currently funded by the National Institutes of Health National Institute on Alcohol Abuse and Alcoholism (AA023781) and the National Institute on Drug Abuse (DA042650). EP-11313 was generously provided by Epigenetix Inc. The Bascom Palmer Eye Institute also received funding from the National Institutes of Health National Eye Institute (Core Grant P30EY014801), Department of Defense (329 Grant #W81XWH-13-1-0048), and a Research to Prevent Blindness Unrestricted Grant.
Authorship
Contribution: F.-C.Y. and M.X. conceived the project; H.Y., S.K., Y.G., I.L, M.A.D., Z.L. J.L., P.Z., S.C., S.Y., Z.L., and F.-C.Y. designed the study. H.Y., S.K., Y.G., I.L, J.L., L.L., S.C., Z.L., S.Y., H.A.-A., Z.C., and P.Z. performed the cellular and molecular experiments; S.K., M.A.D., M.G.F., and Z.C. performed the RNA sequencing and assay for transposase-accessible chromatin with high throughput sequencing and analyzed the data; J.L., S.C., Y.Z., S.Y., M.X., and F.-C.Y. reviewed the blood smears and histopathological sections; H.Y., S.K., Y.G., I.L., M.A.D., Y.Z., S.D.N., J.W.H., C.W., M.X., and F.-C.Y. reviewed, analyzed, and discussed the data and edited the manuscript; H.Y., S.K., Y.G., M.X., and F.-C.Y. wrote the manuscript. All authors reviewed and approved the manuscript.
Conflict-of-interest disclosure: The authors declare no competing interests.
Correspondence: Feng-Chun Yang, Sylvester Comprehensive Cancer Center, Department of Biochemistry and Molecular Biology, University of Miami Miller School of Medicine, 1011 15th St, Gautier Building, Room 417, Miami, FL 33136; e-mail: fxy37@med.miami.edu; or Mingjiang Xu, Sylvester Comprehensive Cancer Center, Department of Biochemistry and Molecular Biology, University of Miami Miller School of Medicine, 1011 15th St, Gautier Building, Room 417, Miami, FL 33136; e-mail: mxx51@miami.edu; or Claes Wahlestedt, Sylvester Comprehensive Cancer Center, Center for Therapeutic Innovation and Department of Psychiatry and Behavioral Sciences, University of Miami Miller School of Medicine, Miami, FL 33136; e-mail: clawah@gmail.com.
References
Author notes
H.Y., S.K., and Y.G. contributed equally to this work.