Key Points
BM stroma-derived conditions protect AML patient cells against topoisomerase II and BCL2 inhibitors, as well as several classes of TKIs.
JAK1/2 inhibitor ruxolitinib reverses cytoprotection against BCL2 antagonist venetoclax, suggesting a novel combinatorial treatment.
Abstract
The bone marrow (BM) provides a protective microenvironment to support the survival of leukemic cells and influence their response to therapeutic agents. In acute myeloid leukemia (AML), the high rate of relapse may in part be a result of the inability of current treatment to effectively overcome the protective influence of the BM niche. To better understand the effect of the BM microenvironment on drug responses in AML, we conducted a comprehensive evaluation of 304 inhibitors, including approved and investigational agents, comparing ex vivo responses of primary AML cells in BM stroma-derived and standard culture conditions. In the stroma-based conditions, the AML patient cells exhibited significantly reduced sensitivity to 12% of the tested compounds, including topoisomerase II, B-cell chronic lymphocytic leukemia/lymphoma 2 (BCL2), and many tyrosine kinase inhibitors (TKIs). The loss of TKI sensitivity was most pronounced in patient samples harboring FLT3 or PDGFRB alterations. In contrast, the stroma-derived conditions enhanced sensitivity to Janus kinase (JAK) inhibitors. Increased cell viability and resistance to specific drug classes in the BM stroma-derived conditions was a result of activation of alternative signaling pathways mediated by factors secreted by BM stromal cells and involved a switch from BCL2 to BCLXL-dependent cell survival. Moreover, the JAK1/2 inhibitor ruxolitinib restored sensitivity to the BCL2 inhibitor venetoclax in AML patient cells ex vivo in different model systems and in vivo in an AML xenograft mouse model. These findings highlight the potential of JAK inhibitors to counteract stroma-induced resistance to BCL2 inhibitors in AML.
Introduction
Acute myeloid leukemia (AML) is characterized by an accumulation of proliferative, abnormally differentiated hematopoietic cells in the bone marrow (BM) and other tissues, leading to interference of normal hematopoiesis and BM failure.1 Despite high rates of remission obtained with conventional chemotherapy, incomplete eradication of leukemic cells from the BM and subsequent disease relapse remain major clinical challenges.2,3 The BM microenvironment plays an important role in leukemic cell survival, promoting drug resistance and influencing outcome.4-7
In addition to hematopoietic cells, the BM is composed of fibroblast-like stromal cells, osteoblasts and osteoclasts, which aid leukemic cells in evading the effects of chemotherapy and targeted drugs.8,9 Several tyrosine kinase inhibitors (TKIs) are being developed for the treatment of AML, and resistance to these drugs is an area of concern.10-15 Despite rapid initial response, small numbers of leukemic cells persist in the BM, making remissions short-lived.16-19 In addition, in vitro studies have shown that direct contact to stromal cells is sufficient to protect AML cells from TKIs and other therapies.20-23 For example, coculture of CD34+ progenitor AML patient cells with internal tandem duplication in fms-like tyrosine kinase-3 (FLT3-ITD), together with stromal cells, induced resistance to sorafenib.24 Novel therapeutic strategies are therefore needed that can override stroma-mediated protection of AML cells.
The identification of effective drugs may be facilitated by assays that model the BM microenvironment and tumor–stroma interactions, such as coculture of immortalized stromal cells with AML cell lines.25-27 However, the derived information is limited, as cell lines do not fully represent the heterogeneity observed in patients. Alternatively, ex vivo high-throughput testing of patient-derived AML cells can identify effective drugs for patients with AML resistant to conventional therapy.28,29
Here, we conducted comprehensive evaluation of cell sensitivity ex vivo in patients with AML to 304 oncology drugs and compared responses in standard cell culture and BM stromal cell-derived conditions. In stroma-derived conditions, cells from patients with lost sensitivity to many drugs, but gained sensitivity to Janus kinase (JAK) inhibitors. The combination of JAK1/2 inhibitor ruxolitinib with BCL2 inhibitor venetoclax was more effective against AML cells in stroma-derived conditions and an AML mouse model compared with either drug alone, highlighting a potentially useful combination that can overcome the cytoprotective effects of the BM microenvironment.
Materials and methods
Cell lines and cell culture
The HS-5 human BM stromal cell line was from the American Type Culture Collection (Manassas, VA). HS-5 cells were maintained in RPMI 1640 medium with 10% fetal bovine serum, 2 mM l-glutamine, penicillin (100 U/mL), and streptomycin (100 µg/mL). Conditioned medium (CM) was collected from 70% to 80% confluent HS-5 cell cultures after 72 hours of incubation in supplemented RPMI 1640. The CM was cleared by centrifugation, filtered (0.22 µm), and stored at −80°C.
AML patient cells and healthy donors
BM aspirates or peripheral blood samples (n = 26) were obtained from patients with AML (n = 21) after informed consent, using protocols approved by a local institutional review board of Helsinki University Hospital and Comprehensive Cancer Center and in accordance with the Declaration of Helsinki. Patient characteristics are described in supplemental Table 1, available on the Blood Web site. Mononuclear cells (MNCs) were isolated by density gradient separation (Ficoll-Paque PREMIUM; GE Healthcare, Little Chalfont, Buckinghamshire, UK) and immediately analyzed or vitally frozen for later use. Cells were maintained in mononuclear cell medium (MCM; Promocell, Heidelberg, Germany) or in a 25% HS-5 CM plus 75% RPMI 1640 medium mix. Cell viability was measured using the CellTiter-Glo (CTG) reagent (Promega, Madison, WI), according to the manufacturer’s instructions, with a PHERAstar FS plate reader (BMG LABTECH, Ortenberg, Germany).
Cytokine analysis
HS-5 CM, CM collected from primary AML mesenchymal stem cell (MSC) cultures, and BM supernatant fluid from 1 patient with AML and a healthy donor were analyzed for 174 human cytokines using the RayBio C-Series Human Cytokine Antibody Arrays C2000 and C5, following the manufacturer’s instructions (RayBiotech, Norcross, GA). BM fluid was obtained by centrifuging BM aspirates (3 mL), as previously described.30 Detection was carried out by chemiluminescence, and data quantified with the Odyssey imaging system (LI-COR Biosciences, Lincoln, NE).
Drug sensitivity and resistance testing
AML cells were suspended in MCM or CM at 4 × 105 cells/mL. Cells were added to predrugged plates, which included 304 commercially available small molecule inhibitors (supplemental Table 3). Cell viability was measured with CTG after 72 hours, and drug sensitivity assessed as previously described.29
Data analysis
A drug sensitivity score (DSS) was calculated on the basis of a modified area under the dose–response curve calculation, as described earlier.31 Unsupervised hierarchical Ward-linkage clustering with Spearman and Manhattan distance measures of drug and sample profiles was performed, using the DSS values. Analysis of phylogenetics and evolution R-package was used for generation of the fan plots.32 Prism 5 (GraphPad, La Jolla, CA) was used for statistical analysis. Comparison of DSS values with or without CM across samples was performed using paired Student t tests. A P value < .05 was considered statistically significant.
Gene expression analysis
Gene expression was assessed by RNA sequencing of AML patient samples and validated by reverse transcription polymerase chain reaction. Detailed methods and primer sequences are found in the supplemental Appendix.
Western blot analysis
AML patient cells (n = 5) were treated with 300 nM ruxolitinib, 100 nM venetoclax, or their combination in 25% HS-5 CM for 48 hours. Cell lysates were prepared, separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis, and transferred to nitrocellulose membranes. Detection antibodies included anti-MCL1 (#4572), anti-BCLXL (#2764) (Cell Signaling Technology, Danvers, MA), anti-BCL2 (Dako, Glostrup, Denmark), and anti-β-actin (C4, sc-47778; Santa Cruz Biotechnology, Santa Cruz, CA).
