Key Points
LN-derived CLL cells have increased capacity for T-cell activation and superior immune synapse formation compared with those from PB.
Enhanced CLL cell immunologic function is also linked to PB circulating cells with the propensity to migrate.
Abstract
Several lines of evidence suggest that homing of tumor cells to lymphoid tissue contributes to disease progression in chronic lymphocytic leukemia (CLL). Here, we demonstrate that lymph node (LN)-derived CLL cells possess a distinct phenotype, and exhibit enhanced capacity for T-cell activation and superior immune synapse formation when compared with paired peripheral blood (PB) samples. LN-derived CLL cells manifest a proliferative, CXCR4dimCD5bright phenotype compared with those in the PB and higher expression of T-cell activation molecules including CD80, CD86, and HLA-D–related (DR). In addition, LN-CLL cells have higher expression of α4β1 (CD49d) which, as well as being a co-stimulatory molecule, is required for CLL cells to undergo transendothelial migration (TEM) and enter the proliferation centers of the LNs. Using an in vitro system that models circulation and TEM, we showed that the small population of CLL cells that migrate are CXCR4dimCD5bright with higher CD49d, CD80, CD86, and HLA-DR compared with those that remain circulating; a phenotype strikingly similar to LN-derived CLL cells. Furthermore, sorted CD49dhi CLL cells showed an enhanced capacity to activate T cells compared with CD49dlo subpopulations from the same patient. Thus, although PB-CLL cells have a reduced capacity to form immune synapses and activate CD4+ T cells, this was not the case for LN-CLL cells or those with the propensity to undergo TEM. Taken together, our study suggests that CLL cell immunologic function is not only modulated by microenvironmental interactions but is also a feature of a subpopulation of PB-CLL cells that are primed for lymphoid tissue homing and interaction with T cells.
Introduction
Chronic lymphocytic leukemia (CLL) is a common B-cell malignancy that follows a remarkably diverse clinical course. It is characterized by an accumulation of mature B-lymphocytes in the peripheral blood (PB), bone marrow, and secondary lymphoid organs such as the lymph nodes (LNs).1 Because circulating tumor cells generally have a very low proliferation rate, it was originally assumed that CLL was primarily a disease of failed apoptosis. However, in vivo studies of tumor kinetics, using deuterated water, revealed higher than expected tumor cell turnover, with a birth rate of up to 2% per day.2
The proliferative component of CLL appears to be confined to pseudofollicles or proliferation centers in secondary lymphoid tissues,3,4 where interactions with non-neoplastic T cells5,6 and follicular dendritic cells7 take place, and promote tumor cell growth.4 In contrast, very few CLL cells in the peripheral circulation show features of proliferation and those that do are believed to represent recent emigrants from the LN.8 In the peripheral circulation, CLL cells transiently interact with endothelial cells, which stimulate survival9 but not proliferation.10 These findings suggest a 2-compartment model of disease in which CLL cells traffic between the peripheral vasculature and the lymphoid tissues. In support of this, Herishanu et al compared the gene expression of CLL cells in different compartments and identified the LN as the predominant site of CLL cell activation and proliferation.11 Because disease progression occurs when tumor proliferation outstrips loss, the capacity of tumor cells to migrate into tissues is an important factor in determining outcome. Transit of CLL cells to the tissues is mediated, at least in part, by their expression of CD49d12 and the chemokine receptors such as CXCR4 and CXCR5,13-15 and is controlled by the secretion of the chemokine ligands including CXCL12 and CXCL13.
Work by Calissano et al8 used in vivo deuterium incorporation to study the phenotype and gene expression of the resting and proliferative fraction. They characterized the 2 compartments using differences in CD5 expression (which is upregulated following B-cell activation) and CXCR4 (which is raised in CLL cells with high surface immunoglobulin M, and downregulated following B-cell receptor [BCR] engagement16 or binding of CXCL12). They concluded that distinct subsets of CLL cells exist within PB-CLL cells, including small populations of CXCR4dimCD5bright and CXCR4brightCD5dim cells. They hypothesized that the former are proliferative, recent emigrants from the LN and the later “older” resting cells attempting to re-enter the LN.
Several types of interaction are thought to occur in the CLL tissue microenvironment. There is good evidence that co-stimulatory signals from activated CD4+ T cells5,17 play an important role in promoting tumor growth. Contact with activated autologous CD4+ T cells is sufficient to induce proliferation of CLL cells in vitro and analysis of tissue samples from CLL patients reveals that proliferating leukemic cells frequently contact activated CD4+ T cells.6,18
The objective of the present study was to reconcile these findings with the extensive previous evidence that CLL cells strongly inhibit T-cell activation.19-21 Previous studies assessing the T-cell activation capabilities of CLL cells have used cells derived from the PB, which it has been suggested induces anergy.22 However, these cells are known to have different properties to those residing within the LNs.6,11 Here, we used fine-needle aspiration (FNA) to perform functional assays and determine whether matched CLL cells from LN-CLL and PB-CLL had a distinct compartment-specific phenotype and T-cell activation function. In addition, we used our novel circulation system to study migration23 in order to compare LN-derived CLL cells with CLL cells that migrated in vitro.
Materials and methods
Patient samples
Matched PB and LN FNA sampling was undertaken simultaneously on 11 patients with a diagnosis of CLL and palpable lymphadenopathy. The FNA was performed by the passage of a 23-gauge needle through the skin once and sampling 6 to 8 times within the node. PB only was taken from another 36 patients. CLL PB mononuclear cells (PBMCs) were isolated from whole blood of CLL patients by density gradient centrifugation with Lymphoprep (Axis-Shield) or Histopaque (Sigma). All were taken with the patients’ informed consent in accordance with the Declaration of Helsinki. Normal T and B cells were derived from healthy volunteers.
