Key Points
Anti-sclerostin treatment increases bone mass and fracture resistance in MM
Anti-sclerostin in combination with zoledronic acid is superior to zoledronic acid alone in increasing fracture resistance.
Abstract
Multiple myeloma (MM) is a plasma cell cancer that develops in the skeleton causing profound bone destruction and fractures. The bone disease is mediated by increased osteoclastic bone resorption and suppressed bone formation. Bisphosphonates used for treatment inhibit bone resorption and prevent bone loss but fail to influence bone formation and do not replace lost bone, so patients continue to fracture. Stimulating bone formation to increase bone mass and fracture resistance is a priority; however, targeting tumor-derived modulators of bone formation has had limited success. Sclerostin is an osteocyte-specific Wnt antagonist that inhibits bone formation. We hypothesized that inhibiting sclerostin would prevent development of bone disease and increase resistance to fracture in MM. Sclerostin was expressed in osteocytes from bones from naive and myeloma-bearing mice. In contrast, sclerostin was not expressed by plasma cells from 630 patients with myeloma or 54 myeloma cell lines. Mice injected with 5TGM1-eGFP, 5T2MM, or MM1.S myeloma cells demonstrated significant bone loss, which was associated with a decrease in fracture resistance in the vertebrae. Treatment with anti-sclerostin antibody increased osteoblast numbers and bone formation rate but did not inhibit bone resorption or reduce tumor burden. Treatment with anti-sclerostin antibody prevented myeloma-induced bone loss, reduced osteolytic bone lesions, and increased fracture resistance. Treatment with anti-sclerostin antibody and zoledronic acid combined increased bone mass and fracture resistance when compared with treatment with zoledronic acid alone. This study defines a therapeutic strategy superior to the current standard of care that will reduce fractures for patients with MM.
Introduction
Multiple myeloma (MM) is a neoplastic disease of B cells that develops in the skeleton. About 86 000 new cases of myeloma are diagnosed globally each year,1 and 95% of patients develop bone disease, which leads to a 16-fold increase in the risk of skeletal fractures, most commonly in the vertebrae.1-6 The bone disease results from tumor cell production of paracrine factors that increase osteoclast-mediated bone resorption and suppress bone formation by osteoblasts.7,8 Inhibiting the osteoclastic bone resorption component prevents development of myeloma bone disease.9-13 The bisphosphonate zoledronic acid (ZA), which inhibits osteoclasts directly, is now the standard of care for treating myeloma bone disease. Although this prevents further bone loss and can reduce skeletal-related events, bisphosphonates do not stimulate bone formation, and patients continue to suffer skeletal-related events, including fractures.14 Thus, comprehensive treatment of MM bone disease requires not only inhibition of osteoclastic resorption but also stimulation of osteoblastic bone formation.
Wnt signaling is important in regulating osteoblastic bone formation.15 Soluble Wnt antagonists are critical components of this system and inhibit bone formation.15 The Wnt antagonist Dickkopf-1 (DKK1) is expressed by myeloma cells, and serum levels are elevated in some patients.16-19 Furthermore, inhibiting DKK1 in experimental models of myeloma prevents bone disease, suggesting that this soluble Wnt antagonist has a role in osteolysis.20-22 Activin, a member of the transforming growth factor β superfamily, is also expressed by myeloma cells; inhibiting activin increases bone formation and prevents bone loss in models of myeloma.23-25 However, these approaches that target tumor-derived factors have yet to be translated successfully into the clinic, with only a portion of patients responding to treatment,26 which likely reflects their heterogeneous expression within myeloma tumors and between patients. A superior strategy that has yet to be explored is to target molecules in the bone microenvironment that are tumor independent and more likely common to all patients. One clear candidate with expression restricted to osteocytes embedded within bone matrix is sclerostin, a soluble Wnt antagonist that controls bone formation.27,28 Concentrations of sclerostin are elevated in the serum of patients with myeloma.29 Anti-sclerostin antibodies powerfully promote bone formation and increase bone mass in models of osteoporosis and bone repair.30-34 In patients with osteoporosis, treatment with anti-sclerostin antibody increases bone mass and reduces fracture incidence across large clinical cohorts.35,36 We hypothesized that combining existing antiresorptive therapy with inhibition of sclerostin will increase bone formation and bone mass and thus decrease fracture susceptibility in MM, thereby defining an important new therapeutic strategy to treat myeloma bone disease. To address this, we determined whether sclerostin was expressed by myeloma cells or restricted to osteocytes and evaluated the effect of anti-sclerostin antibody alone and in combination with bisphosphonate therapy on the skeleton in a series of well-characterized murine and human xenograft models of myeloma bone disease.
Patients and methods
Analysis of samples from human myeloma patients
Patients with previously untreated therapy-requiring or relapsed myeloma and healthy donors were included in the study approved by the University of Heidelberg ethics committees (#229/2003 and #S-152/2010) after written informed consent. Normal bone marrow plasma cells (BMPCs) and myeloma cells were purified by using anti-CD138.37-39
Myeloma cell lines and murine primary cell harvests
Murine 5TGM1-enhanced green fluorescent protein (5TGM1-eGFP), human MM1.S-luc-eGFP, and OPM2 myeloma cells were cultured as previously described.40,41 Murine 5T2MM cells were maintained by serial passage in C57BL/KaLwRijHsd (BKAL) mice.42
Peripheral blood CD27+ memory B cells were subjected to fluorescence-activated cell sorting, and polyclonal plasmablast cells (PPCs) were generated as described.43 Human myeloma cell lines and sources are reported in supplemental Information (available on the Blood Web site). Primary osteocytes were isolated as previously described.44
SOST and DKK1 gene expression
Gene expression profiling was performed by using U133 2.0 plus arrays (Affymetrix).38,45 For RNA sequencing (RNA-seq), complementary DNA was amplified by using the SMARTer Ultra Low RNA Kit (Illumina). NEBNext ChIP-Seq Library Prep protocol (New England BioLabs) was used for library preparation. Samples were sequenced on an Illumina HiSeq2000 sequencing system (Illumina).
Osteocyte RNA-seq analysis
Osteocyte enrichment of gene expression was determined by comparing transcriptome sequencing data from osteocyte-enriched bones void of bone marrow with samples that had intact bone marrow. Transcriptome analysis of osteocyte gene expression from tumor-bearing bones relative to naive samples was performed following RNA extraction from homogenized (TriReagent, Sigma-Aldrich) osteocyte-enriched bone, quality checks by Total RNA Pico Chip (Agilent Technologies), and depletion of ribosomal RNA using RNaseH (Epicentre) and ribosomal RNA–targeting oligonucleotides.46 Total-RNA library preparation was performed by using the TruSeq Stranded Total RNA LT Sample Prep Kit (Illumina). Samples were sequenced on a HiSequation 2500 sequencer (Illumina). Details are described in supplemental Information.
Measuring sclerostin protein
Secreted sclerostin (SOST) protein was assayed in media from 5TGM1, MM1.S, OPM2, and primary osteocyte cultures by using an enzyme-linked immunosorbent assay (R&D Systems).
Animal experiments
Animal experiments were performed in accordance with approved protocols from St. Vincent’s Hospital Animal Ethics Committee (ARA 14/43) and Dana-Farber Cancer Institute Animal Care and Use Committee (14-001). BKAL mice (Harlan, The Netherlands) were injected intravenously with syngeneic 5TGM1-eGFP or 5T2MM murine myeloma cells.40,42 SCID-beige or NOD.Cg-Prkdcscid/Il2rgtm1Wjl/SzJ (NSG) mice were injected intravenously with MM1.S-luc-eGFP human myeloma cells. Vehicle buffer or anti-sclerostin antibody (100 mg/kg intravenously once per week; BPS804, Novartis Pharma), phosphate-buffered saline, or ZA (0.1 mg/kg intraperitoneally once) commenced on day 1. Bioluminescent imaging (BLI) for whole body tumor was performed on an IVIS imaging system (Xenogen, Caliper Life Sciences) or IVIS Spectrum imaging system (Perkin Elmer) and analyzed by using Living Image software (Perkin Elmer). Bone marrow tumor burden was assessed by flow cytometry using GFP expression (for 5TGM1 and MM1.S) or allophycocyanin-conjugated 5T2 idiotype antibody (5T2MM).42 See supplemental Information for details.
Micro computed tomography analysis of bone structure in myeloma-bearing mice
Femora and lumbar vertebrae were imaged in a micro computed tomography (microCT) scanner (Model 1172; Skyscan) and analyzed by using CTAn software (Skyscan).47 Details are provided in supplemental Information.
Histology and immunohistochemistry of femora from myeloma-bearing mice
To identify osteoclasts, femora sections were stained for tartrate-resistant acid phosphatase. Immunohistochemistry was used to identify tumor cells with CD138 and osteocytes with sclerostin protein as described in supplemental Information.47 Osteomeasure (Osteometrics) quantified osteoblasts and osteoclast parameters on endosteal surfaces and dynamic osteoblast activity on trabecular surfaces by using standard methods.20,23,48,49
Compression testing of L4 vertebra
L4 vertebrae were biomechanically tested by compression testing on an Instron 5944 load frame (Instron, Norwood, MA), and data were collected by using BlueHill 3 software (Instron).
Statistical analysis
Data were analyzed by using Prism (GraphPad). One-way analysis of variance and multiple comparisons were performed by using Tukey’s correction. Unpaired Student t tests were performed when comparing 2 populations. A two-sample Student t test using the R function was performed when studies involved small groups of independent samples. All data are expressed as mean ± standard error of the mean. Details for RNA-seq and microarray analyses are listed in supplemental Information.