Phospho-flow analysis
AML patient cells were stimulated with medium, granulocyte/macrophage colony-stimulating factor (GM-CSF), G-CSF, interleukin 6 (IL-6), IL-8, or macrophage inflammatory protein 3α (MIP-3α; 10 ng/mL; Peprotech, Rocky Hill, NJ) for 20 minutes at 37°C, fixed with Lyse/Fix Buffer (BD Biosciences, San Jose, CA) for 10 minutes at 37°C, and permeabilized with Perm Buffer III (BD Biosciences) for 30 minutes at −20°C. Cells were washed, stained with Alexa 647-anti-phospho-Stat5 (pY694), PE-CF594-anti-phospho-Stat3 (pY705), BV421-anti-phospho-Akt (pS473), and PE-anti-phospho-Erk1/2 (pT202/pY204, all antibodies from BD Biosciences), and analyzed on the iQUE Plus instrument (Intellicyt, Albuquerque, NM). Data were analyzed with Cytobank (Fluidigm, San Francisco, CA).
Drug combination studies
Drugs (venetoclax, ruxolitinib, quizartinib; ChemieTek, Indianapolis, IN) were added simultaneously at fixed concentrations to AML patient cells and incubated for 72 hours in either MCM or CM. Cell viability was measured using the CTG assay. Data were analyzed with the Zero Interaction Potency (ZIP) model by considering a dose–response matrix in which 2 drugs are tested in a serial dilution of 8 concentrations.33 The δ synergy score for each dose pair was determined as the difference between the observed and expected response given by the ZIP model, and scores were represented by pseudocoloring a 2-dimensional contour plot over the dose matrix, resulting in a synergy landscape enabling identification of strong synergistic dose regions.33
Colony-forming cell assay
For colony-forming cell (CFC) assays, AML patient cells were treated with vehicle (0.1% dimethyl sulfoxide), ruxolitinib (300 nM), venetoclax (100 nM), or their combination in MCM or 25% CM for 72 hours. After treatment, cells were plated in MethoCult H4435 Enriched Methylcellulose Medium (STEMCELL Technologies, Vancouver, BC, Canada), and the CFC output was recorded after 14 days.
Coculture assays
BM stromal cells from AML patients were prepared at 1.5 × 105 cells/mL and seeded onto 96-well plates (Corning, Corning, NY) at 100 µL/well. After overnight incubation, AML patient cells were added directly to the adherent stroma (1 × 105 cells/100 µL/well) or separated by a 0.4-µm pore membrane (Corning). Vehicle (0.1% dimethyl sulfoxide), ruxolitinib (300 nM), venetoclax (100 nM), or their combination were added and the cocultures incubated 48 hours. AML cells were labeled with PE-Annexin V, 7AAD, PE-Cy7-CD34, and BV605-CD45 (all from BD Biosciences) and analyzed using the iQUE Plus instrument and FlowJo Software version 7.2.5 (Ashland, OR).
In vivo drug efficacy in an AML mouse model
The protocol for animal studies was approved by the Norwegian State Commission for Laboratory Animals and the experiments performed according to the European Convention for the Protection of Vertebrates Used for Scientific Purposes. Twenty-four female nonobese diabetic/severe combined immunodeficient γ IL2rγnull (NSG) mice were inoculated IV with 5 × 106 MOLM-13luc AML cells and divided into control, venetoclax (25 mg/kg, intraperitoneally), ruxolitinib (50 mg/kg BID, by mouth), and combination groups (all n = 6; equally distributed based on bioluminescence intensity at day 7). All groups were treated for 3 weeks, 5 days a week, with 2 days off. The health and weight of the mice were monitored daily, and the mice were imaged weekly. Briefly, anesthetized mice were injected intraperitoneally with 50 mg/kg firefly d-luciferin (BIOSYNTH, Staad, Switzerland) 10 minutes before whole-body imaging, using the IVIS Spectrum Imager (PerkinElmer, Waltham, MA). Images were acquired and analyzed with Living Image acquisition software version 4.5.2 (PerkinElmer). The mice were humanely killed when moribund, as defined by institutional guidelines.
Results
BM stromal cell–CM enhances survival of AML patient cells ex vivo and leads to activation of JAK/STAT signaling
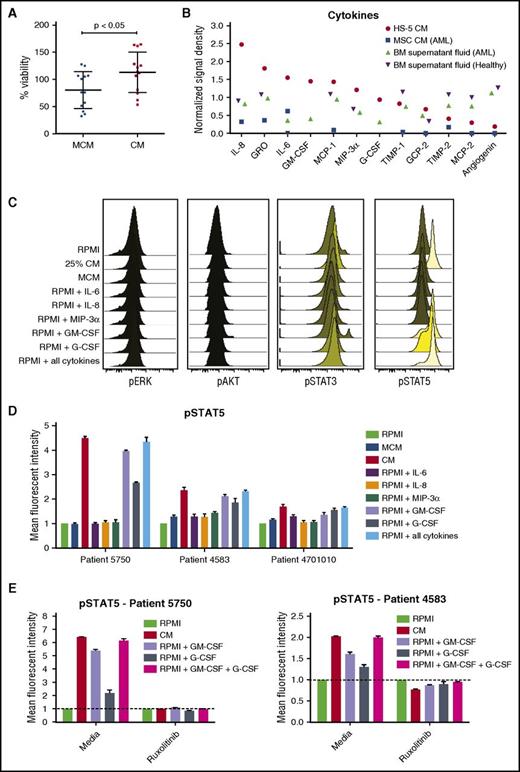
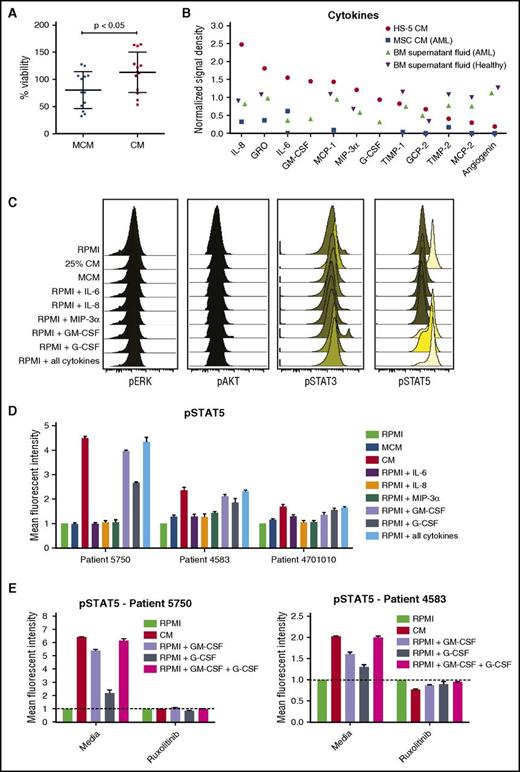
BM stromal cell line HS-5 was previously shown to induce expansion of hematopoietic progenitor cells in coculture.34 To determine whether soluble factors from HS-5 cells are sufficient to support primary AML cell growth ex vivo, the viability of AML patient cells was measured over the course of 3 days when cultured in different dilutions of HS-5 CM. Compared with RPMI 1640 medium with 10% fetal bovine serum or a commercially available MCM, viability of primary AML cells was greater with all CM dilutions tested (supplemental Figure 1). A statistically significant increase in viability was observed after 3 days in 12 of 13 AML patient samples cultured in 25% CM compared with MCM, with mean viability of 113% in 25% CM and 80% in MCM (113.0 ± 10.36 in CM and 80.21 ± 9.43 in MCM; n = 13; P < .05) (Figure 1A). Because 25% CM was sufficient to support AML cell viability ex vivo, this concentration was used for subsequent assays.