Circulation system
A hollow fiber bioreactor system (FiberCell Systems Inc) was adapted to generate an in vitro model of circulating CLL previously described23 using human umbilical vein endothelial cells and human microvascular endothelial cells (Life Technologies) at 5% CO2 at 37°C. PB-CLL cells were introduced into the circulating system through one of the access ports in the circulating compartment, and were allowed to circulate for 48 hours before samples were removed from port D (circulating) and port C (migrated). CLL cells were subsequently immunophenotyped as described below.
Immunophenotyping
CLL cells recovered from the circulation system, following FNA or PB density gradient centrifugation were labeled using the panels shown in supplemental Table 1, available on the Blood Web site. For the FNA/PB staining, a whole blood staining method was performed as per the manufacturer’s instructions and a red cell lysis buffer (eBiosciences) was used. For each antigen, the mean fluorescent intensity (MFI) of the CD19+/CD5+ CLL cells was recorded.
Mixed lymphocyte reaction (MLR)
T and B cells were purified by negative selection (StemCell Technologies), checked for purity by flow cytometry, and resuspended to 106 cells/mL in RPMI complete medium (CM) with 1% bovine serum albumin (CM). Enriched (>95%) CLL B cells from both the LN and PB, or CD49dhi and CD49dlo (top and bottom 20%), were sorted using a BD FACS Aria (gating strategy shown in supplemental Figure 1), resuspended in CM at 106 cells/mL, and for the thymidine-incorporation assay irradiated at 30 Gy. CLL and T cells were plated out in triplicate at 1:1 and 1:10, and incubated for 48 hours at 5% CO2 at 37°C and then harvested as previously described.21 When natalizumab was added, CLL cells were pre-incubated for 20 minutes at 20 μg/106 cells before coculture with T cells and a further 20 μg/106 cells added every 24 hours. For T-cell flow cytometry (antibody list: supplemental Table 1, panels E-F) CD3+CD4+/CD8+ T cells were tightly gated on and expression of HLA-D–related (DR), Ki67, and CD69 assessed. For the Ki67 assay, Fix and Perm (Invitrogen) was used as per the manufacturer’s instructions except that 0.5 μL of 10% NP40 was added per 50 μL of perm buffer. The thymidine incorporation assay was performed as previously described.21
Synapse assays
Quantitative CLL:T-cell synapse assays were performed and analyzed as previously described.24 Blinded confocal images were analyzed and CD4+ T-cell/antigen-presenting cell (APC) conjugates were identified only when T cells were in direct contact interaction with CLL APCs (blue fluorescent channel). The area analysis tool was then used to measure the total area (μm2) of filamentous-actin (red fluorescent channel) accumulation at all T-cell contact sites and synapses with APCs.
Statistical analysis
Statistical analyses were performed using GraphPad Prism 4.0 and 7.0 (GraphPad Software, San Diego, CA). Data were assessed for Gaussian distribution using the D’Agostino-Pearson normality test and appropriate tests applied.
Results
Comparison of the phenotype of PB and LN-CLL cells
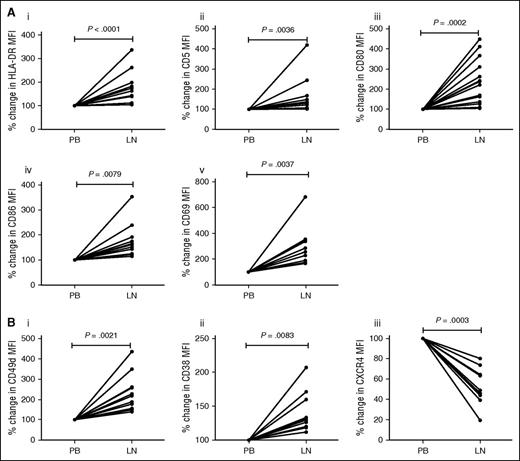
In order to directly compare the phenotype of CLL cells derived from LNs and the peripheral circulation, we performed matched FNA and PB sampling on 11 CLL patients with lymphadenopathy. Only patients with lymphadenopathy were selected and these patients typically have high-risk disease and manifest other markers of poor prognosis (supplemental Table 2). Using multicolor flow cytometry, CD5+/CD19+ CLL cells were gated and MFI of HLA-DR, CD5, CD80, CD86, and CD69 established. In all cases, LN-CLL cells had significantly higher expression of the markers associated with antigen presentation, co-stimulation, and activation: HLA-DR (P < .0001), CD5 (P = .0036), CD80 (P = .0002), CD86 (P = .0079), and CD69 (P = .0037; Figure 1A). These findings are in keeping with previous work showing activation of CLL cells in the LN11 and support our hypothesis that LN-CLL cells have better T-cell activation potential than those from the PB. Interestingly, compared with PB-CLL cells, these LN-CLL cells also had a phenotype associated with adhesion and migration, namely increased CD49d (P = .0021) and CD38 (P = .0083), and decreased CXCR4 expression (P = .0003) (Figure 1B). This may be because BCR activation occurs within the LN and is associated with downregulation of CXCR4. In addition, although raised CXCR4 expression is associated with the propensity to migrate, following migration the CLL cells encounter high local concentrations of CXCL12, which promotes rapid downregulation of this chemokine receptor. Immunosuppressive markers were also analyzed, but expression of programmed death-ligand 1 (PD-L1) and PD-L2 was absent in both PB and LN-CLL cells in 5/8 patients, and the remaining 3 patients showed very low levels with no difference between PB and LN-CLL cells. CD200 expression was high in both LN-CLL and PB-CLL cells (data not shown).