Results
Osteocytes but not myeloma cells express sclerostin
To demonstrate that sclerostin expression is restricted to bone tissue and is not present in myeloma cells, we examined sclerostin expression in tumor cells from patients with myeloma. We analyzed microarray data sets from CD138+ plasma cells isolated from 630 patients with untreated MM, 82 patients with relapsed myeloma, and 54 myeloma cell lines.50 CD138+ BMPCs from 10 normal donors, memory B cells (MBCs), and PPCs served as controls (Figure 1A). A cohort of CD138+ plasma cells isolated from 263 patients with myeloma and 19 myeloma cells lines along with BMPCs, MBCs, and PPCs were also examined by RNA-seq analysis (Figure 1B). DKK1 was expressed by CD138+ myeloma cells isolated from patients with myeloma and human myeloma cell lines but not normal BMPCs or B-lineage cells (Figure 1A-B). SOST was not expressed by myeloma cells, myeloma cell lines, or other B-lineage cells (Figure 1A-B). Immunohistochemical analysis of 5TGM1-eGFP myeloma cells confirmed the absence of sclerostin in murine myeloma cells (Figure 1C). Furthermore, analysis of the sclerostin protein in media conditioned by human myeloma cells (MM1.S and OPM2), murine myeloma cells (5TGM1-eGFP), and primary osteocyte cultures demonstrated sclerostin production by osteocytes but not myeloma cells (Figure 1D). RNA-seq analysis of osteocytes isolated from non-tumor–bearing murine bones revealed osteocyte enrichment of genes encoding the soluble Wnt antagonists, sclerostin (Sost), dickkopf1 (Dkk1), soluble frizzled-related proteins 1 and 2 (Sfrp1 and Sfrp2), and frizzled-b (Frzb) compared with bone marrow (Figure 1E). These Wnt antagonists were also expressed in osteocytes isolated from mice bearing 5TGM1-eGFP cells. Despite a reduction in Sost and Dkk1 messenger RNA expression in osteocytes isolated from the bones of 5TGM1-eGFP–bearing mice compared with naive non-tumor–bearing mice (Figure 1F), immunohistochemical analysis demonstrated that sclerostin protein was present in osteocytes from naive and disease-bearing mice (Figure 1G). Together these data demonstrate that sclerostin is expressed by osteocytes but not by myeloma cells, supporting the notion that sclerostin is a tumor-independent regulator of bone formation in myeloma.
Sclerostin is expressed by osteocytes but not myeloma cells. (A) Gene expression profiling using DNA microarray analysis of memory B cells (MBCs [n = 5]), in vitro–generated PPCs (n = 5), normal BMPCs (n = 10), malignant plasma cells from patients with newly diagnosed MM (n = 630) and relapsed/refractory MM (RMM [n = 82]), as well as human myeloma cell lines (HMCLs [n = 54]). Gray dots represent the absence of expression, and black dots represent the presence of expression according to the presence-absence calls with negative probesets (PANP) algorithm. (B) RNA-seq analysis of DKK1 and SOST of MBCs (n = 4), PPCs (n = 4), BMPCs (n = 10), MM cells (n = 263), and HMCLs (n = 19). DKK1 (204602_at) is expressed by the majority of malignant plasma cell samples from previously untreated and relapsed myeloma patients. In contrast, SOST (223869_at) expression is absent in all normal and malignant plasma cells. (C) Immunohistochemical staining for CD138 (top panel) and sclerostin (bottom panel) counter-stained with hematoxylin in bone marrow of naive mice and mice bearing 5TGM1-eGFP myeloma cells. Slides were scanned on Scanscope CS2 (Aperio) up to original magnification ×40, and images were captured by using Aperio Imagescope at digital magnification ×20. Scale bar represents 50 μm. (D) Sclerostin protein was measured in media conditioned by MM1.S, OPM2 human myeloma cells lines, 5TGM1 murine myeloma cells, and primary osteocytes (n = 4; data are mean ± 1 standard error of the mean [SEM]; ****P < .0001). (E) Density plot highlighting the osteocyte-enriched expression of secreted Wnt signaling antagonists (n = 6). (F) Tumor burden significantly alters the expression of the secreted Wnt signaling pathway antagonists Sost, Dkk1, and Frzb (n = 6; data are mean ± 1 standard deviation [SD]; multiple comparison–adjusted P value *P < .05; **P < .01; ****P < .0001). (G) Immunohistochemical staining for sclerostin in osteocytes from naive and 5TGM1-bearing mice; arrow heads denote positive-stain osteocytes with positive-stained canaliculi (inset). Slides were scanned on Scanscope CS2 (Aperio) up to original magnification ×40, and images were captured by using Aperio Imagescope at digital magnification ×40. Scale bar represents 50 µm.
Sclerostin is expressed by osteocytes but not myeloma cells. (A) Gene expression profiling using DNA microarray analysis of memory B cells (MBCs [n = 5]), in vitro–generated PPCs (n = 5), normal BMPCs (n = 10), malignant plasma cells from patients with newly diagnosed MM (n = 630) and relapsed/refractory MM (RMM [n = 82]), as well as human myeloma cell lines (HMCLs [n = 54]). Gray dots represent the absence of expression, and black dots represent the presence of expression according to the presence-absence calls with negative probesets (PANP) algorithm. (B) RNA-seq analysis of DKK1 and SOST of MBCs (n = 4), PPCs (n = 4), BMPCs (n = 10), MM cells (n = 263), and HMCLs (n = 19). DKK1 (204602_at) is expressed by the majority of malignant plasma cell samples from previously untreated and relapsed myeloma patients. In contrast, SOST (223869_at) expression is absent in all normal and malignant plasma cells. (C) Immunohistochemical staining for CD138 (top panel) and sclerostin (bottom panel) counter-stained with hematoxylin in bone marrow of naive mice and mice bearing 5TGM1-eGFP myeloma cells. Slides were scanned on Scanscope CS2 (Aperio) up to original magnification ×40, and images were captured by using Aperio Imagescope at digital magnification ×20. Scale bar represents 50 μm. (D) Sclerostin protein was measured in media conditioned by MM1.S, OPM2 human myeloma cells lines, 5TGM1 murine myeloma cells, and primary osteocytes (n = 4; data are mean ± 1 standard error of the mean [SEM]; ****P < .0001). (E) Density plot highlighting the osteocyte-enriched expression of secreted Wnt signaling antagonists (n = 6). (F) Tumor burden significantly alters the expression of the secreted Wnt signaling pathway antagonists Sost, Dkk1, and Frzb (n = 6; data are mean ± 1 standard deviation [SD]; multiple comparison–adjusted P value *P < .05; **P < .01; ****P < .0001). (G) Immunohistochemical staining for sclerostin in osteocytes from naive and 5TGM1-bearing mice; arrow heads denote positive-stain osteocytes with positive-stained canaliculi (inset). Slides were scanned on Scanscope CS2 (Aperio) up to original magnification ×40, and images were captured by using Aperio Imagescope at digital magnification ×40. Scale bar represents 50 µm.
Inhibiting sclerostin prevents myeloma bone disease
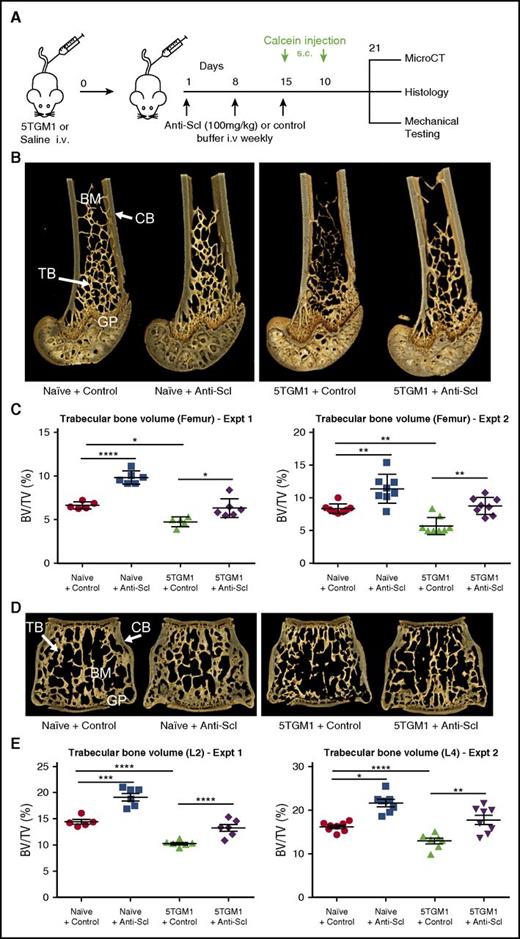
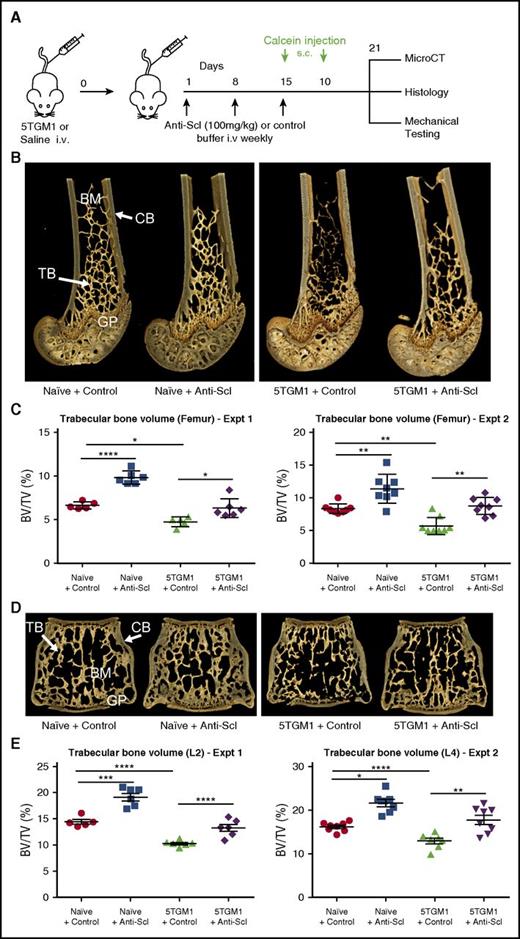
To investigate whether inhibition of sclerostin prevents the development of myeloma bone disease, we treated non-myeloma–bearing mice and mice bearing either murine 5TGM1-eGFP or human MM1.S myeloma cells with an anti-sclerostin antibody and measured changes in bone mass by microCT analysis (Figure 2A). Treatment of naive BKAL mice with an anti-sclerostin antibody increased trabecular bone volume and trabecular thickness, as well as the volume and thickness of cortical bone in the femur and lumbar vertebra (Figure 2B-E; supplemental Table I). This was replicated in 2 independent experiments, confirming that anti-sclerostin antibody exerts direct anabolic effects on bone.