HS-5 CM is enriched in inflammatory cytokines that promote the proliferation and survival of primary leukemic AML cells. (A) Freshly isolated AML cells were cultured in MCM or 25% HS-5 CM for 3 days, and cell viability assessed with the CTG assay. AML cells had a mean viability of 113% after 3 days in CM compared with 80% in MCM (113.0 ± 10.4 in CM and 80.2 ± 9.4 in MCM; n = 13; P < .05). Graphs show mean ± SEM. (B) Cytokine levels measured from HS-5 CM, primary MSCs CM from a patient with AML and BM supernatant fluid from a patient with AML and a healthy donor. (C) Phosphoflow analysis of phospho-ERK, phospho-AKT, phospho-STAT3, and phospho-STAT5 in AML patient cells treated with RPMI, 25% HS-5 CM, MCM, or RPMI supplemented with 10 ng/mL of IL-6, IL-8, MIP-3α, GM-CSF, G-CSF, or a combination of all the cytokines for 20 minutes, showing stimulation of STAT5 phosphorylation. (D) Detection of STAT5 phosphorylation in AML cells from 3 patients after 20 minutes of stimulation with different media conditions or individual cytokines (10 ng/mL). (E) Detection of phospho-STAT5 in AML patient cells treated for 1 hour with or without ruxolitinib (300 nM) after 20 minutes of stimulation with 25% CM or RPMI supplemented with 10 ng/mL GM-CSF, G-CSF, or a combination of the 2 cytokines. Error bars represent standard deviation of duplicates.
HS-5 CM is enriched in inflammatory cytokines that promote the proliferation and survival of primary leukemic AML cells. (A) Freshly isolated AML cells were cultured in MCM or 25% HS-5 CM for 3 days, and cell viability assessed with the CTG assay. AML cells had a mean viability of 113% after 3 days in CM compared with 80% in MCM (113.0 ± 10.4 in CM and 80.2 ± 9.4 in MCM; n = 13; P < .05). Graphs show mean ± SEM. (B) Cytokine levels measured from HS-5 CM, primary MSCs CM from a patient with AML and BM supernatant fluid from a patient with AML and a healthy donor. (C) Phosphoflow analysis of phospho-ERK, phospho-AKT, phospho-STAT3, and phospho-STAT5 in AML patient cells treated with RPMI, 25% HS-5 CM, MCM, or RPMI supplemented with 10 ng/mL of IL-6, IL-8, MIP-3α, GM-CSF, G-CSF, or a combination of all the cytokines for 20 minutes, showing stimulation of STAT5 phosphorylation. (D) Detection of STAT5 phosphorylation in AML cells from 3 patients after 20 minutes of stimulation with different media conditions or individual cytokines (10 ng/mL). (E) Detection of phospho-STAT5 in AML patient cells treated for 1 hour with or without ruxolitinib (300 nM) after 20 minutes of stimulation with 25% CM or RPMI supplemented with 10 ng/mL GM-CSF, G-CSF, or a combination of the 2 cytokines. Error bars represent standard deviation of duplicates.
To identify effector molecules in the HS-5 CM that enhanced AML cell viability, we analyzed the cytokine content of the HS-5 CM, CM collected from primary BM MSCs, and BM fluid. Using an antibody panel detecting 174 human cytokines, we identified high levels of IL-8, IL-6, GM-CSF, MCP-1, MIP-3α, and GRO in the HS-5 CM, BM fluid, and CM from primary MSCs (Figure 1C). Additional cytokines in the HS-5 CM exhibiting at least 2-fold or higher difference relative to baseline values in RPMI 1640 medium were GCP-2/CXCL6, G-CSF, MCP-2, TIMP-1 and TIMP-2, and angiogenin. Of the detected cytokines, only IL-8, IL-6, GM-CSF, and G-CSF were detected in the original study describing the HS-5 cell line, in which 19 different cytokines were analyzed.34 A complete list of the cytokines and detection values are presented in supplemental Table 2.
Expression levels of the corresponding receptors for the detected cytokines in the AML patient samples were measured by quantitative reverse transcription polymerase chain reaction. Although expression varied between patient samples, the majority expressed CXCR1, IL6R, CCR6, CSF2RA, and CSF3R, which bind IL-8, IL-6, MIP-3α, GM-CSF, and G-CSF, respectively (supplemental Figure 2). Many of the detected cytokines are predicted to induce proliferation of cells expressing the corresponding receptor; thus, the expression results suggest the cytokines may directly affect AML cell growth.35
Primary AML cells were stimulated with CM and phosphorylation of STAT3, STAT5, ERK, and AKT measured to assess the effect of stroma-derived factors on cellular signaling. Compared with control conditions, CM rapidly induced phosphorylation of STAT5 (Figure 1D). When individual cytokines were tested, we noted that GM-CSF and G-CSF alone could mimic the effect of CM on cellular signaling (Figure 1E). Taken together, these results show that BM stromal cell-derived cytokines increase STAT signaling, which may lead to enhanced survival of AML cells.
Stroma-derived factors alter primary AML cell drug responses
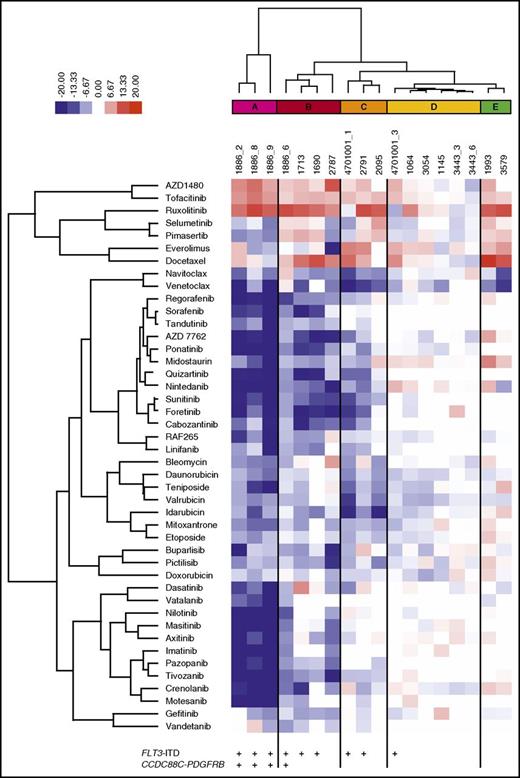
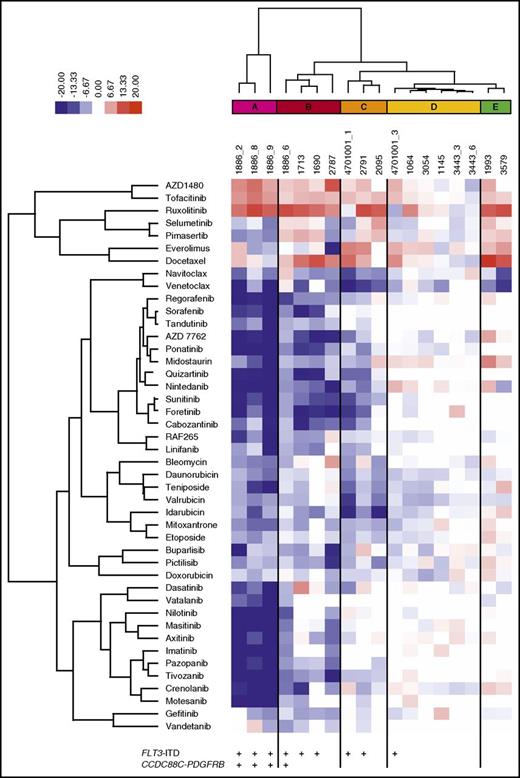
To determine the effect of BM stroma-derived factors on drug sensitivity, we tested 18 samples from 13 patients with AML collected at diagnosis (n = 4), relapse (n = 11), or refractory setting (n = 3). In addition, 304 anticancer compounds, including standard-of-care drugs cytarabine and anthracyclines (supplemental Table 3), were tested in 5 concentrations in both MCM and 25% CM to generate dose–response curves for each drug and sample (supplemental Table 4). A DSS was calculated, which was used as the metric to quantify the sensitivity of the samples to each drug, with higher DSS values indicating greater sensitivity (supplemental Figure 3).31 The mean DSS across all samples was determined for each drug in both culture conditions. The difference between the mean DSS for each drug in MCM and CM was quantified as a deltaDSS, which was used for hierarchical clustering of the tested drugs, resulting in 15 drug clusters (supplemental Figure 4). Across all samples, 12% of the 304 drugs were significantly affected (FDR < 0.05) by the screening conditions (Table 1; supplemental Table 5). When the analysis was performed separately, with samples harboring the activating internal tandem duplication in FLT3 (FLT3-ITD) or FLT3 wild-type samples, differential drug sensitivity was only observed in samples with FLT3-ITD mutations (15% of drugs affected by the conditions with FLT3-ITD and 0.3% without FLT3-ITD; n = 9; FDR < 0.05; supplemental Tables 5 and 6).