LN-derived CLL cells have a phenotype associated with T-cell activation and migration. Matched LN and PB samples from 11 CLL patients were analyzed using multicolor flow cytometry, and the percent change between the LN and PB MFI calculated. (A) Compared with PB-derived CLL cells, LN-derived CD19+CD5+ CLL cells showed higher expression of the markers associated with antigen-presentation, co-stimulation, and activation: HLA-DR (Ai), CD5 (Aii), CD80 (Aiii), CD86 (Aiv), and CD69 (Av). (B) In addition, they have a phenotype associated with recent migration: raised CD49d (Bi) and CD38 (Bii), and reduced CXCR4 (Biii).
LN-derived CLL cells have a phenotype associated with T-cell activation and migration. Matched LN and PB samples from 11 CLL patients were analyzed using multicolor flow cytometry, and the percent change between the LN and PB MFI calculated. (A) Compared with PB-derived CLL cells, LN-derived CD19+CD5+ CLL cells showed higher expression of the markers associated with antigen-presentation, co-stimulation, and activation: HLA-DR (Ai), CD5 (Aii), CD80 (Aiii), CD86 (Aiv), and CD69 (Av). (B) In addition, they have a phenotype associated with recent migration: raised CD49d (Bi) and CD38 (Bii), and reduced CXCR4 (Biii).
Enhanced capacity of LN-derived CLL cells to activate T cells
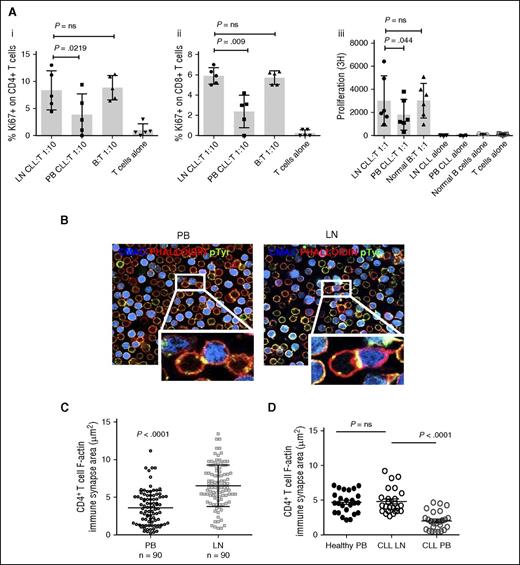
Because LN-CLL cells have increased expression of molecules associated with T-cell activation compared with their PB counterparts, we next sought to determine whether they also had an increased capacity to stimulate normal allogeneic T cells. Paired LN and PB-CLL cells plus normal B cells were mixed with purified CD3+ T cells from a healthy donor in an MLR, and the proliferation and activation status of the T cells was assessed by measuring 3H thymidine incorporation and the expression of Ki67, CD69, and HLA-DR by flow cytometry. LN-CLL cells induced greater activation of both CD4+ and CD8+ T cells as measured by their higher expression of Ki67, CD69, and HLA-DR when compared with PB-CLL cells, and this was equivalent to that induced by normal B cells (Figure 2A and supplemental Figure 2A). Furthermore, enhanced T-cell proliferation was confirmed in the presence of irradiated LN-CLL cells, again equivalent to that induced by normal B cells, as evidenced by significantly increased thymidine incorporation (Figure 2A). The CLL cells were purified by negative selection to avoid modification of properties by antibody binding; the resultant cells were >95% CD5+CD19+. To control for the potential that the small residual non-CLL cell pool contained different numbers of APCs, we evaluated the proportion of CD5−CD19−HLA-DR+ cells in the LN and the PB residual populations, and showed there was no difference: LN 1.4% ± 1.2% and PB 1.9% ± 1.8 (P = .52; data not shown).
LN-CLL cells are functionally better at T-cell activation and induce superior CLL:T-cell synapses. (A) Paired LN and PB-CLL cells (irradiated for thymidine-incorporation assays) from 6 patients and B cells from 5 normal donors were mixed at a 1:1 or 1:10 ratio with allogeneic T cells in triplicate. Compared with PB-CLL cells, LN-CLL cells and normal B cells have an increased ability to stimulate T-cell activation and proliferation, as shown by increased expression of Ki67 on gated CD4+ (Ai) cells and CD8+ cells (Aii) after a 48-hour coculture, and increased thymidine-incorporation by CD3+ T cells in a 5-day MLR (Aiii). (B) To investigate the ability of the LN-derived CLL cells to induce autologous T-cell synapse formation compared with PB-CLL cells and normal B cells, we measured the area of filamentous actin polymerization in 90 CLL or B-cell:CD4+ T-cell conjugates in 4 paired patient samples and 1 paired normal sample. This representative figure from 1 patient shows the increased synapse formation induced by the LN-derived CLL cells compared with matched PB-CLL cells. (C) Representative result from 1 patient showing that synapse area was significantly greater when LN-derived CLL cells were used (mean synapse area induced by LN-CLL 6.534 μm2 ± 2.7 vs PB-CLL 3.594 μm2 ± 2.3; P < .0001). (D) Representative result from another patient demonstrating the synapse area generated by LN-CLL cells is comparable to that of normal B cells. ns, not significant; pTyr, phosphotyrosine.