Treatment with anti-sclerostin antibody prevents myeloma-induced bone loss in the 5TGM1 murine model of myeloma. (A) Schematic depicting study design for 5TGM1 studies. (B) 3D microCT reconstructions of distal femora representing each treatment group in experiment (Expt) 2. (C) Dot plot of the ratio of trabecular bone volume to total volume (BV/TV) from distal femora of experiment 1 (n = 5-6) and experiment 2 (n = 8) (data are mean ± 1 SEM). (D) 3D microCT reconstructions of L4 vertebrae representing L4 from each treatment group in experiment 2. (E) Dot plot of trabecular BV/TV from lumbar vertebra 2 (L2) from experiment 1 (n = 6) and lumbar vertebra 4 (L4) from experiment 2 (n = 8) (data are mean ± 1 SEM; *P < .05; **P < .01; ***P < .001; ****P < .0001). BM, bone marrow; CB, cortical bone; GP, growth plate; i.v., intravenous; s.c., subcutaneous; Scl, sclerostin; TB, trabecular bone.
Treatment with anti-sclerostin antibody prevents myeloma-induced bone loss in the 5TGM1 murine model of myeloma. (A) Schematic depicting study design for 5TGM1 studies. (B) 3D microCT reconstructions of distal femora representing each treatment group in experiment (Expt) 2. (C) Dot plot of the ratio of trabecular bone volume to total volume (BV/TV) from distal femora of experiment 1 (n = 5-6) and experiment 2 (n = 8) (data are mean ± 1 SEM). (D) 3D microCT reconstructions of L4 vertebrae representing L4 from each treatment group in experiment 2. (E) Dot plot of trabecular BV/TV from lumbar vertebra 2 (L2) from experiment 1 (n = 6) and lumbar vertebra 4 (L4) from experiment 2 (n = 8) (data are mean ± 1 SEM; *P < .05; **P < .01; ***P < .001; ****P < .0001). BM, bone marrow; CB, cortical bone; GP, growth plate; i.v., intravenous; s.c., subcutaneous; Scl, sclerostin; TB, trabecular bone.
Injection of BKAL mice with 5TGM1-eGFP murine myeloma cells resulted in a significant decrease in femur trabecular volume and reduction in trabecular number (Figure 2B-E; supplemental Table I). MicroCT analysis of the lumbar vertebra, a common site of fracture in patients with myeloma, showed that 5TGM1-eGFP cells also caused bone loss at this site (Figure 2B-E; supplemental Table I). Treatment with anti-sclerostin antibody prevented 5TGM1-eGFP–induced bone loss and increased trabecular and cortical bone thickness in both the femur and vertebra (Figure 2B-E; supplemental Table I).
To determine whether the inhibition of sclerostin also prevents bone loss induced by human myeloma cells, we injected NSG mice with either human MM1.S-luc-eGFP myeloma cells or saline via the tail vein and treated them with anti-sclerostin antibody or control buffer (supplemental Figure 2A). Anti-sclerostin antibody increased trabecular bone volume and trabecular number in the femur and vertebra of NSG non-myeloma–bearing mice in a manner similar to that in BKAL mice (supplemental Figure 2). Injection of human MM1.S-luc-eGFP myeloma cells induced bone loss and a reduction in trabecular thickness and number (supplemental Figure 2B-C). Treatment with anti-sclerostin antibody prevented bone loss in both femur and vertebra (supplemental Figure 2).
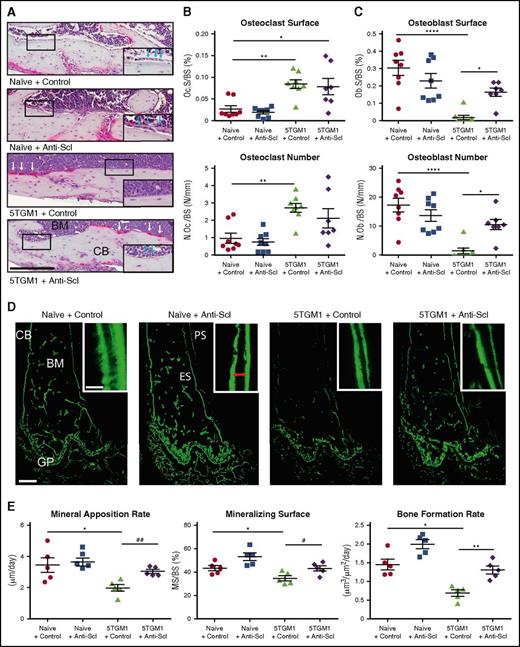
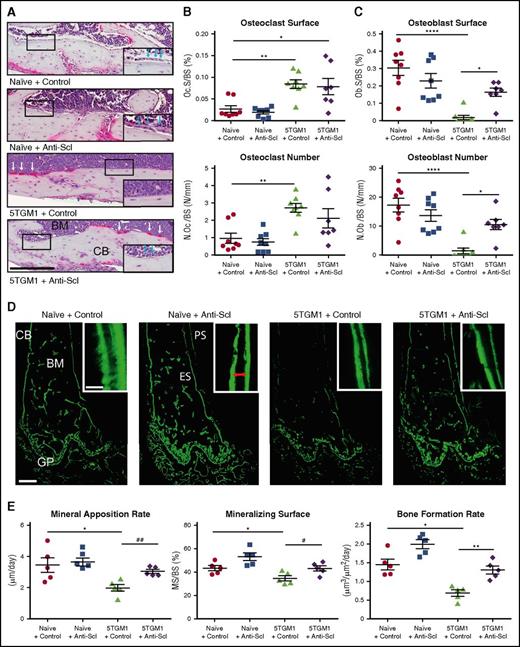
To determine the mechanism underlying the ability of anti-sclerostin antibody to prevent myeloma bone disease, we performed histomorphometric analysis of BKAL mice bearing 5TGM1-eGFP cells. Consistent with previous studies, osteoclast number and surface were increased in BKAL mice bearing 5TGM1-eGFP cells (Figure 3A-B).11 Furthermore, the presence of tumor cells resulted in a decreased number of osteoblasts and the extent of endosteal bone surface covered by osteoblasts (Figure 3A,C), consistent with the uncoupling of bone resorption and bone formation that occurs in patients with myeloma. Treatment with anti-sclerostin antibody increased both osteoblast number and the surface occupied by osteoblasts compared with control but did not affect osteoclastic resorption (Figure 3A-C). To investigate osteoblast function, we injected mice with calcein 6 and 2 days before the mice were euthanized. The distance between the 2 time-separated calcein labels was used to determine the mineral apposition rate (MAR) and bone formation rate (BFR). Consistent with the reduced osteoblast numbers in BKAL mice bearing 5TGM1-eGFP cells, osteoblast mineralizing surfaces (MSs), MAR, and BFR were all decreased (Figure 3D-E). Treatment with anti-sclerostin antibody prevented 5TGM1-eGFP–induced suppression of MAR, BFR, and MSs normalizing bone formation to levels seen in control non-myeloma–bearing mice. These data demonstrate that treatment with anti-sclerostin antibody does not affect the increased osteoclastic resorption but does prevent myeloma cell–mediated inhibition of bone formation. This suggests that treatment with anti-sclerostin antibody prevents myeloma bone disease by ensuring the coupling between bone resorption and formation.
Treatment with anti-sclerostin antibody prevents 5TGM1 myeloma-induced suppression of bone formation. (A) Tartrate-resistant acid phosphatase (TRAP)/hematoxylin-stained histologic sections from representative mice from each treatment group showing osteoblasts on the endocortical bone surface (inset, blue arrows) and osteoclasts reacted for TRAP and stained red (white arrows); scale bar represents 200 µm. Slides were scanned on a Scanscope CS2 (Aperio) up to original magnification ×40, and images were captured by using an Aperio Imagescope at digital magnification ×20. (B) Dot plots of osteoclast surface/bone surface (Oc.S/BS) and number of osteoclasts/bone surface (N.Oc/BS) (data are mean ± 1 SEM; *P < .05; **P < .001). (C) Dot plots of osteoblast surface/bone surface (Ob.S/BS) and number of osteoblasts/bone surface (N.Ob/BS) (n = 8) (data are mean ± 1 SEM; *P < .05; **P < .001). (D) Representative sections from each group showing mineralized bone surfaces labeled with calcein. Images were captured on a Leica DMI 5500 at ×2.5 by using LAS X software (Leica); scale bar represents 500 µm. Double-label bone surfaces were used to measure mineral apposition rate (inset: red arrows show the distance between 2 labels separated by a fixed time interval). Images were captured on a Leica DMI 5500 at ×40 using LAS X software (Leica). Scale bar represents 20 µm. (E) Dot plots of MAR, mineralizing surface (MS), and BFR (n = 5; data are mean ± 1 SEM; *P < .05; **P < .01; #P < .05 (Student t test); ##P < .01 (Student t test). ES, endosteal bone surface; PS, periosteal bone surface.