Stromal conditions protect AML cells from several TKIs
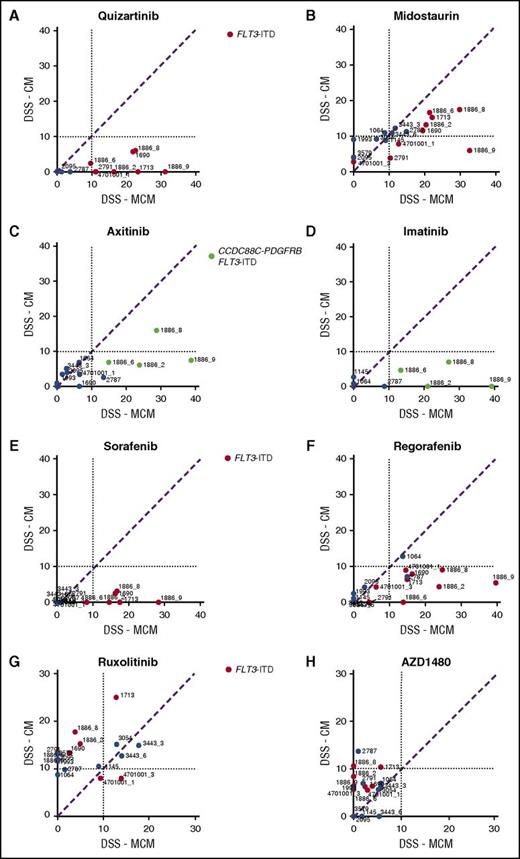
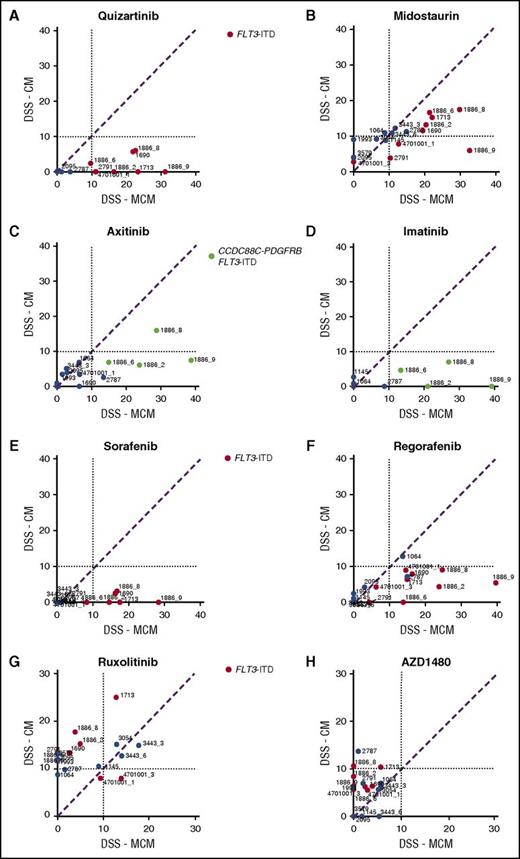
AML cells exhibited significantly lower sensitivity to TKIs targeting FLT3, PDGFRs, VEGFRs, ABL, and KIT when tested in CM compared with MCM (Figure 2A-F). The difference was particularly striking in AML cells harboring FLT3-ITD mutations (Figure 2A-B) or the CCDC88C-PDGFRB gene rearrangement (Figure 2C-D), in accordance with previous reports.21 These samples (groups A-C) were distinguished from samples without the alterations (groups D-E) by unsupervised clustering based on drug sensitivity differences between the CM and MCM conditions (Figure 3). Many samples were sensitive to JAK inhibitors (eg, ruxolitinib and AZD1480) when tested in CM, but not in MCM, regardless of FLT3-ITD status (Figure 2G-H). This was in line with the cytokines present in CM, which stimulate cell growth and differentiation by inducing pathways such as JAK/STAT.35
Stroma-derived factors protect AML MNCs from broad-spectrum TKIs. HS-5 CM induces resistance to drugs targeting (A-B) FLT3 and (C-D) PDGFR in samples from patients with AML carrying FLT3-ITD (red-colored samples vs blue-colored samples without the alteration) and/or CCDC88C-PDGFRB alterations (samples 1886_2, 1886_6, 1886_8, 1886_9, green-colored samples). (E-F) Likewise, sensitivity to broad-spectrum TKIs sorafenib and regorafenib is lost in the presence of CM, whereas (G-H) the samples with a FLT3-ITD show increased response to JAK kinase inhibitors ruxolitinib and AZD1480 in the presence of CM.
Stroma-derived factors protect AML MNCs from broad-spectrum TKIs. HS-5 CM induces resistance to drugs targeting (A-B) FLT3 and (C-D) PDGFR in samples from patients with AML carrying FLT3-ITD (red-colored samples vs blue-colored samples without the alteration) and/or CCDC88C-PDGFRB alterations (samples 1886_2, 1886_6, 1886_8, 1886_9, green-colored samples). (E-F) Likewise, sensitivity to broad-spectrum TKIs sorafenib and regorafenib is lost in the presence of CM, whereas (G-H) the samples with a FLT3-ITD show increased response to JAK kinase inhibitors ruxolitinib and AZD1480 in the presence of CM.
Functional taxonomy of AML patient samples based on differential drug response profiles between stroma-derived CM and standard culture conditions. Unsupervised clustering of the patient samples based on drug sensitivity differences between CM and MCM (deltaDSS) separates the samples into 5 subgroups (A-E). AML samples with a FLT3-ITD (groups A-C) are distinguished from samples without the alteration (groups D-E).
Functional taxonomy of AML patient samples based on differential drug response profiles between stroma-derived CM and standard culture conditions. Unsupervised clustering of the patient samples based on drug sensitivity differences between CM and MCM (deltaDSS) separates the samples into 5 subgroups (A-E). AML samples with a FLT3-ITD (groups A-C) are distinguished from samples without the alteration (groups D-E).