LN-CLL cells are functionally better at T-cell activation and induce superior CLL:T-cell synapses. (A) Paired LN and PB-CLL cells (irradiated for thymidine-incorporation assays) from 6 patients and B cells from 5 normal donors were mixed at a 1:1 or 1:10 ratio with allogeneic T cells in triplicate. Compared with PB-CLL cells, LN-CLL cells and normal B cells have an increased ability to stimulate T-cell activation and proliferation, as shown by increased expression of Ki67 on gated CD4+ (Ai) cells and CD8+ cells (Aii) after a 48-hour coculture, and increased thymidine-incorporation by CD3+ T cells in a 5-day MLR (Aiii). (B) To investigate the ability of the LN-derived CLL cells to induce autologous T-cell synapse formation compared with PB-CLL cells and normal B cells, we measured the area of filamentous actin polymerization in 90 CLL or B-cell:CD4+ T-cell conjugates in 4 paired patient samples and 1 paired normal sample. This representative figure from 1 patient shows the increased synapse formation induced by the LN-derived CLL cells compared with matched PB-CLL cells. (C) Representative result from 1 patient showing that synapse area was significantly greater when LN-derived CLL cells were used (mean synapse area induced by LN-CLL 6.534 μm2 ± 2.7 vs PB-CLL 3.594 μm2 ± 2.3; P < .0001). (D) Representative result from another patient demonstrating the synapse area generated by LN-CLL cells is comparable to that of normal B cells. ns, not significant; pTyr, phosphotyrosine.
We also compared the ability of normal PB B cells, LN-CLL cells, and matched PB-CLL cells to form immune synapses with autologous CD4+ T cells. Previous work has shown that PB-CLL cells exhibit impaired T-cell synapse formation.20 Here, we measured synapse assembly (CLL:CD4+ T-cell conjugates) in 4 patient samples and demonstrated for the first time that LN-CLL cells showed enhanced autologous T-cell synapse formation, equivalent to that induced by healthy B cells,24 when compared with their matched PB-CLL cells (patients 1-4 = P < .0001, P = .03, P = .05, and P < .0001, respectively; Figure 2B-D).
PB-CLL cells that migrate in our in vitro model possess a strikingly similar phenotype to LN-CLL cells
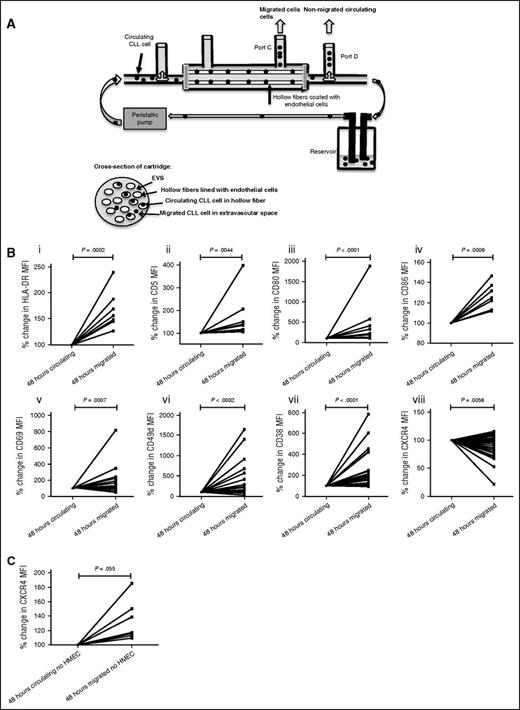
We investigated whether the phenotype manifested by LN-CLL cells was dependent on their residence in the LN microenvironment or could be identified in a subset of PB-CLL cells with a propensity to migrate. We used a physiologically relevant in vitro circulation system of CLL (Figure 3A)23 into which PBMCs from 36 CLL patients were then individually introduced for 48 hours before samples were harvested. Matched samples were obtained from port D (CLL cells remaining in circulation) and port C (those that had migrated through the endothelial cell-coated fibers into the extravascular space [EVS]). In keeping with our previous report,23 a small percentage of CLL cells migrated into the EVS after 48 hours (1.37% ± 2.32%). Compared with CLL cells remaining in the circulating compartment, migrated cells showed lower expression of CXCR4 (P = .0058), as well as increased expression of CD49d (P < .0002), CD38 (P < .0001), HLA-DR (P = .0002), CD5 (P = .0044), CD80 (P < .0001), CD86 (P = .0006), and CD69 (P = .0007) (Figure 3B). This phenotype was strikingly similar to that of LN-derived CLL cells. It is worthy of note that when we repeated these experiments without endothelial cells lining the hollow fibers, there was significantly reduced migration but the tiny number that migrated manifested higher levels of CXCR4 (P = .055; Figure 3C) and no difference in CD5. The other phenotypic markers showed similar increases in the absence of endothelial cells (data not shown). These results confirm our previous observation that PB-CLL cells with high CXCR4 and CD49d have an increased propensity to migrate,25 but imply that following transendothelial migration (TEM), the CXCR4 expression is reduced and CD5 is increased.