Treatment with anti-sclerostin antibody prevents 5TGM1 myeloma-induced suppression of bone formation. (A) Tartrate-resistant acid phosphatase (TRAP)/hematoxylin-stained histologic sections from representative mice from each treatment group showing osteoblasts on the endocortical bone surface (inset, blue arrows) and osteoclasts reacted for TRAP and stained red (white arrows); scale bar represents 200 µm. Slides were scanned on a Scanscope CS2 (Aperio) up to original magnification ×40, and images were captured by using an Aperio Imagescope at digital magnification ×20. (B) Dot plots of osteoclast surface/bone surface (Oc.S/BS) and number of osteoclasts/bone surface (N.Oc/BS) (data are mean ± 1 SEM; *P < .05; **P < .001). (C) Dot plots of osteoblast surface/bone surface (Ob.S/BS) and number of osteoblasts/bone surface (N.Ob/BS) (n = 8) (data are mean ± 1 SEM; *P < .05; **P < .001). (D) Representative sections from each group showing mineralized bone surfaces labeled with calcein. Images were captured on a Leica DMI 5500 at ×2.5 by using LAS X software (Leica); scale bar represents 500 µm. Double-label bone surfaces were used to measure mineral apposition rate (inset: red arrows show the distance between 2 labels separated by a fixed time interval). Images were captured on a Leica DMI 5500 at ×40 using LAS X software (Leica). Scale bar represents 20 µm. (E) Dot plots of MAR, mineralizing surface (MS), and BFR (n = 5; data are mean ± 1 SEM; *P < .05; **P < .01; #P < .05 (Student t test); ##P < .01 (Student t test). ES, endosteal bone surface; PS, periosteal bone surface.
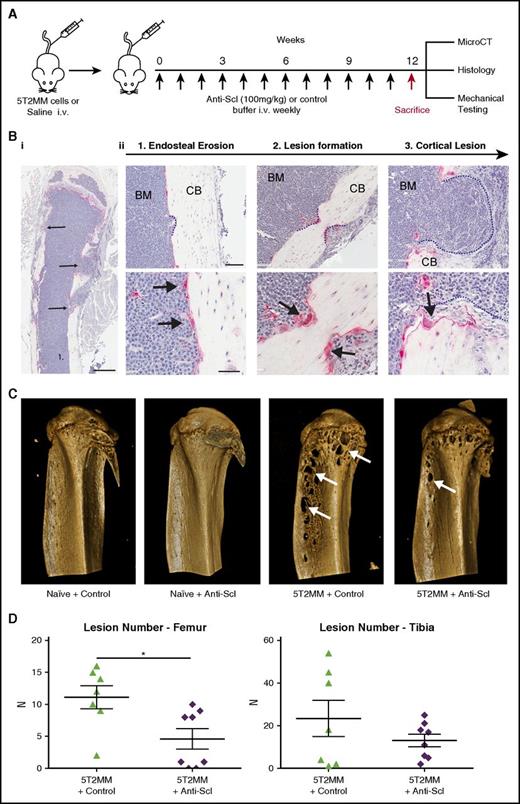
Inhibiting sclerostin stops osteolytic lesion formation
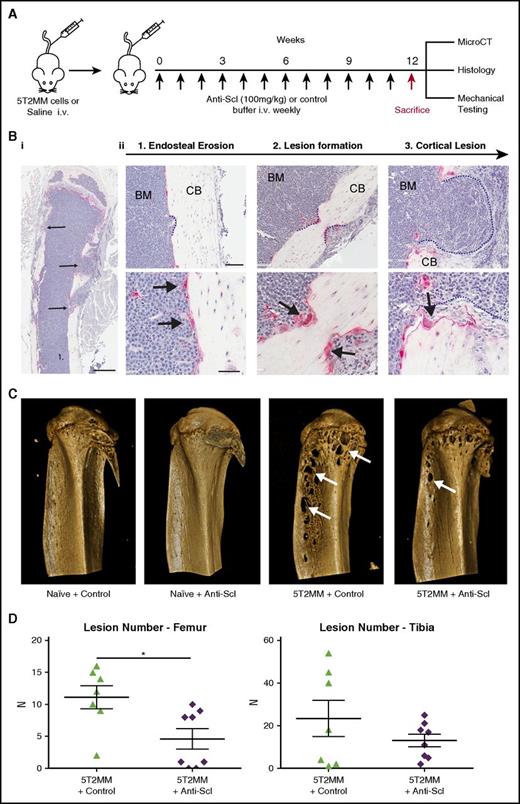
Although generalized bone loss is a common feature in myeloma, one of the defining features in patients with MM is the development of osteolytic bone lesions. To investigate whether inhibiting sclerostin can prevent development of osteolytic lesions, we used the 5T2MM syngeneic model, which develops osteolytic bone lesions similar to those seen in patients.42 Injection of 5T2MM murine myeloma cells into BKAL mice resulted in the development of defined osteolytic bone lesions (Figure 4A-D). Histological analysis demonstrated that tartrate resistant acid phosphatase–positive osteoclasts on the cortico-endosteal surface were associated with multiple steps in the development of bone lesions. This included sites of initial cortical erosion (Figure 4Bii1), lesion development (Figure 4Bii2), and overt osteolytic lesions that penetrate the cortical bone (Figure 4Bii3). Osteoblasts were absent from these sites. MicroCT analysis demonstrated the presence of clearly defined bone lesions not seen in naive mice (Figure 4C). Importantly, treatment with anti-sclerostin antibody reduced the number of osteolytic bone lesions in the femora of 5T2MM-bearing mice (Figure 4D).
Treatment with anti-sclerostin antibody reduces formation of osteolytic bone lesions. (A) Schematic describing the study design for investigations of bone lesions in 5T2MM-bearing mice. (Bi) TRAP/hematoxylin-stained histologic section of a tibia from a 5T2MM-bearing mouse. Slides were scanned on a Scanscope CS2 (Aperio) up to original magnification ×40, and images were captured by using an Aperio Imagescope at digital magnification ×2. Scale bar represents 500 µm; black arrows point to cortical lesions. (Bii) Higher magnification (×12 and ×30) images that demonstrate the temporal development of CB lesions in the 5T2MM model. (1) Initial erosion of the endosteal surface of the CB by red TRAP-positive osteoclasts (black arrows) adjacent to tumor cells in the BM. (2) Formation of a CB lesion (blue dotted line) by osteoclasts (black arrows). (3) A cortical lesion that has penetrated the cortex with the tumor (blue dotted line) extending into the surrounding soft tissue. (Top panel) scale bar represents 100 µm; (bottom panel) scale bar represents 50 µm. (C) Representative 3D reconstruction of tibia from each treatment group showing full cortex penetration lesions (white arrows) in the 5T2MM+ control and 5T2MM+ anti-sclerostin antibody groups. (D) Dot plots of lesion number per bone for femora and tibiae (n = 7-8 per group; data are mean ± 1 SEM; *P < .05).
Treatment with anti-sclerostin antibody reduces formation of osteolytic bone lesions. (A) Schematic describing the study design for investigations of bone lesions in 5T2MM-bearing mice. (Bi) TRAP/hematoxylin-stained histologic section of a tibia from a 5T2MM-bearing mouse. Slides were scanned on a Scanscope CS2 (Aperio) up to original magnification ×40, and images were captured by using an Aperio Imagescope at digital magnification ×2. Scale bar represents 500 µm; black arrows point to cortical lesions. (Bii) Higher magnification (×12 and ×30) images that demonstrate the temporal development of CB lesions in the 5T2MM model. (1) Initial erosion of the endosteal surface of the CB by red TRAP-positive osteoclasts (black arrows) adjacent to tumor cells in the BM. (2) Formation of a CB lesion (blue dotted line) by osteoclasts (black arrows). (3) A cortical lesion that has penetrated the cortex with the tumor (blue dotted line) extending into the surrounding soft tissue. (Top panel) scale bar represents 100 µm; (bottom panel) scale bar represents 50 µm. (C) Representative 3D reconstruction of tibia from each treatment group showing full cortex penetration lesions (white arrows) in the 5T2MM+ control and 5T2MM+ anti-sclerostin antibody groups. (D) Dot plots of lesion number per bone for femora and tibiae (n = 7-8 per group; data are mean ± 1 SEM; *P < .05).
The impact of treatment with anti-sclerostin antibody on generalized bone loss induced by 5T2MM myeloma cells was also examined. Injection of 5T2MM murine cells resulted in a significant decrease in trabecular and cortical bone in the femur. Treatment with anti-sclerostin antibody prevented the 5T2MM-induced bone loss in the femur and increased bone volume in 5T2MM-bearing vertebra (supplemental Figure 3A-B; supplemental Tables IIIA-B). This is consistent with data from the 5TGM1-eGFP and MM1.S-luc-eGFP models and confirms the robust response to anti-sclerostin antibody.