Stromal conditions reduce sensitivity to BCL2 inhibitors
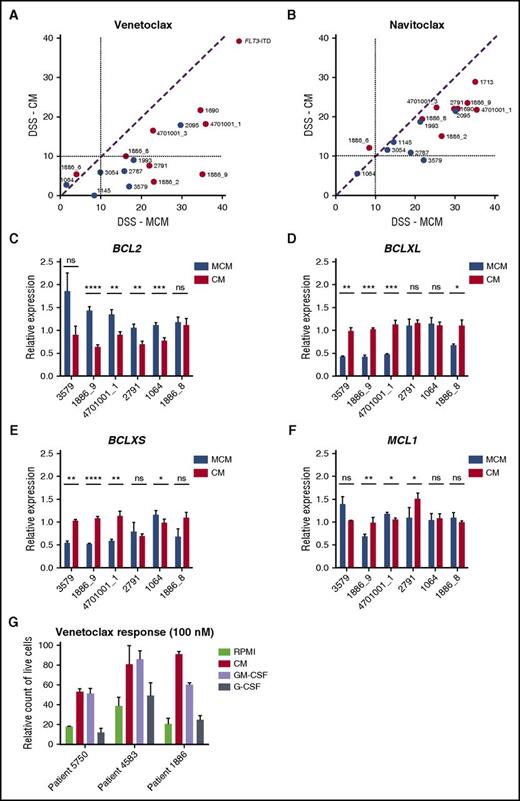
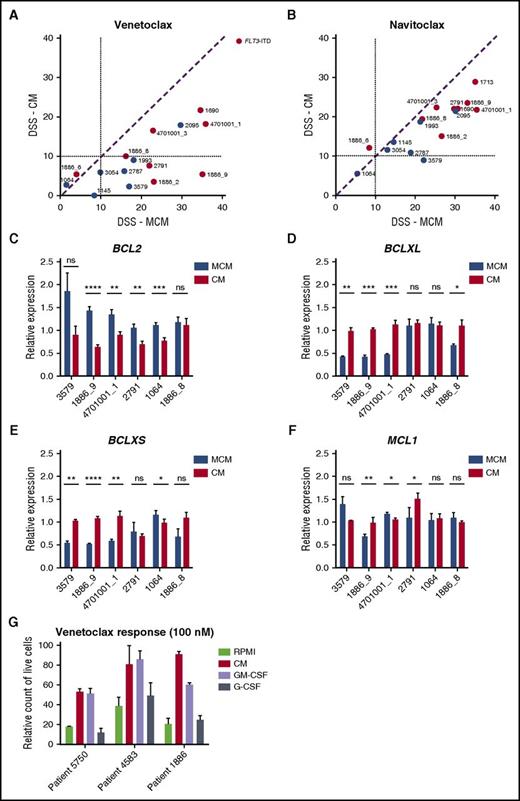
AML cells in HS-5 CM were less sensitive to BCL2 inhibitor venetoclax and slightly less sensitive to BCL2/BCLXL inhibitor navitoclax (Figure 4A-B). These results suggest that stroma-derived factors stimulate signals that make the leukemia cells resistant to BCL2 inhibition. To determine whether prosurvival factors such as BCL2, BCLXL, and MCL1 are affected by the culture conditions, we measured expression of these genes after incubating cells in either MCM or CM for 48 hours. All samples tested expressed lower levels of BCL2 after incubation in CM compared to MCM, with 4/6 showing a statistically significant difference in expression (Figure 4C). In contrast, most samples (4/6) had significantly higher expression of BCLXL and BCLXS in CM compared with MCM (Figure 4D-E). The expression of MCL1, however, was unaffected (Figure 4F). Taken together, BM stromal conditions switched the AML cells to be less dependent on BCL2-driven survival.
The effect of stroma-based conditions vs MCM on AML cell response to BCL2 inhibitors. (A-B) Samples show decreased sensitivity to BCL2-specific inhibitor venetoclax and to BCL2/BCLXL inhibitor navitoclax in the presence of HS-5 CM. (C-E) CM results in decreased BCL2 expression and induction of BCLXL and BCLXS expression in AML patient cells. (F) No difference in MCL1 expression was detected in the 2 conditions. Bar plots represent the mRNA expression for BCL2 genes after 48 hours of incubation of AML cells (n = 6) in 25% HS-5 CM and MCM medium. Data are normalized against GAPDH expression and error bars represent standard deviation of at least 2 replicates. (G) Flow cytometry analysis showing an amount of live CD45+ AML cells cultured in RPMI, 25% HS-5 CM, or RPMI supplemented with 10 ng/mL GM-CSF or G-CSF after 48 hours of treatment with venetoclax (100 nM). Error bars represent standard deviation of 3 replicates. ns, not significant. *P < .05; **P < .01; ***P < .001; ****P < .0001.
The effect of stroma-based conditions vs MCM on AML cell response to BCL2 inhibitors. (A-B) Samples show decreased sensitivity to BCL2-specific inhibitor venetoclax and to BCL2/BCLXL inhibitor navitoclax in the presence of HS-5 CM. (C-E) CM results in decreased BCL2 expression and induction of BCLXL and BCLXS expression in AML patient cells. (F) No difference in MCL1 expression was detected in the 2 conditions. Bar plots represent the mRNA expression for BCL2 genes after 48 hours of incubation of AML cells (n = 6) in 25% HS-5 CM and MCM medium. Data are normalized against GAPDH expression and error bars represent standard deviation of at least 2 replicates. (G) Flow cytometry analysis showing an amount of live CD45+ AML cells cultured in RPMI, 25% HS-5 CM, or RPMI supplemented with 10 ng/mL GM-CSF or G-CSF after 48 hours of treatment with venetoclax (100 nM). Error bars represent standard deviation of 3 replicates. ns, not significant. *P < .05; **P < .01; ***P < .001; ****P < .0001.
To identify a factor or factors contributing to CM-induced protection of AML cells from BCL2 inhibition, we analyzed the effect of IL-6, IL-8, MIP-3α, GM-CSF, and G-CSF on viability when added individually to MNCs collected from patients with AML in the presence of venetoclax. GM-CSF, and to some extent G-CSF, alone mimicked the reduced sensitivity to venetoclax conferred by HS-5 CM (Figure 4G; supplemental Figure 5). Both GM-CSF and G-CSF are known to activate JAK/STAT signaling.36,37 To determine the contribution of STAT activation on venetoclax sensitivity, we knocked-down expression of STAT3 in the venetoclax-resistant HEL cell line,38 which resulted in increased venetoclax sensitivity (supplemental Figure 6). This indicates that JAK/STAT signaling may contribute to venetoclax resistance.
Ruxolitinib restores activity of venetoclax against AML cells in stromal conditions
To determine whether stroma-induced resistance to BCL2 inhibitor venetoclax and FLT3 inhibitor quizartinib could be counteracted by chemical inhibition of JAK/STAT signaling, the drugs were tested in combination with ruxolitinib, using AML patient cells (n = 13) cultured in MCM or CM. Ruxolitinib rescued AML cell sensitivity to venetoclax in the presence of CM, and the combination of the 2 drugs exhibited synergistic activity in this setting. The combinatorial activity, however, was not recapitulated in the MCM condition (Figure 5A-B). In contrast to earlier studies,22,23 we did not detect synergism between ruxolitinib and quizartinib in the presence or absence of CM (data not shown).
JAK1/2 inhibitor restores activity of a BCL2 antagonist against AML cells in stroma-based conditions. (A-B) Combinatorial treatment of AML cells with ruxolitinib and venetoclax shows synergistic activity between the inhibitors in the presence of CM, whereas the synergy is less pronounced in MCM. AML cells were cultured either in 25% HS-5 CM or MCM for 72 hours in the presence of various concentrations of ruxolitinib, venetoclax, or a combination of the 2 agents, and cell viability was measured using the CTG assay. For the combination matrices, the interaction landscapes are shown in 2 dimensional. δ, the difference in percentage inhibition compared with the expected additive compound effect calculated by the ZIP model. (C) Ruxolitinib and venetoclax combination reduces colony-forming ability of primary AML cells in CM. AML cells from 2 patients were left untreated (0.1% dimethyl sulfoxide [DMSO] = control) or treated with ruxolitinib (300 nM), venetoclax (100 nM), or their combination in MCM or 25% CM medium for 72 hours and plated in methylcellulose progenitor assay. Total CFC output was recorded after 14 days. (D) CD34+ expression after culture of primary AML cells for 48 hours with or without drug treatment (300 nM ruxolitinib, 100 nM venetoclax, or their combination) with RPMI, 25% HS-5 CM, direct contact with AML-derived BM MSCs, or separated from stroma with a 0.4-µm pore membrane. Error bars represent standard deviation from 2 independent experiments.