CLL cells that migrate have a strikingly similar phenotype to those derived from the LN. (A) CLL PBMCs were introduced into the circulating model system coated with human endothelial cells (human umbilical vein endothelial cell23 or human microvascular endothelial cell-19,10 ), and samples were collected from port C (migrated) and port D (circulating) after 48 hours. Matched CD5+/CD19+ CLL cells from each compartment were analyzed using multicolor flow cytometry. (B) Compared with CLL cells that remained circulating, migrated CLL cells had a phenotypic pattern strikingly similar to LN-CLL cells: higher HLA-DR (n = 7, Ai), CD5 (n = 12, Aii), CD80 (n = 19, Aiii), CD86 (n = 7, Aiv), CD69 (n = 26, Av), CD49d (n = 36, Avi), and CD38 (n = 32, Avii), and reduced CXCR4 (n = 34, Aviii). CLL cells that remained circulating had a pattern reflective of PB-CLL cells. (C) PBMCs from 11 patients were introduced into the circulating model system in the absence of endothelial cell coating. The numbers of CLL cells migrating was much lower than in the presence of endothelial cells and only sufficient for analysis migrated from 8 patients. In these 8 cases, the migrated CLL cells had increased CXCR4 expression compared with those that remained circulating. HMEC, human microvascular endothelial cell.
CLL cells that migrate have a strikingly similar phenotype to those derived from the LN. (A) CLL PBMCs were introduced into the circulating model system coated with human endothelial cells (human umbilical vein endothelial cell23 or human microvascular endothelial cell-19,10 ), and samples were collected from port C (migrated) and port D (circulating) after 48 hours. Matched CD5+/CD19+ CLL cells from each compartment were analyzed using multicolor flow cytometry. (B) Compared with CLL cells that remained circulating, migrated CLL cells had a phenotypic pattern strikingly similar to LN-CLL cells: higher HLA-DR (n = 7, Ai), CD5 (n = 12, Aii), CD80 (n = 19, Aiii), CD86 (n = 7, Aiv), CD69 (n = 26, Av), CD49d (n = 36, Avi), and CD38 (n = 32, Avii), and reduced CXCR4 (n = 34, Aviii). CLL cells that remained circulating had a pattern reflective of PB-CLL cells. (C) PBMCs from 11 patients were introduced into the circulating model system in the absence of endothelial cell coating. The numbers of CLL cells migrating was much lower than in the presence of endothelial cells and only sufficient for analysis migrated from 8 patients. In these 8 cases, the migrated CLL cells had increased CXCR4 expression compared with those that remained circulating. HMEC, human microvascular endothelial cell.
Migrated and LN-derived CLL cells are CXCR4dimCD5bright compared with their circulating and PB-derived counterparts
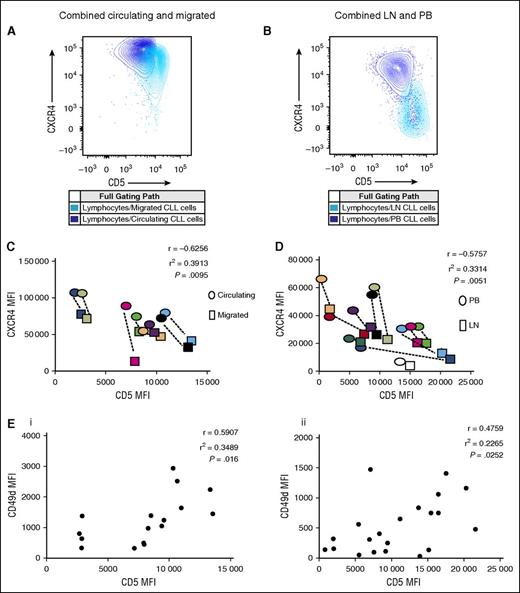
The work by Calissano et al8 identified small intraclonal subpopulations of CLL cells with different proliferative characteristics. A small number of CXCR4dimCD5bright CLL cells were identified as being the proliferative subpopulation and it was hypothesized that these had recently emigrated from the LN. In this study, we demonstrate that the small number of CLL cells that have migrated our in vitro model were enriched for a CXCR4dimCD5bright phenotype when compared with those that remained circulating. In addition, we showed a clear negative correlation between the expression of CD5 and CXCR4 (P = .0095; Figure 4A,C). Whether these cells are recent emigrants from the LN that are better primed to migrate in this system due to their enhanced activation status, or whether they are a small population with an increased migratory potential remains unknown. However, we repeated this analysis on LN-CLL cells and clearly showed that, compared with matched PB-CLL cells, LN-CLL cells also had this CXCR4dimCD5bright phenotype (P = .0051; Figure 4B,D).
Both models show a negative correlation between CD5 and CXCR4 but a positive correlation between CD5 and CD49d. (A) A representative figure showing matched circulating and migrated CD19+CD5+ CLL cells from a single patient harvested after 48 hours in the circulation system. Compared with each other, the migrated CLL cells are CD5brightCXCR4dim and the circulating cells CD5dimCXCR4bright. (B) A representative figure showing matched PB and LN CD19+CD5+ CLL cells from a single patient. The LN-CLL cells are CD5brightCXCR4dim and the PB cells CD5dimCXCR4bright. (C) For both the migrated and circulating CD19+CD5+ CLL cells, the MFI of the CD5 and CXCR4 were established and show a negative correlation. This was done on 8 patients and a color assigned to each patient. For each case, the dots surrounded by a colored box represents the result for the migrated and that surrounded by an oval in the same color represents the matched circulating result. In each case, the migrated cells have higher CD5 and lower CXCR4 than the circulating ones. (D) The same was done for the LN and PB CD19+CD5+ CLL cells from 11 patients. In each case, the LN-derived CLL cells have higher CD5 and lower CXCR4 (box) than those from the PB (oval). (E) In both the circulating/migrated model (Ei) and the LN/PB model (Eii), CD5 and CD49d positively correlate. Each solid circle represents the CD5 MFI plotted against the CD49 MFI for an individual patient sample.