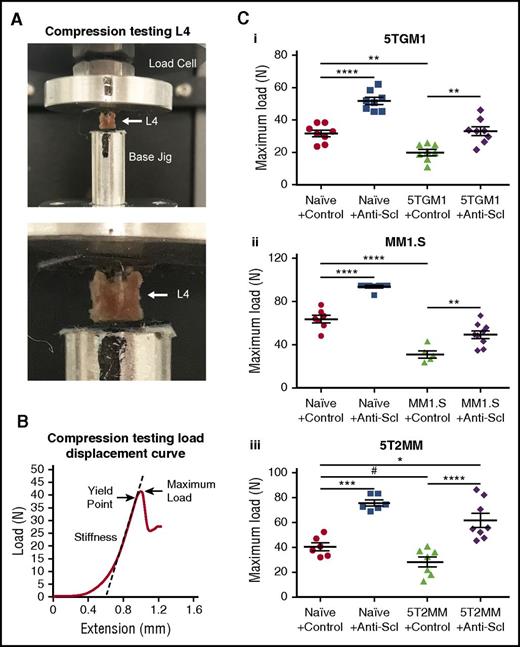
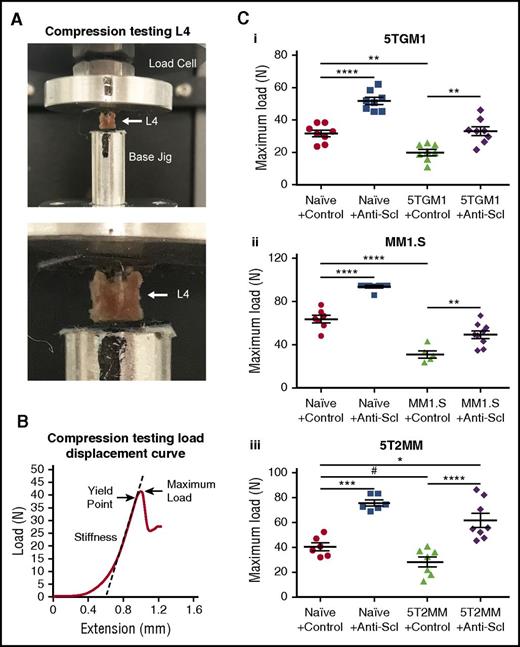
Inhibiting sclerostin increases resistance to fracture
Pathological fracture is a major cause of morbidity in MM and although pharmacologic interventions reduce further bone loss, they do not increase bone strength. To investigate whether treatment with anti-sclerostin antibody would increase bone strength and resistance to fracture, we performed biomechanical compression testing of the vertebra from the 5TGM1-eGFP and 5T2MM mouse models and MM1.S human xenograft models after anti-sclerostin antibody treatment. In naive non-tumor–bearing BKAL and NSG mice, treatment with anti-sclerostin antibody increased the maximum load to failure sustained by lumbar vertebrae (L4) (Figure 5A-C). The myeloma-induced bone loss in 5TGM1-eGFP–, 5T2MM-, and MM1.S-bearing mice was associated with 37%, 30%, and 51% reductions in maximum load to failure, respectively (Figure 5C). Importantly, treatment with anti-sclerostin antibody prevented reduction in bone strength in each model (Figure 5C). In 5TGM1-eGFP and MM1.S mice, treatment with anti-sclerostin antibody returned the maximum load to failure to levels found in naive mouse controls, whereas values were significantly higher in the 5T2MM model (Figure 5C).
Treatment with anti-sclerostin antibody prevents loss of bone mechanical strength in myeloma-burdened bones. (A) Images demonstrating the positioning of L4 vertebrae for compression testing between the load cell and the custom-designed jig in the Instron 5944 load frame. (B) Representative load displacement curve of an L4 vertebra from the naive control group showing the outcomes of stiffness, yield point, and maximum load at the first point of failure. (C) Dot plots of maximum load for L4 vertebrae from the (i) 5TGM1-eGFP, (ii) MM1.S, and (iii) 5T2MM models of myeloma (5TGM1: n = 8; MM1.S: n = 5-9; 5T2MM: n = 6-8). Data are mean ± 1 SEM; *P < .05; **P < .01; ***P < .001; ****P < .0001; #P < .05 (Student t test).
Treatment with anti-sclerostin antibody prevents loss of bone mechanical strength in myeloma-burdened bones. (A) Images demonstrating the positioning of L4 vertebrae for compression testing between the load cell and the custom-designed jig in the Instron 5944 load frame. (B) Representative load displacement curve of an L4 vertebra from the naive control group showing the outcomes of stiffness, yield point, and maximum load at the first point of failure. (C) Dot plots of maximum load for L4 vertebrae from the (i) 5TGM1-eGFP, (ii) MM1.S, and (iii) 5T2MM models of myeloma (5TGM1: n = 8; MM1.S: n = 5-9; 5T2MM: n = 6-8). Data are mean ± 1 SEM; *P < .05; **P < .01; ***P < .001; ****P < .0001; #P < .05 (Student t test).
Additive effects of anti-sclerostin antibody and ZA
Bisphosphonates, which inhibit bone resorption, are the standard of care for patients with symptomatic MM; however, the effect of combining an agent that stimulates bone formation with a bisphosphonate that inhibits bone resorption is unknown. The consequence of combining the anti-sclerostin antibody with the bisphosphonate ZA was investigated in the 5TGM1-eGFP model (Figure 6A). Anti-sclerostin antibody alone and ZA alone both prevented 5TGM1-eGFP–induced bone loss in the femur and vertebra but likely by contrasting mechanisms (Figure 6B-E). Treatment with the combination of anti-sclerostin antibody and ZA resulted in a fourfold increase in trabecular bone volume when compared with control-treated 5TGM1-eGFP–bearing mice and a 142% and 112% increase, respectively, when compared with mice treated with anti-sclerostin antibody or ZA alone (Figure 6C). Treatment with the combination of anti-sclerostin antibody and ZA increased trabecular thickness when compared with ZA given alone but not anti-sclerostin antibody, whereas trabecular number was increased relative to anti-sclerostin antibody but not ZA (Figure 6C). This difference likely reflects the different mechanism of action of the 2 agents: treatment with anti-sclerostin antibody promoted new bone formation on existing trabecular elements and ZA prevented osteoclastic resorption and loss of individual trabeculae. The combination of anti-sclerostin antibody and ZA also increased vertebral bone volume more than anti-sclerostin antibody (37%) or ZA (97%) alone (Figure 6D-E).
Combination treatment with anti-sclerostin antibody and ZA is superior in preventing bone loss in the 5TGM1 murine model of myeloma. (A) Schematic describing the study design for investigations of bone loss in 5TGM1-eGFP–bearing mice treated with both anti-sclerostin antibody and ZA. (B) Examples of 3D microCT reconstructions of distal femora from each treatment group. (C) Dot plots of BV/TV trabecular thickness and number and cortical bone thickness in femora for each treatment group (n = 8 per group). Data are mean ± 1 SEM; *P < .05; **P < .01; ***P < .001; ****P < .0001. (D) Dot plot of L4 vertebra BV/TV for each treatment group (data are mean ± 1 SEM; **P < .01; ***P < .001; ****P < .0001; #P < .05 [Student t test]). (E) Structural parameters from microCT scans of L4 vertebrae (n = 8 per group). Data are mean ± 1 SEM; aP < .001; bP < .01; dP < .05 compared with naive plus control mice; eP < .001; fP < .05 compared with 5TGM1 plus control mice; gP < .001 compared with 5TGM1 plus ZA; hP < .001 compared with 5TGM1 plus anti-sclerostin antibody. (F) Dot plot of maximum load from L4 vertebra for each treatment group (n = 8 per group). Data are mean ± 1 SEM; **P < .01; ***P < .001; #P < .05 [Student t test].
Combination treatment with anti-sclerostin antibody and ZA is superior in preventing bone loss in the 5TGM1 murine model of myeloma. (A) Schematic describing the study design for investigations of bone loss in 5TGM1-eGFP–bearing mice treated with both anti-sclerostin antibody and ZA. (B) Examples of 3D microCT reconstructions of distal femora from each treatment group. (C) Dot plots of BV/TV trabecular thickness and number and cortical bone thickness in femora for each treatment group (n = 8 per group). Data are mean ± 1 SEM; *P < .05; **P < .01; ***P < .001; ****P < .0001. (D) Dot plot of L4 vertebra BV/TV for each treatment group (data are mean ± 1 SEM; **P < .01; ***P < .001; ****P < .0001; #P < .05 [Student t test]). (E) Structural parameters from microCT scans of L4 vertebrae (n = 8 per group). Data are mean ± 1 SEM; aP < .001; bP < .01; dP < .05 compared with naive plus control mice; eP < .001; fP < .05 compared with 5TGM1 plus control mice; gP < .001 compared with 5TGM1 plus ZA; hP < .001 compared with 5TGM1 plus anti-sclerostin antibody. (F) Dot plot of maximum load from L4 vertebra for each treatment group (n = 8 per group). Data are mean ± 1 SEM; **P < .01; ***P < .001; #P < .05 [Student t test].
To determine whether the additive effects of anti-sclerostin antibody and ZA treatment on bone volume were associated with increased bone strength, biomechanical testing of the lumbar vertebrae was performed. Treatment with anti-sclerostin antibody and ZA combined increased the maximum load to failure sustained by the lumbar vertebrae when compared with treatment with ZA alone but did not differ from treatment with anti-sclerostin antibody alone (Figure 6F). Treatment with ZA alone did not increase vertebral maximum load to failure compared with control. This indicates that the increase in bone formation has the predominant effect on bone strength. Together these data demonstrate that treating with combined anti-sclerostin antibody and ZA increases bone volume and bone structural parameters beyond that seen with ZA alone, and this improvement is associated with an increase in bone strength and resistance to fracture.