JAK1/2 inhibitor restores activity of a BCL2 antagonist against AML cells in stroma-based conditions. (A-B) Combinatorial treatment of AML cells with ruxolitinib and venetoclax shows synergistic activity between the inhibitors in the presence of CM, whereas the synergy is less pronounced in MCM. AML cells were cultured either in 25% HS-5 CM or MCM for 72 hours in the presence of various concentrations of ruxolitinib, venetoclax, or a combination of the 2 agents, and cell viability was measured using the CTG assay. For the combination matrices, the interaction landscapes are shown in 2 dimensional. δ, the difference in percentage inhibition compared with the expected additive compound effect calculated by the ZIP model. (C) Ruxolitinib and venetoclax combination reduces colony-forming ability of primary AML cells in CM. AML cells from 2 patients were left untreated (0.1% dimethyl sulfoxide [DMSO] = control) or treated with ruxolitinib (300 nM), venetoclax (100 nM), or their combination in MCM or 25% CM medium for 72 hours and plated in methylcellulose progenitor assay. Total CFC output was recorded after 14 days. (D) CD34+ expression after culture of primary AML cells for 48 hours with or without drug treatment (300 nM ruxolitinib, 100 nM venetoclax, or their combination) with RPMI, 25% HS-5 CM, direct contact with AML-derived BM MSCs, or separated from stroma with a 0.4-µm pore membrane. Error bars represent standard deviation from 2 independent experiments.
The effects of ruxolitinib and venetoclax as single agents and in combination were analyzed on the CFC capacity of AML cells in CM and MCM conditions. Although venetoclax treatment in MCM inhibited CFC of AML cells, it did not lead to significant reduction in colonies when AML cells were grown in CM. However, the combination of venetoclax and ruxolitinib significantly reduced the CFC output in CM, showing the combination is more effective than venetoclax alone in eradicating AML cells in the stroma-based condition (Figure 5C).
To determine whether the protective effect of stroma on BCL2 inhibition was dependent on cell-to-cell interactions, we cultured AML patient cells either in direct contact with AML-derived BM MSCs or separated from stroma with a 0.4-µm pore membrane. Forty-eight hours of treatment with 100 nM venetoclax did not result in significant reduction of CD34+ AML cells, regardless of whether AML cells were directly cultured with stroma or separated by a membrane, further indicating that stroma-derived soluble factors are sufficient to reduce sensitivity to venetoclax. Combined treatment with venetoclax and ruxolitinib, however, significantly reduced the number of CD34+ AML cells in both assays (Figure 5D).
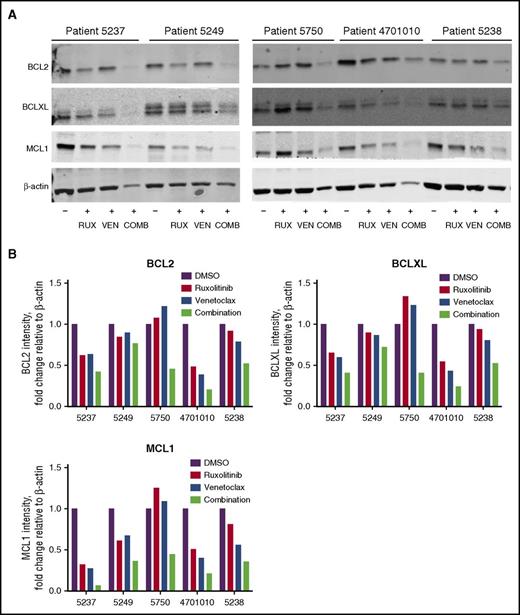
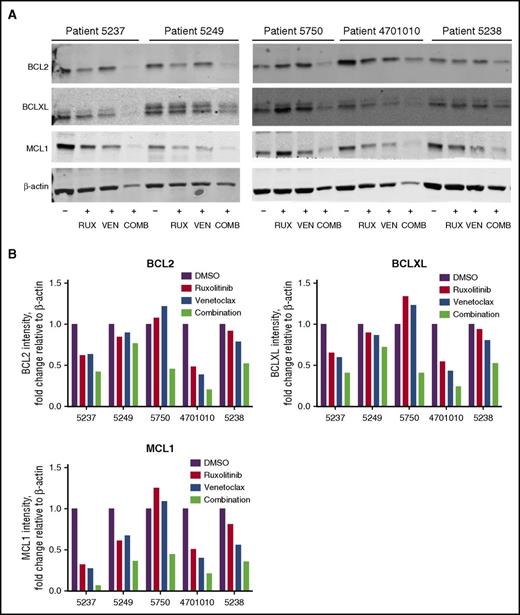
To determine drug effects on BCL2 family member expression, BCL2, BCLXL, and MCL1 protein levels were assessed in AML patient cells after treatment with ruxolitinib, venetoclax, or their combination when cultured in CM. In general, the combination resulted in a greater decrease in BCL2, BCLXL, and MCL1 protein levels compared with the single agents (Figure 6A-B).
Reduced BCL2, BCLXL, and MCL1 protein in AML patient cells treated with ruxolitinib and venetoclax. (A) Western blot analysis of BCL2, BCLXL, and MCL1 proteins in AML patient cells after treatment with 300 nM ruxolitinib (RUX), 100 nM venetoclax (VEN), or a combination of both drugs (COMB) in 25% HS-5 CM. Protein lysates for patient cells (n = 5) were collected 48 hours after drug treatment and analyzed by immunoblotting, using antibodies to the antigens indicated. Results are shown for patients 5237, 5249, 5750, 4701010, and 5238. (B) Quantification of relative protein levels for BCL2, BCLXL, and MCL1 by analysis of chemiluminescence signals, using Odyssey v2.0 software corrected to the intensities obtained with the β-actin antibody.
Reduced BCL2, BCLXL, and MCL1 protein in AML patient cells treated with ruxolitinib and venetoclax. (A) Western blot analysis of BCL2, BCLXL, and MCL1 proteins in AML patient cells after treatment with 300 nM ruxolitinib (RUX), 100 nM venetoclax (VEN), or a combination of both drugs (COMB) in 25% HS-5 CM. Protein lysates for patient cells (n = 5) were collected 48 hours after drug treatment and analyzed by immunoblotting, using antibodies to the antigens indicated. Results are shown for patients 5237, 5249, 5750, 4701010, and 5238. (B) Quantification of relative protein levels for BCL2, BCLXL, and MCL1 by analysis of chemiluminescence signals, using Odyssey v2.0 software corrected to the intensities obtained with the β-actin antibody.
Combined treatment with ruxolitinib and venetoclax is more effective than either agent alone in an AML xenograft mouse model
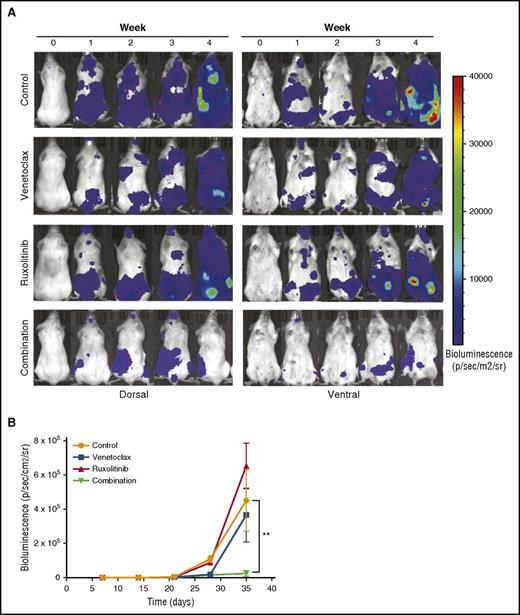
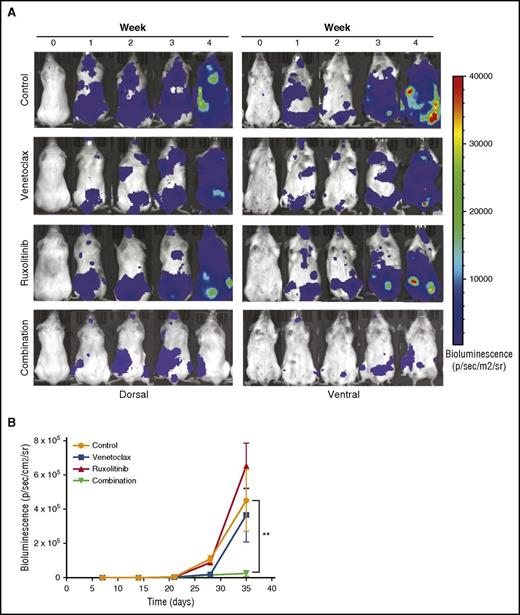
To evaluate the efficacy of the ruxolitinib and venetoclax combination in vivo, we inoculated NSG mice IV with MOLM-13luc cells and, after engraftment, divided the mice into control, venetoclax, ruxolitinib, and combination groups. The animals were imaged for bioluminescence and followed for weight loss and overall survival for 4 weeks. At the end of the treatment period, bioluminescence imaging showed similar antitumor effects for venetoclax and the combination, whereas a significant difference was observed between the treatment groups at week 4, 1 week after treatment termination (Figure 7A-B). At this time, the single-drug treatments did not have an effect on tumor burden, whereas the combination significantly prevented MOLM-13luc cell growth in the NSG mice (Figure 7A-B). Overall survival, however, was not significantly increased in the combination group compared with in the single-drug-treated groups. This could have been a result of toxicity issues, which warrant further optimization in future studies (supplemental Figure 7).