Both models show a negative correlation between CD5 and CXCR4 but a positive correlation between CD5 and CD49d. (A) A representative figure showing matched circulating and migrated CD19+CD5+ CLL cells from a single patient harvested after 48 hours in the circulation system. Compared with each other, the migrated CLL cells are CD5brightCXCR4dim and the circulating cells CD5dimCXCR4bright. (B) A representative figure showing matched PB and LN CD19+CD5+ CLL cells from a single patient. The LN-CLL cells are CD5brightCXCR4dim and the PB cells CD5dimCXCR4bright. (C) For both the migrated and circulating CD19+CD5+ CLL cells, the MFI of the CD5 and CXCR4 were established and show a negative correlation. This was done on 8 patients and a color assigned to each patient. For each case, the dots surrounded by a colored box represents the result for the migrated and that surrounded by an oval in the same color represents the matched circulating result. In each case, the migrated cells have higher CD5 and lower CXCR4 than the circulating ones. (D) The same was done for the LN and PB CD19+CD5+ CLL cells from 11 patients. In each case, the LN-derived CLL cells have higher CD5 and lower CXCR4 (box) than those from the PB (oval). (E) In both the circulating/migrated model (Ei) and the LN/PB model (Eii), CD5 and CD49d positively correlate. Each solid circle represents the CD5 MFI plotted against the CD49 MFI for an individual patient sample.
CD49d expression is associated with expression of activation and co-stimulatory molecules, and an increased capacity to activate T cells
The phenotype of migrated CLL cells suggests that, as well as having a greater propensity to migrate, their increased expression of co-stimulatory molecules could also potentially have a greater affect on T-cell activation. It has been previously shown that CD49d expression identifies CLL cells that have an increased capacity to undergo TEM12,23 and interestingly, it is also a co-stimulatory molecule.26 In order to investigate whether there is a link between migration and co-stimulation in CLL, we correlated the expression of CD49d with a variety of activation and co-stimulatory molecules in the LN and PB of patients with CLL, and in the circulating and extravascular compartment of the in vitro circulation system. We found that CD49d expression positively correlated with CD5 expression in both the circulating and EVS compartments of the in vitro system, and in the matched LN/PB ex vivo samples (P = .016 and P = .0252, respectively; Figure 4E). There was also a strong and statistically significant correlation between CD49d levels and CD80, CD86, HLA-DR, CD69, and CD38 in both in vitro and in vivo systems (Figure 5), and once again the correlation patterns from the circulating model system and the matched LN-CLL and PB-CLL cells were strikingly similar.
Both models show a strikingly similar positive correlation between CD49d and markers associated with antigen presentation and activation. (A) PBMCs were introduced into the circulating model system for 48 hours, and the MFI values for both the migrated and circulating CLL cells recorded. There is a positive correlation between the expression of CD49d and CD80 (Ai), CD86 (Aii), HLA-DR (Aiii), CD69 (Aiv), and CD38 (Av). (B) MFI data from matched LN and PB-CLL cells from 11 patients were analyzed for correlation. There was a positive correlation between expression of CD49d and CD80 (Ai), CD86 (Aii), HLA-DR (Aiii), CD69 (Aiv), and CD38 (Av). Each solid box represents the CD80, CD86, HLA-DR, CD69 or CD38 MFI plotted against the CD49 MFI for an individual patient sample.
Both models show a strikingly similar positive correlation between CD49d and markers associated with antigen presentation and activation. (A) PBMCs were introduced into the circulating model system for 48 hours, and the MFI values for both the migrated and circulating CLL cells recorded. There is a positive correlation between the expression of CD49d and CD80 (Ai), CD86 (Aii), HLA-DR (Aiii), CD69 (Aiv), and CD38 (Av). (B) MFI data from matched LN and PB-CLL cells from 11 patients were analyzed for correlation. There was a positive correlation between expression of CD49d and CD80 (Ai), CD86 (Aii), HLA-DR (Aiii), CD69 (Aiv), and CD38 (Av). Each solid box represents the CD80, CD86, HLA-DR, CD69 or CD38 MFI plotted against the CD49 MFI for an individual patient sample.
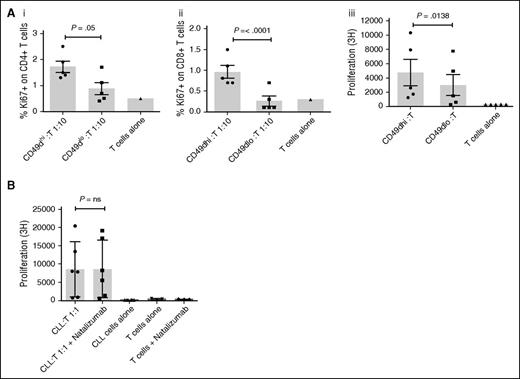
Functional assays were not possible with cells harvested from the EVS due to the limited number of cells that could be obtained. We instead exploited the observation that CD49dhi CLL cells have the highest migratory potential. We sorted the top and bottom 20% of the CD49d-expressing CLL cells (based on MFI) from the PB of 5 CLL patients and compared their ability to activate alloreactive T cells in an MLR. CD49dhi and CD49dlo CLL cells were mixed with purified CD3+ T cells from a healthy donor, and the proliferation and activation status of the T cells assessed by measuring their expression of Ki67, CD69, and HLA-DR by flow cytometry and incorporation of 3H thymidine as described previously. CD49dhi CLL cells induced greater activation of CD4+ and CD8+ T cells as evidenced by higher expression of Ki67 (P = .05 and P < .001), CD69 (P = .05 and P = .04), and HLA-DR (P = .03 and P = .006) when compared with T cells cocultured with CD49dlo CLL cells (Figure 6A and supplemental Figure 2B). Furthermore, enhanced T-cell proliferation was confirmed in the presence of irradiated CD49dhi cells by significantly increased thymidine incorporation (P = .013; Figure 6A). These results show that, for CLL cells, there is a clear relationship between the propensity to migrate and the ability to activate T cells. We have previously demonstrated that blocking CD49d using natalizumab prevented CLL migration in our circulating system.23 Here, we investigated whether natalizumab could also inhibit the ability of CLL cells to activate alloreactive T cells in an MLR. CLL cells from 6 CD49dhi CLL patients were pre-incubated with natalizumab prior to irradiation and coculture with purified CD3+ T cells from a healthy donor. Following a 5-day MLR, there was no difference in the thymidine incorporation of the T cells incubated with CLL cells in the presence or absence of natalizumab (Figure 6B). This suggests that the functional role of CD49d on these cells relates to migratory potential rather than T-cell activation.