Inhibiting sclerostin does not promote myeloma burden
Wnt signaling promotes tumor growth,51,52 and aberrant Wnt signaling has been reported to increase myeloma cell proliferation.53 Soluble Wnt antagonists inhibit endogenous Wnt signaling and therefore the anti-sclerostin antibody might promote tumor proliferation by stopping Wnt suppression in the local bone microenvironment. To confirm that treatment with anti-sclerostin antibody did not alter myeloma growth directly, we treated MM1.S-luc-eGFP and 5TGM1-eGFP cells in vitro with increasing concentrations of anti-sclerostin antibody (up to 2.25 µg/mL). Treatment with anti-sclerostin antibody had no effect on the proliferation of these myeloma cells (data not shown). To determine whether treatment with anti-sclerostin antibody had indirect effects on myeloma burden, we measured myeloma burden in the skeleton and spleen as extraosseous sites in all models (Figure 7). Fluorescence-activated cell sorting analysis of 5TGM1-eGFP cells demonstrated the presence of myeloma cells in both bone marrow and spleen of 5TGM1-eGFP–bearing mice. Importantly, there was no difference in myeloma burden in either the bone marrow or spleen of mice treated with anti-sclerostin antibody compared to control mice (Figure 7A-C). Furthermore, treatment with anti-sclerostin antibody did not alter the proportion of idiotype-positive cells in either the bone marrow or spleen of the 5T2MM murine model (Figure 7D). Finally, we evaluated the effect of treatment with anti-sclerostin antibody on myeloma burden in the MM1.S human xenograft model. BLI imaging of whole body myeloma burden demonstrated a reduction in total flux in 1 of 2 experiments (Figure 7E-F). However, flow cytometric analysis of MM1.S-GFP+ cells in the bone marrow showed no difference between anti-sclerostin antibody treatment and control treatment (Figure 7F). The reason for the discrepancy between the BLI analysis and the direct bone marrow flow cytometric assessment of myeloma burden in the MM1.S model may reflect a reduction in extraskeletal tumor growth; however, at least in the spleen, tumor burden was unaltered in the MM1.S model (data not shown). This more likely reflects the increase in cortical bone thickness caused by treatment with anti-sclerostin antibody attenuating the bioluminescence signal. Together these data demonstrate that despite profoundly affecting the bone environment, inhibiting sclerostin with the anti-sclerostin antibody does not affect myeloma burden.
Treatment with anti-sclerostin antibody does not have an impact on tumor burden. (A) Representative flow cytometry plots of 5TGM1-eGFP control-treated bone marrow and 5TGM1-eGFP anti-sclerostin antibody–treated bone marrow showing the percentage of 5TGM1-eGFP+ cells in the sample (black box). (B) Dot plots of bone marrow tumor burden as a percent of total bone marrow cells (% GFP+) in experiments 1 and 2 of the 5TGM1 study (n = 6 [experiment 1]; n = 8 [experiment 2]). Data are mean ± 1 SEM. (C) Dot plots of spleen tumor burden as a percent of total cells (% GFP+) in experiments 1 and 2 of the 5TGM1 study (n = 6 [experiment 1]; n = 8 [experiment 2]). Data are mean ± 1 SEM. (D) Dot plots of bone marrow and spleen tumor burden (% idiotype+) in the 5T2MM study (n = 6-7). Data are mean ± 1 SEM. (E) Dot plots of whole body tumor burden (total flux p/s) and bone marrow percent GFP+ cells in MM1.S experiment 1 (n = 9 per group). Data are mean ± 1 SEM; *P < .05. (F) Dot plots of whole body tumor burden (total flux p/s) at 3 weeks and bone marrow percent GFP+ cells in MM1.S experiment 2 (n = 8-9 for whole body tumor; n = 5-9 for bone marrow tumor). Data are mean ± 1 SEM.
Treatment with anti-sclerostin antibody does not have an impact on tumor burden. (A) Representative flow cytometry plots of 5TGM1-eGFP control-treated bone marrow and 5TGM1-eGFP anti-sclerostin antibody–treated bone marrow showing the percentage of 5TGM1-eGFP+ cells in the sample (black box). (B) Dot plots of bone marrow tumor burden as a percent of total bone marrow cells (% GFP+) in experiments 1 and 2 of the 5TGM1 study (n = 6 [experiment 1]; n = 8 [experiment 2]). Data are mean ± 1 SEM. (C) Dot plots of spleen tumor burden as a percent of total cells (% GFP+) in experiments 1 and 2 of the 5TGM1 study (n = 6 [experiment 1]; n = 8 [experiment 2]). Data are mean ± 1 SEM. (D) Dot plots of bone marrow and spleen tumor burden (% idiotype+) in the 5T2MM study (n = 6-7). Data are mean ± 1 SEM. (E) Dot plots of whole body tumor burden (total flux p/s) and bone marrow percent GFP+ cells in MM1.S experiment 1 (n = 9 per group). Data are mean ± 1 SEM; *P < .05. (F) Dot plots of whole body tumor burden (total flux p/s) at 3 weeks and bone marrow percent GFP+ cells in MM1.S experiment 2 (n = 8-9 for whole body tumor; n = 5-9 for bone marrow tumor). Data are mean ± 1 SEM.
Discussion
Cancers that develop in bone, such as MM, result in devastating bone disease and fractures. This is an important cause of morbidity and mortality, and a major clinical problem. Therapies that inhibit osteoclasts do not restore bone mass and thus patients continue to suffer fractures. Inhibiting tumor-derived products is also likely to have limited efficacy because of the heterogeneous behavior of tumor cells. In contrast, anabolic agents that stimulate bone formation by targeting bone cell–specific pathways offer a potential new modality for treating myeloma-induced bone disease. We demonstrated that sclerostin is an osteocyte-specific protein that is not expressed by myeloma cells. Treatment of myeloma-bearing mice with an anti-sclerostin antibody (1) prevented myeloma-induced suppression of osteoblastic bone formation, (2) prevented myeloma-induced bone loss, (3) reduced development of osteolytic lesions, and (4) increased bone strength and resistance to fracture. Importantly, the combination of anti-sclerostin antibody and the anti-resorptive agent ZA increased bone mass, bone strength, and resistance to fracture more than treatment with ZA alone.
Soluble Wnt antagonists are pivotal regulators of bone formation and have been implicated in suppressing bone formation in myeloma.53 We demonstrated that the Wnt antagonists Dkk1 and Sost were strongly expressed in osteocytes, which are master regulators of bone homeostasis. DKK1 was also shown to be expressed by plasma cells isolated from a large cohort of patients with myeloma, which supports previous observations.18,54 However, SOST was not expressed by myeloma cells isolated from the same cohort of patients (those who were undergoing relapse or those who were refractory to disease) nor was it expressed by an extensive panel of myeloma cell lines. This contrasts with reports of sclerostin expression detected by reverse transcription polymerase chain reaction in plasma cells isolated from a small cohort of patients with myeloma.55,56 The reason for this is unclear, but it likely reflects technical differences among studies. We also showed that global Dkk1 and Sost expression was decreased in bones isolated from mice bearing myeloma. The mechanism for this is unclear but might reflect alterations in load detected by osteocytes in response to the presence of tumor cells. Interestingly, local osteocyte production of sclerostin has been shown to be increased when osteocytes interact directly with cancer cells.57 This demonstrates that regulation of sclerostin expression in bone in the presence of tumor is complex, with both local and generalized responses, suggesting that sclerostin expression may be more heterogeneous in the presence of myeloma. However, the strong expression of Sost in both myeloma-bearing and control bones provided the rationale for investigating the ability of anti-sclerostin antibody to prevent myeloma bone disease.
To establish whether inhibiting sclerostin and thus enhancing Wnt signaling would increase bone formation, we treated mice bearing 5TGM1-eGFP cells with an anti-sclerostin antibody. Treatment with anti-sclerostin antibody prevented osteoblast suppression, ensuring that bone formation was maintained. Inhibiting sclerostin had no effect on the myeloma-induced increase in osteoclastic bone resorption, which suggests that treatment retained the coupling between bone resorption and formation. The stimulation of bone formation caused an increase in bone mass in the 3 models of myeloma bone disease. This was associated with increases in trabecular bone thickness, demonstrating that inhibiting sclerostin resulted in new bone formation on existing bone rather than stopping further bone loss, which is seen with antiresorptive treatments. This is supported by the recent demonstration that treatment with an alternative anti-sclerostin antibody can increase bone mass in mice bearing MM1.S human myeloma cells.58 Importantly, our data are consistent with the anti-sclerostin antibody–induced increase in bone mass seen in models of osteoporosis30,31 and bone repair.32,33 Because osteolytic bone lesions are a hallmark of MM, we also investigated whether treatment with anti-sclerostin antibody could prevent development of osteolytic lesions. We discovered that treatment with anti-sclerostin antibody reduced development of osteolytic bone lesions. This has also been observed when inhibiting Dkk1 and activin signaling.20,23 These data argue that retaining the coupling between resorption and formation, even in the presence of increased osteoclastic resorption, is sufficient to prevent development of both generalized myeloma bone disease and osteolytic lesions.
Although anti-sclerostin antibody could offer a new approach to treating myeloma bone disease, this must to be considered in the context of current gold-standard therapy, which is the antiresorptive bisphosphonate ZA. We showed that anti-sclerostin antibody alone and ZA alone both prevented myeloma-induced bone loss and that anti-sclerostin antibody alone increased bone strength. In addition, anti-sclerostin antibody was superior to ZA at improving bone strength. Treatment by adding anti-sclerostin antibody to ZA increased bone volume over treatment with either agent alone. Importantly, treatment with the combination of both agents also increased resistance to fracture when compared with ZA alone. These findings suggest that addition of anti-sclerostin antibody to ZA therapy will result in additional clinically relevant benefits above and beyond the current gold-standard of treatment.
Before anti-sclerostin antibody treatment can be evaluated in a clinical program, its possible impact on tumor growth needs to be considered. Inhibiting a soluble Wnt antagonist may increase local Wnt signaling and promote tumor growth.52 Although Wnt signaling has been shown to stimulate myeloma cell proliferation in vitro,53 treatment with anti-sclerostin antibody had no effect on myeloma burden in the skeleton or spleen of the 5TGM1-eGFP, 5T2MM, or MM1.S models. The absence of effect across multiple models suggests that sclerostin does not regulate pathways that control tumor growth. These data reinforce the hypothesis that sclerostin represents a tractable new therapeutic target in the treatment of myeloma bone disease.