Inhibition of JAK/STAT and BCL2 signaling by ruxolitinib and venetoclax reduces tumor burden in an AML xenograft mouse model. Twenty-four NSG mice engrafted with luciferase expressing MOLM-13 cells were treated with vehicle, venetoclax (25 mg/kg, intraperitoneally), ruxolitinib (50 mg/kg, by mouth), or both for 3 weeks. (A) Each row represents an individual mouse from each treatment group. Bioluminescence images shown before the start of the treatment, each week of the treatment period, and 1 week after the end of the treatment. (B) Mean bioluminescence of all mice in each treatment group (n = 6 for each group) recorded before the start of the treatment, during the 3-week treatment period, and 1 week after the end of the treatment. Error bars represent standard error of the mean. Statistically significant difference is represented by asterisk, which signify **P < .01 by Mann-Whitney test.
Inhibition of JAK/STAT and BCL2 signaling by ruxolitinib and venetoclax reduces tumor burden in an AML xenograft mouse model. Twenty-four NSG mice engrafted with luciferase expressing MOLM-13 cells were treated with vehicle, venetoclax (25 mg/kg, intraperitoneally), ruxolitinib (50 mg/kg, by mouth), or both for 3 weeks. (A) Each row represents an individual mouse from each treatment group. Bioluminescence images shown before the start of the treatment, each week of the treatment period, and 1 week after the end of the treatment. (B) Mean bioluminescence of all mice in each treatment group (n = 6 for each group) recorded before the start of the treatment, during the 3-week treatment period, and 1 week after the end of the treatment. Error bars represent standard error of the mean. Statistically significant difference is represented by asterisk, which signify **P < .01 by Mann-Whitney test.
Discussion
The BM microenvironment supports the survival of leukemic cells and influences response to therapeutic agents, which can contribute to development of drug resistance. Although new methods and models have been developed that better reflect the tumor microenvironment for diseases such as AML, the effect of the microenvironment on responses to different drug classes has not previously been comprehensively evaluated. Here, we compared sensitivity of primary AML patient cells with 304 small molecule anticancer agents in a standard culture medium and CM from a BM stromal cell line. We identified drug types affected by the culture conditions that would presumably be affected by different microenvironments. Of the tested drugs, 12% were significantly affected by the culture conditions, with many of these being developed as therapies for AML.
Earlier studies showed that direct contact with HS-5 monolayers improves AML cell viability and protects leukemic cells from spontaneous and drug-induced cell death against agents such as cytarabine and daunorubicin.20 In our study, we observed that CM from HS-5 cultures was sufficient to maintain AML cell viability and induced better growth ex vivo compared with other commercial media. Notably, the response of primary AML cells to standard induction agents idarubicin and daunorubicin was muted when cells were incubated in HS-5 CM, whereas cytarabine responses were similar. Previous studies indicated that protection from cytarabine may be a result of direct cell–cell contact, whereas stroma-secreted soluble factors are sufficient to reduce sensitivity to topoisomerase II inhibitors.20,39 As for other chemotherapeutic agents, primary AML cells were more sensitive to antimitotic taxanes and vinca alkaloids when tested in CM, presumably as a result of increased proliferation of the cells in that condition.
Interestingly, a number of TKIs were less potent against primary AML cells in HS-5 CM, whereas serine-threonine kinase inhibitors were not affected. In earlier studies, stromal cells were reported to protect FLT3-ITD-positive AML cells from FLT3 inhibitors SU614, sorafenib, and midostaurin, as well as BCR-ABL inhibitor nilotinib.22-24 Weisberg and colleagues noted that both direct adherence to stroma and CM led to quizartinib and midostaurin resistance.22,23 We expanded these observations to other broad-spectrum TKIs targeting VEGFR, PDGFR, MET, ABL, and KIT, which exhibited reduced efficacy against AML cells in the presence of HS-5 CM. The difference in activity was most obvious in AML cells with FLT3-ITD or an activating PDGFRB alteration, suggesting HS-5CM–induced alternate pathways not targeted by the TKIs. These observations are supported by clinical studies showing depletion of circulating blasts in the peripheral blood, but lack of long-term efficacy in patients treated with FLT3 inhibitors, suggesting cytoprotection conferred by the tumor microenvironment.10,11,13
BCL2 inhibitors were also less effective against primary AML cells when tested in HS-5 CM. Earlier reports showed high sensitivity to BCL2 inhibitors navitoclax and venetoclax with primary patient samples and AML cell lines.29,38 In this study, primary AML cells were less sensitive to venetoclax in CM compared with standard culture conditions. Depending on the microenvironment, the actual anticancer efficacy of venetoclax in AML may differ. Similar observations have been made with chronic lymphocytic leukemia cells residing within lymph nodes.40 Mechanistically, the resistance of chronic lymphocytic leukemia cells to BCL2 inhibitors was explained by upregulation of additional BCL2 family members, such as BCLXL, MCL1, and BFL1, which are not inhibited by venetoclax.40 A similar shift from BCL2 to BCLXL expression was observed when primary AML cells were cultured in CM, which would explain the loss of sensitivity to BCL2-specific venetoclax. Navitoclax, however, retained activity in both conditions, possibly as a result of simultaneous targeting of BCLXL and BCL2. Hence, inhibition of both BCL2 and BCLXL is likely required for overcoming stroma-mediated resistance to BCL2 inhibitors. However, navitoclax leads to severe thrombocytopenia, which has stopped clinical exploration of the drug in leukemia. Therefore, an alternate strategy may be the combination of venetoclax with an agent that blocks the switch from BCL2 to BCLXL dependence.
The observed drug responses of AML cells in HS-5 CM are likely explained by the presence of cytokines that lead to activation of alternate signaling pathways. Drugs with the largest gain in activity when tested in CM included the JAK1/2 inhibitor ruxolitinib. Notably, of the most abundant cytokines secreted by HS-5 cells, G-CSF and GM-CSF led to increased phosphorylation of STAT5, a downstream effector of JAKs. This observation led us to test JAK1/2 inhibitors as a combination therapy partner against AML cells. We found that ruxolitinib potentiated sensitivity to venetoclax when tested with AML patient cells in HS-5 CM, and the combination effectively inhibited growth of CD34+ AML cells in a CFC assay, and in coculture and transwell assays with AML patient-derived MSCs. Significantly, combined treatment with ruxolitinib and venetoclax was more effective at reducing tumor burden in an AML mouse model than either drug alone, although optimal dosing and the pharmacokinetics of the drugs require further investigation.