CD49dhi CLL cells are superior stimulators of T-cell activation. Paired CD49dhi and CD49dlo CLL cells (irradiated for thymidine-incorporation assays) from 5 patients were mixed at a 1:1 or 1:10 ratio with allogeneic T cells in triplicate. (A) Compared with CD49dlo cells, CD49dhi cells have an increased ability to stimulate T-cell activation and proliferation, as shown by the increased expression of Ki67 on gated CD4+ (Ai) and CD8+ (Aii) cells after 48 hours and increased thymidine-incorporation in a 5-day MLR (Aiii). (B) Irradiated CLL cells from 6 patients known to express CD49d were mixed at a 1:1 ratio with allogeneic T cells in triplicate and in the absence or presence of natalizumab, and proliferation measured by thymidine-incorporation in a 5-day MLR. Blocking of CD49d had no effect on the ability of CLL cells to induce T-cell proliferation. The solid circles, squares, and bars represent the mean result for each individual patient MLR.
CD49dhi CLL cells are superior stimulators of T-cell activation. Paired CD49dhi and CD49dlo CLL cells (irradiated for thymidine-incorporation assays) from 5 patients were mixed at a 1:1 or 1:10 ratio with allogeneic T cells in triplicate. (A) Compared with CD49dlo cells, CD49dhi cells have an increased ability to stimulate T-cell activation and proliferation, as shown by the increased expression of Ki67 on gated CD4+ (Ai) and CD8+ (Aii) cells after 48 hours and increased thymidine-incorporation in a 5-day MLR (Aiii). (B) Irradiated CLL cells from 6 patients known to express CD49d were mixed at a 1:1 ratio with allogeneic T cells in triplicate and in the absence or presence of natalizumab, and proliferation measured by thymidine-incorporation in a 5-day MLR. Blocking of CD49d had no effect on the ability of CLL cells to induce T-cell proliferation. The solid circles, squares, and bars represent the mean result for each individual patient MLR.
Discussion
In this study, we set out to compare the phenotypic and functional properties of PB-CLL cells with those that have undergone TEM into LNs. Traditionally, investigations into the pathophysiology of CLL were largely restricted to PB-derived cells, but recent data demonstrating the key role of the LN microenvironment in this disease has highlighted the importance of understanding the differences in cells residing within the LNs compared with those in the PB.
In this study, we used FNA to access simultaneous LN and PB-CLL cells, and used this material to answer some important questions regarding differences in phenotype and function between the two compartments. Unfortunately, no matched bone marrow samples were available for this study, but previous work by Herishanu et al11 suggests that this microenvironment is not as pro-proliferative or activation-inducing as the LN.
Previous comparisons have used disaggregated LN biopsies from cases when there is diagnostic doubt or atypical disease behavior. Our study recruited typical cases of CLL and the LN sample was fresh suspension cells. Using these samples, we clearly demonstrated that LN-CLL cells had enhanced expression of markers that would induce T-cell activation. In contrast to previous studies, which suggest that PB-CLL cells are poor APCs,22,27 induce T-cell anergy, and inhibit T-cell activation,20,21,28 we showed that LN-CLL cells can induce T-cell activation and proliferation. We also showed that, in contrast to PB-CLL cells, those from the LN were capable of forming immune synapses with autologous CD4+ T cells that were comparable to those formed by healthy B and T cells. These results provide a plausible explanation for the observation that CLL LNs contain significant numbers of activated T cells, despite the known inhibitory effects of the tumor.21 It has been suggested that CLL cells might present antigen to T cells,29 and thereby initiate a self-sustaining stimulatory loop in which CLL cells cause T-cell activation, which in turn leads to activation and proliferation of the tumor. The observation that CLL cells are capable of presenting red cell-derived rhesus antigen, causing the expansion of auto-reactive T-cell clones in patients with autoimmune hemolytic anemia,30 provides further in vivo evidence in support of this theory. Whether the interactions promote an antitumor response or a self-stimulatory tumor survival loop remains unknown, but the nature of the disease suggests it is the latter.