The data presented here provide the rationale for a new approach to treating the bone disease that occurs in MM. Inhibiting sclerostin with an anti-sclerostin antibody increases bone mass and resistance to fracture, and when combined with ZA, is superior to the current standard of care. This supports the rationale for evaluating the efficacy of treatment with anti-sclerostin antibody in clinical studies in patients with myeloma. The prevention of fractures would transform prognosis for patients with myeloma, decrease morbidity, enhance quality of life, and ultimately reduce mortality. This new understanding has implications for treating not only patients with MM but also patients with other cancers that develop in the skeleton.
Gene expression data from myeloma patient samples reported in this article have been deposited in the ArrayExpress database (accession numbers E-MTAB-4715, E-MTAB-4717, E-TABM-937, and E-TABM-1088); the osteocyte-enrichment gene expression data are deposited in the ArrayExpress repository (E-MTAB-5533); and data for gene expression analysis of osteocytes from tumor burdened bones relative to naive samples is deposited in the ArrayExpress repository (E-MTAB-5555).
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the Biological Testing Facility for Animal Husbandry at the Garvan Institute of Medical Research; the Australian BioResources Centre, Mossvale, Australia, for animal housing; the The Kinghorn Cancer Centre for RNA sequencing; Aldo Roccaro, Yawara Kawano, Shokichi Tsukamoto, and Antonio Sacco (Dana-Farber Cancer Institute) for assistance with sample harvests; Andrew Kung (Columbia University Medical Centre, New York, NY) for supplying the MM1.S-luc-eGFP cell line; Shruti Shah and Jenny Down (Garvan Institute of Medical Research) for histology sample preparation and immunohistochemistry staining; and Novartis Pharma (Basel, Switzerland) for providing anti-sclerostin antibody and vehicle buffer.
This work was supported by: Janice Gibson and the Ernest Heine Family Foundation (P.I.C.); an Australian Research Council Future Fellowship (P.A.B.); the National Institutes of Health, National Institute of General Medical Sciences (grant GM106391) and National Institute of Diabetes and Digestive and Kidney Diseases (grant R24 DK092759-01); the American Cancer Society Research (grant IRG-16-191-33); start-up funding from the Maine Medical Center Research Institute (M.R.R.); a Kay Stubbs Cancer Research Grant, Cancer Council New South Wales; and the Deutsche Forschungsgemeinschaft (grant SFB/TRR79, TP B1, B12, M9) and the German Federal Ministry of Education (Clinically-Applicable, Omics-based Assessment of Survival, Side Effects, and Targets in Multiple Myeloma [CLIOMMICS] [grant 01ZX1309] and Clinical Applicable Multimodal Prediction of Survival in Multiple Myeloma [CAMPSIMM] [grant 01ES1103]).
Authorship
Contribution: P.I.C., T.G.P., and M.M.M. conceived the study; M.R.R., M.M.M., S.E.Y., S.T.M., A.S., and D.H. designed and performed experiments and analyzed data; R.L.T., J.A.P., M.K.S., T.L.C., A.M., L.M.T.L., D.A.-H., C.F., and H.F. performed experiments; P.A.B., D.G.L., K.V., I.K., I.M.G., M.K., J.H.D.B., G.R.W., B.O.O., and T.G.P. analyzed and interpreted data; B.O.O. contributed reagent; P.A.B., M.R.R., J.H.D.B., and G.R.W. revised the manuscript; M.M.M. and P.I.C. wrote the manuscript; and all authors read and approved the manuscript.
Conflict-of-interest disclosure: M.K. and I.K. are employed by Novartis Pharma. The remaining authors declare no competing financial interests.
Correspondence: Peter I. Croucher, Garvan Institute of Medical Research, 384 Victoria St, Sydney, NSW 2010, Australia; e-mail: p.croucher@garvan.org.au.

![Figure 1. Sclerostin is expressed by osteocytes but not myeloma cells. (A) Gene expression profiling using DNA microarray analysis of memory B cells (MBCs [n = 5]), in vitro–generated PPCs (n = 5), normal BMPCs (n = 10), malignant plasma cells from patients with newly diagnosed MM (n = 630) and relapsed/refractory MM (RMM [n = 82]), as well as human myeloma cell lines (HMCLs [n = 54]). Gray dots represent the absence of expression, and black dots represent the presence of expression according to the presence-absence calls with negative probesets (PANP) algorithm. (B) RNA-seq analysis of DKK1 and SOST of MBCs (n = 4), PPCs (n = 4), BMPCs (n = 10), MM cells (n = 263), and HMCLs (n = 19). DKK1 (204602_at) is expressed by the majority of malignant plasma cell samples from previously untreated and relapsed myeloma patients. In contrast, SOST (223869_at) expression is absent in all normal and malignant plasma cells. (C) Immunohistochemical staining for CD138 (top panel) and sclerostin (bottom panel) counter-stained with hematoxylin in bone marrow of naive mice and mice bearing 5TGM1-eGFP myeloma cells. Slides were scanned on Scanscope CS2 (Aperio) up to original magnification ×40, and images were captured by using Aperio Imagescope at digital magnification ×20. Scale bar represents 50 μm. (D) Sclerostin protein was measured in media conditioned by MM1.S, OPM2 human myeloma cells lines, 5TGM1 murine myeloma cells, and primary osteocytes (n = 4; data are mean ± 1 standard error of the mean [SEM]; ****P < .0001). (E) Density plot highlighting the osteocyte-enriched expression of secreted Wnt signaling antagonists (n = 6). (F) Tumor burden significantly alters the expression of the secreted Wnt signaling pathway antagonists Sost, Dkk1, and Frzb (n = 6; data are mean ± 1 standard deviation [SD]; multiple comparison–adjusted P value *P < .05; **P < .01; ****P < .0001). (G) Immunohistochemical staining for sclerostin in osteocytes from naive and 5TGM1-bearing mice; arrow heads denote positive-stain osteocytes with positive-stained canaliculi (inset). Slides were scanned on Scanscope CS2 (Aperio) up to original magnification ×40, and images were captured by using Aperio Imagescope at digital magnification ×40. Scale bar represents 50 µm.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/129/26/10.1182_blood-2017-03-773341/4/m_blood773341f1.jpeg?Expires=1769449759&Signature=EDndNPISg3N2UdksK5wC6bOxM-BvSiidaqEAtNdmsbOh3Fib-Eo3957BGcudB4unmM726UN8mBVAq~j2M4bJsgI8q7TcWrEo9n95i2WoMIL8es3kCf-gPO8mqiGoUahkVCUwmrIiReEc76xHZuUsWooB5uFdZCwZgcXh0t3Xww8mSltm6vOHYOZ3phjrq92r03Y~HwhCANMnxhE51l51Ugbm5oL9GzL9KcKN8~vbALd-8FIXs8b3ADHPLWY-AYD7WhZTUzhK55xjCVUyxGSJJ3yKsKQVH3gIdxiZS4WyNKC58sGB6nlvN4lfOgLNyYw7p3ZMsIW6i92c8TglL90Aug__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 6. Combination treatment with anti-sclerostin antibody and ZA is superior in preventing bone loss in the 5TGM1 murine model of myeloma. (A) Schematic describing the study design for investigations of bone loss in 5TGM1-eGFP–bearing mice treated with both anti-sclerostin antibody and ZA. (B) Examples of 3D microCT reconstructions of distal femora from each treatment group. (C) Dot plots of BV/TV trabecular thickness and number and cortical bone thickness in femora for each treatment group (n = 8 per group). Data are mean ± 1 SEM; *P < .05; **P < .01; ***P < .001; ****P < .0001. (D) Dot plot of L4 vertebra BV/TV for each treatment group (data are mean ± 1 SEM; **P < .01; ***P < .001; ****P < .0001; #P < .05 [Student t test]). (E) Structural parameters from microCT scans of L4 vertebrae (n = 8 per group). Data are mean ± 1 SEM; aP < .001; bP < .01; dP < .05 compared with naive plus control mice; eP < .001; fP < .05 compared with 5TGM1 plus control mice; gP < .001 compared with 5TGM1 plus ZA; hP < .001 compared with 5TGM1 plus anti-sclerostin antibody. (F) Dot plot of maximum load from L4 vertebra for each treatment group (n = 8 per group). Data are mean ± 1 SEM; **P < .01; ***P < .001; #P < .05 [Student t test].](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/129/26/10.1182_blood-2017-03-773341/4/m_blood773341f6.jpeg?Expires=1769449759&Signature=vYllB-mTPDyRv5MVHFvZqgLvYQ59rOuUAAQccIA0a9~0b6bmkDHv7~tFYWDXdWPfbOsOglACRI3iklGI-VcSv03G~9yzFzmHXjGN3xLrOsZp3-R6zp85oUlLN569BM28hJ26qXzUnjk8u~LPZ5PrCZaWzLU-jwR4bbJEP0zk1TxkAHTQFfWMAPJZqFrsWhaXu-0o~cjrq33VO9Ax0lMKQxKTWIl7UJrwM1ZZTk1LxWf6XULJhTKZlUlCEtdTVrHVaYq1Fomk7CqiDaKd~u710P7gv27FKzHqOstbjLxdT6cCXrD-E24K~Vs0WwYSKQf9ftou9xg1qtyA0mEyN41kHQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 