In conclusion, data from comprehensive drug sensitivity testing indicate that ex vivo drug responses of AML cells are highly affected by BM derived factors, which confer resistance to several agents, including TKIs, topoisomerase II, and BCL2 inhibitors. Although our approach has limitations and does not identify selected drug-resistant subclones, the results underscore the need to use drug sensitivity testing methods that take into account tumor–microenvironment interactions. Testing drugs in different conditions also provides an index of the effect of the BM and other culture conditions on the drug’s efficacy and could help predict drug effects that may be difficult to translate from in vitro and ex vivo conditions to the clinic. Importantly, we identified an effective novel drug combination of ruxolitinib and venetoclax, which helped overcome BM microenvironment-mediated cytoprotection to BCL2 inhibitors and warrants further investigation.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the patients and healthy donors for their samples, our research staff of the Institute for Molecular Medicine Finland Technology Center, High Throughput Biomedicine Unit, the Breeze team, and the Hematology Research Unit for their excellent technical assistance.
The study was financially supported by the Finnish Funding Agency for Technology and Innovation–Tekes and the European Regional Development Funds, as well as grants from the Academy of Finland, Cancer Society of Finland, and Sigrid Juselius Foundation. R.K., M.M.M., and B.Y. are supported by grants from the University of Helsinki Doctoral School Programs in Health. D.T. is supported by the People Programme (Marie Curie Actions) of the Seventh Framework Programme of the European Union (FP7/2007-2013) under Research Executive Agency grant agreement number 600388, and the Agency of Competitiveness for Companies of the Government of Catalonia, Agency of Competitiveness for Companies of the Government of Catalonia. E.M. is supported by grants from the Norwegian Cancer Society (grant number 732200) and the Western Health Board of Norway (grant numbers 911779 and 911182).
Authorship
Contribution: R.K., T.P., K.W., and C.A.H. designed research; R.K., T.P., M.L., K.K.J., M.M.M., and A.P. performed experiments; R.K., M.P., M.M.S., and E.M. contributed to the mouse model experiments; R.K., T.P., and M.M.M. obtained patient sample data; M.K., B.T.G., and K.P. collected clinical samples and obtained ethical permits; all authors contributed to data analysis and interpretation; J.K. and C.A.H. supervised the study; R.K. and C.A.H. wrote the paper; and all authors contributed to and approved the final version of the manuscript.
Conflict-of-interest disclosure: B.T.G. is a member of the board of directors/advisory committees and has an equity ownership of BerGenBio AS and is a member of the board of directors/advisory committees of Boehringer Ingelheim. B.T.G. and E.M. also have equity ownerships of KinN Therapeutics AS. K.P. has received honoraria and grant support from Bristol-Myers Squibb, Celgene, Novartis, and Pfizer. O.K. has received commercial research support from Bayer, Pfizer, Roche, and the Innovative Medicines Initiatives (a consortium for pharmaceutical companies) project PREDECT and is a consultant/advisory board member of Medisapiens and received royalty from Vysis-Abbot. K.W. has received research funding from Novartis and Pfizer. C.A.H. has received research funding from Celgene, Novartis, Orion, Pfizer, and Innovative Medicines Initiatives 2 project HARMONY. The remaining authors declare no competing financial interests.
The current affiliation for T.P. is Research Center for Molecular Medicine of the Austrian Academy of Sciences, Vienna, Austria.
Correspondence: Caroline A. Heckman, Institute for Molecular Medicine Finland, P.O. Box 20 (Tukholmankatu 8), University of Helsinki, FI-00014 Helsinki, Finland; e-mail: caroline.heckman@helsinki.fi.

![Figure 5. JAK1/2 inhibitor restores activity of a BCL2 antagonist against AML cells in stroma-based conditions. (A-B) Combinatorial treatment of AML cells with ruxolitinib and venetoclax shows synergistic activity between the inhibitors in the presence of CM, whereas the synergy is less pronounced in MCM. AML cells were cultured either in 25% HS-5 CM or MCM for 72 hours in the presence of various concentrations of ruxolitinib, venetoclax, or a combination of the 2 agents, and cell viability was measured using the CTG assay. For the combination matrices, the interaction landscapes are shown in 2 dimensional. δ, the difference in percentage inhibition compared with the expected additive compound effect calculated by the ZIP model. (C) Ruxolitinib and venetoclax combination reduces colony-forming ability of primary AML cells in CM. AML cells from 2 patients were left untreated (0.1% dimethyl sulfoxide [DMSO] = control) or treated with ruxolitinib (300 nM), venetoclax (100 nM), or their combination in MCM or 25% CM medium for 72 hours and plated in methylcellulose progenitor assay. Total CFC output was recorded after 14 days. (D) CD34+ expression after culture of primary AML cells for 48 hours with or without drug treatment (300 nM ruxolitinib, 100 nM venetoclax, or their combination) with RPMI, 25% HS-5 CM, direct contact with AML-derived BM MSCs, or separated from stroma with a 0.4-µm pore membrane. Error bars represent standard deviation from 2 independent experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/130/6/10.1182_blood-2016-02-699363/4/m_blood699363f5.jpeg?Expires=1769300567&Signature=BcTTl09V734GdIt8EJJEidZwnHLixSK1yIg~k8t-xMQhil0dJ6Gbe1v10JvgaDY1xsTk65K5rRMHcFgyqwkqNtExG3rHycO634NhIth3oonytaaVaiejBRgmBcWQ-ifa~z2KkItAYXbHyLr2Br6On5cv-gtsgxWxcjIDNZ9faahDLWqFjNfXiPyLBFKNy-XkQc6cHDDn2EUL4SuCuaxrsGa4~~xwSz8l27bilgunfuZ0ZSvioErHrtFaBHcPsur7G22g8aKlmDW2xzfE05dxuZAfRfT9-1VtQr9DvKZ7TjveN2DX1NDY~cyHf93rXHJPN1uIV5HnKVHtTYdyqvD5QA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Figure 5. JAK1/2 inhibitor restores activity of a BCL2 antagonist against AML cells in stroma-based conditions. (A-B) Combinatorial treatment of AML cells with ruxolitinib and venetoclax shows synergistic activity between the inhibitors in the presence of CM, whereas the synergy is less pronounced in MCM. AML cells were cultured either in 25% HS-5 CM or MCM for 72 hours in the presence of various concentrations of ruxolitinib, venetoclax, or a combination of the 2 agents, and cell viability was measured using the CTG assay. For the combination matrices, the interaction landscapes are shown in 2 dimensional. δ, the difference in percentage inhibition compared with the expected additive compound effect calculated by the ZIP model. (C) Ruxolitinib and venetoclax combination reduces colony-forming ability of primary AML cells in CM. AML cells from 2 patients were left untreated (0.1% dimethyl sulfoxide [DMSO] = control) or treated with ruxolitinib (300 nM), venetoclax (100 nM), or their combination in MCM or 25% CM medium for 72 hours and plated in methylcellulose progenitor assay. Total CFC output was recorded after 14 days. (D) CD34+ expression after culture of primary AML cells for 48 hours with or without drug treatment (300 nM ruxolitinib, 100 nM venetoclax, or their combination) with RPMI, 25% HS-5 CM, direct contact with AML-derived BM MSCs, or separated from stroma with a 0.4-µm pore membrane. Error bars represent standard deviation from 2 independent experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/130/6/10.1182_blood-2016-02-699363/4/m_blood699363f5.jpeg?Expires=1769524602&Signature=nNxoSfdrhCZfCUPC61y-gDEOzQguc~RJ0l7TOhqSLAUwkDb74EMUqjd02FMYolIDITIOt3B82YWjAyGp0I6vwd1Qup4~2DG30Trch~lwzo-VWbToqpjWtE2YALXHwhZcctOAde98gnwWLQMsjZubYRl4UlMgE25uOWKLbMZUZOuKGKuYcS~lA82TRbU2fdSxo4y-nBaO7WopWOr80CUYK2j0QspJzdMm66VwsnoPnU6V3TeGZPZJoAldFFSic8ykqbrTTB-BYnxBigonoW0AmtfoDqqIWmkB3b1o6JWxpbeDSNCW-HsgrpfYo9DFye8AoEadSMSlzpcPc45lndv2zQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)