In addition, LN-CLL cells also expressed higher levels of CD49d and CD38, which are both involved in TEM. This suggests there is a link between the enhanced ability to activate T cells and the capacity to migrate. Tissue invasion of CLL requires TEM of the malignant cells, but a full understanding of the mechanisms behind this is not yet available. However, it has been established that CLL clones differ from normal B cells in that they require α4β1 (CD49d) engagement to undergo TEM and enter the proliferation centers of LNs.12 In addition, CD38 is associated with CD49d31,32 and homing from the blood to the lymphoid organs.33 In this study, we showed that both of these poor prognostic markers were expressed at higher levels in LN-CLL cells. In much the same way that we now know that CD38 expression is temporal,6,34 it seems likely that CD49d, CXCR4, and CD5 are also temporally regulated, and this could give rise to intraclonal subsets with a higher and lower predisposition to migrate depending on their phenotype. In support of this theory, we demonstrated that CD38hi/CD49dhi LN-CLL cells were CXCR4dimCD5bright compared with PB-CLL cells that supports the Calissano model of them being the proliferative “robust” fraction.8
In order to investigate whether these cells could be identified as a subset in PB-CLL and, if so, whether they have an increased propensity to migrate, we used our novel in vitro circulation system. We demonstrated that compared with the majority of cells that remained circulating, the small population of CLL cells that migrated had significantly higher expression of CD38, CD49d, HLA-DR, CD80, and CD86. In addition, following migration through endothelium, they also possessed a CXCR4dimCD5bright phenotype. Importantly, this CXCR4dimCD5bright phenotype was not seen in CLL cells that migrated in the absence of endothelial cells. We therefore hypothesized that CXCR4 and CD5 are modulated, at least in part, by endothelial cell contact, their secretion of CXCL12, and the process of TEM. Although the data presented here does not completely validate the Calissano model, it does support the concept that there is a small and distinct population of CLL cells with a propensity to migrate in the peripheral circulation and these cells could well be the recent LN emigrants (supplemental Figure 3).
Importantly, we demonstrated a strong positive correlation between the expression of CD49d and that of CD5, HLA-DR, CD80, and CD86, further supporting a link between migration and the potential for increased contact with, and activation of, T cells. These correlations were confirmed in matched LN-CLL and PB-CLL cells, adding weight to the argument that these phenotypes are physiologically relevant.
The limited numbers of cells that migrated in our in vitro model prevented functional assays from being performed, but because it is CLL cells with the highest expression of CD49d that migrate, we compared the T-cell stimulatory properties of CD49dhi with CD49dlo CLL cells derived from the same patient. These assays demonstrated that CD49dhi CLL cells induce superior T-cell activation. We have previously shown that blocking CD49d with natalizumab prevents CLL cell migration but, interestingly, here we have established that CD49d itself is not directly responsible for T-cell activation. This supports the hypothesis that the poor prognosis associated with CD49d expression in CLL is predominately caused by its ability to modulate tumor cell migration rather than directly induce T-cell activation.
CLL is a disease characterized by immune suppression that is exemplified by poor responses to vaccination. However, a number of studies have shown that this suppression is not irreversible35,36 ; PD-1 is not always a marker of terminal exhaustion and immune responses can be re-invigorated by antibody blockade.37 Previous contradictory literature indicated that T-cell activation in CLL is in equilibrium between pro- and anti-activation signals, but here we show that there appears to be a balance shift toward pro-activation in the LNs. In support of this, Herishanu et al11 demonstrated that LN-CLL cells had the signature of BCR activation and Buhmann et al27 showed that CD40L expression by CLL cells upregulated T-cell stimulatory activity.
Our results add to this by suggesting a role for TEM in the T-cell activation capabilities of the LN-CLL cells. Previously, we have shown that the interaction of CLL cells with endothelium in static culture activated NF-κB, resulting in enhanced transcription and protein expression of NF-κB–regulated genes such as CD38 and CD49d.9 In our novel circulation system, which more closely simulates the situation in vivo, a much larger effect was seen in migrated cells and because CD49d is also a co-stimulatory molecule,26 this further supports the link between migration and T-cell activation by CLL cells.
In conclusion, LN-CLL cells manifest a distinct phenotype to those in the PB, and demonstrate an enhanced capacity for T-cell activation and immunologic synapse formation. Data from our in vitro circulation model implies that there is a link between the process of migration, and these phenotypic and functional differences. Clearly, the microenvironment plays a vital role in the pathology of CLL, but it would appear that, within a patient, a subset of CLL cells with a distinct phenotype are inherently more capable of migrating and are primed for interaction with T cells. Although the striking reduction in tumor bulk observed with drugs like ibrutinib and idelalisib is only partially due to tissue redistribution,38,39 it seems likely that their clinical effect is, at least in part, elicited by inhibiting CLL-cell lymphoid tissue homing, which consequently prevents antigen presentation, T-cell activation, and tumor proliferation.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This study was funded in part by Bloodwise (M.P., E.W., and K.C.), the Kay Kendall Leukaemia Fund (E.C.), and the British Society of Haematology (B.A.).
Authorship
Contribution: A.G.S.B. designed the research, performed experiments, and co-wrote the paper; M.P., E.W., D.Y., E.C., B.A., K.C., P.P., E.P., Y.M., and M.S.L. performed practical work; L.D.B., C.F., and A.G.R. provided analytical tools; and C.P. and S.D. designed research and cowrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Andrea G. S. Buggins, King’s College London, Leukaemia Sciences, Rayne Institute, 123 Coldharbour Ln, London SE5 9RS, United Kingdom; e-mail: andrea.buggins@kcl.ac.uk.
References
Author notes
M.P., E.W., C.P., S.D., and A.G.S.B. contributed equally to the study.