7. Treatment with anti-sclerostin antibody does not have an impact on tumor burden. (A) Representative flow cytometry plots of 5TGM1-eGFP control-treated bone marrow and 5TGM1-eGFP anti-sclerostin antibody–treated bone marrow showing the percentage of 5TGM1-eGFP+ cells in the sample (black box). (B) Dot plots of bone marrow tumor burden as a percent of total bone marrow cells (% GFP+) in experiments 1 and 2 of the 5TGM1 study (n = 6 [experiment 1]; n = 8 [experiment 2]). Data are mean ± 1 SEM. (C) Dot plots of spleen tumor burden as a percent of total cells (% GFP+) in experiments 1 and 2 of the 5TGM1 study (n = 6 [experiment 1]; n = 8 [experiment 2]). Data are mean ± 1 SEM. (D) Dot plots of bone marrow and spleen tumor burden (% idiotype+) in the 5T2MM study (n = 6-7). Data are mean ± 1 SEM. (E) Dot plots of whole body tumor burden (total flux p/s) and bone marrow percent GFP+ cells in MM1.S experiment 1 (n = 9 per group). Data are mean ± 1 SEM; *P < .05. (F) Dot plots of whole body tumor burden (total flux p/s) at 3 weeks and bone marrow percent GFP+ cells in MM1.S experiment 2 (n = 8-9 for whole body tumor; n = 5-9 for bone marrow tumor). Data are mean ± 1 SEM.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/129/26/10.1182_blood-2017-03-773341/4/m_blood773341f7.jpeg?Expires=1769449759&Signature=e~KfunbuL3eW5PU4z5J6g450nTI25inNsK-9O3Vhr7nM8MuJkGr2mf9WNJsVfVWIy3uK~NgcHYhXH3Ok3zfS~pULsZBzLgJ7HG08pG-k9ZAFwNuhYNIfNc9YRQqh6YpF0i8vh8lpQUfdKh81MNWrkX0d1MmRsxbiwfPW0rNXxaL4Nc7l07x7hf4OdIYXuulIFPfshS74ujUA-5BJesINHo5y5b9Ryt0QMPjHvLTpcFnBXq0yuaoyG0yikCvWZheqOgYPE8XW78qR0QOzhjV~RxSD15aaDuEGMb10i7dKKGi63guAkC-dz7DX1Wzh96PRaUzkRdl8ZbDy6pgXhxl5FQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Figure 1. Sclerostin is expressed by osteocytes but not myeloma cells. (A) Gene expression profiling using DNA microarray analysis of memory B cells (MBCs [n = 5]), in vitro–generated PPCs (n = 5), normal BMPCs (n = 10), malignant plasma cells from patients with newly diagnosed MM (n = 630) and relapsed/refractory MM (RMM [n = 82]), as well as human myeloma cell lines (HMCLs [n = 54]). Gray dots represent the absence of expression, and black dots represent the presence of expression according to the presence-absence calls with negative probesets (PANP) algorithm. (B) RNA-seq analysis of DKK1 and SOST of MBCs (n = 4), PPCs (n = 4), BMPCs (n = 10), MM cells (n = 263), and HMCLs (n = 19). DKK1 (204602_at) is expressed by the majority of malignant plasma cell samples from previously untreated and relapsed myeloma patients. In contrast, SOST (223869_at) expression is absent in all normal and malignant plasma cells. (C) Immunohistochemical staining for CD138 (top panel) and sclerostin (bottom panel) counter-stained with hematoxylin in bone marrow of naive mice and mice bearing 5TGM1-eGFP myeloma cells. Slides were scanned on Scanscope CS2 (Aperio) up to original magnification ×40, and images were captured by using Aperio Imagescope at digital magnification ×20. Scale bar represents 50 μm. (D) Sclerostin protein was measured in media conditioned by MM1.S, OPM2 human myeloma cells lines, 5TGM1 murine myeloma cells, and primary osteocytes (n = 4; data are mean ± 1 standard error of the mean [SEM]; ****P < .0001). (E) Density plot highlighting the osteocyte-enriched expression of secreted Wnt signaling antagonists (n = 6). (F) Tumor burden significantly alters the expression of the secreted Wnt signaling pathway antagonists Sost, Dkk1, and Frzb (n = 6; data are mean ± 1 standard deviation [SD]; multiple comparison–adjusted P value *P < .05; **P < .01; ****P < .0001). (G) Immunohistochemical staining for sclerostin in osteocytes from naive and 5TGM1-bearing mice; arrow heads denote positive-stain osteocytes with positive-stained canaliculi (inset). Slides were scanned on Scanscope CS2 (Aperio) up to original magnification ×40, and images were captured by using Aperio Imagescope at digital magnification ×40. Scale bar represents 50 µm.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/129/26/10.1182_blood-2017-03-773341/4/m_blood773341f1.jpeg?Expires=1769449760&Signature=KkjFlA~tl3jL0PI8C6KC9bFfX9hQWnHHzyJanbyOHe~pQa91VoLT7rpj0kUyNherakFydepTKahJqA6WapYRV30lrqMT7aRJCe0PteyCopErJ3jChwXRGh4o8Fj3r~dXbEY3KBMhapkTREqj7RWFIs3j-r3WSov4V5wbua3VvonDGF53zdxtf6oigOdqQ791BorCyDnaoX3wkBwGTsEwPzE2eIj9AC0vJpylfM78Au5p0CwhTIp0GCzSlTQKJwv3p-iclBH3aBgvPdH4cR0hmYR-urdjFWHn2vkaf5sqVWtygCMCQX0NbQ~wwNbnhnYmowxeCYT38IacCE9BEvOlRg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 6. Combination treatment with anti-sclerostin antibody and ZA is superior in preventing bone loss in the 5TGM1 murine model of myeloma. (A) Schematic describing the study design for investigations of bone loss in 5TGM1-eGFP–bearing mice treated with both anti-sclerostin antibody and ZA. (B) Examples of 3D microCT reconstructions of distal femora from each treatment group. (C) Dot plots of BV/TV trabecular thickness and number and cortical bone thickness in femora for each treatment group (n = 8 per group). Data are mean ± 1 SEM; *P < .05; **P < .01; ***P < .001; ****P < .0001. (D) Dot plot of L4 vertebra BV/TV for each treatment group (data are mean ± 1 SEM; **P < .01; ***P < .001; ****P < .0001; #P < .05 [Student t test]). (E) Structural parameters from microCT scans of L4 vertebrae (n = 8 per group). Data are mean ± 1 SEM; aP < .001; bP < .01; dP < .05 compared with naive plus control mice; eP < .001; fP < .05 compared with 5TGM1 plus control mice; gP < .001 compared with 5TGM1 plus ZA; hP < .001 compared with 5TGM1 plus anti-sclerostin antibody. (F) Dot plot of maximum load from L4 vertebra for each treatment group (n = 8 per group). Data are mean ± 1 SEM; **P < .01; ***P < .001; #P < .05 [Student t test].](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/129/26/10.1182_blood-2017-03-773341/4/m_blood773341f6.jpeg?Expires=1769449760&Signature=VhD~NSsqFtoChfQi4zENv-HSmTn-cJQOu6AueZaubRQ2DoHnL5lSc2raHCjZRQKU4YU1CLEoXtn-7I6ZFzwwNcd3LrfOu7V7E49bYp8047znU4-TcIuzKIrsfi6D5u3t3xn8CJBYnsbqi2jlmxHiyENP6Th-s9GZOI-Ic~zoD15sU4g70t2aoi~AuJchIVx0kjQm7cSnKe0pEU42kBwd--NNQTGGpebdnV4yteAybtLYA0g~rkb08CYWav8sGSc00DWjGIGQQFd2S-zx1Y2e8UWBdaNXaDCojpKZ1hA-xYDO1PjY2hPNWQJnlSHUfPhWOB7LdIU5fbxUArhP5bWcbg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 7. Treatment with anti-sclerostin antibody does not have an impact on tumor burden. (A) Representative flow cytometry plots of 5TGM1-eGFP control-treated bone marrow and 5TGM1-eGFP anti-sclerostin antibody–treated bone marrow showing the percentage of 5TGM1-eGFP+ cells in the sample (black box). (B) Dot plots of bone marrow tumor burden as a percent of total bone marrow cells (% GFP+) in experiments 1 and 2 of the 5TGM1 study (n = 6 [experiment 1]; n = 8 [experiment 2]). Data are mean ± 1 SEM. (C) Dot plots of spleen tumor burden as a percent of total cells (% GFP+) in experiments 1 and 2 of the 5TGM1 study (n = 6 [experiment 1]; n = 8 [experiment 2]). Data are mean ± 1 SEM. (D) Dot plots of bone marrow and spleen tumor burden (% idiotype+) in the 5T2MM study (n = 6-7). Data are mean ± 1 SEM. (E) Dot plots of whole body tumor burden (total flux p/s) and bone marrow percent GFP+ cells in MM1.S experiment 1 (n = 9 per group). Data are mean ± 1 SEM; *P < .05. (F) Dot plots of whole body tumor burden (total flux p/s) at 3 weeks and bone marrow percent GFP+ cells in MM1.S experiment 2 (n = 8-9 for whole body tumor; n = 5-9 for bone marrow tumor). Data are mean ± 1 SEM.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/129/26/10.1182_blood-2017-03-773341/4/m_blood773341f7.jpeg?Expires=1769449760&Signature=bvlriW~wLW86gzqKb6l0uZvCYovEo~MJinWFzK42Xkc~e7AiWFkOlbQWhCIWtdajOR0ZRCS4sdwpZYCbV8rzzt9RO0qnlFHkTAhsJso6Kw0Q3nJ8YWcC22DpDz4b0RHCEbCNcaquIj3D-JyWgqQ6JtgPiUmIRsqtMM8axG20Li3eTwHisFh557qYuPH6VJ2U9r~KSqTAU3f7QiJa65hZbdOv99ZtrLBBkdV-9SWGoVdHPPgZfQGPQBmXtd9Xu3N~i9flvtQgRNEPdlsOLORn943jmFrrTmpx1UD6JTRTwldWkyip~tpNNWy14Y2zCRlpGZ94prNNy7x30E3vhf3ebg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)