Key Points
Most published SNP associations with chronic GVHD are likely to represent false-positive findings.
HRs for any true-positive SNP associations are likely to be much smaller than reported previously.
Abstract
Previous studies have identified single-nucleotide polymorphisms (SNPs) associated with the risk of chronic graft-versus-host disease (GVHD) after allogeneic hematopoietic cell transplantation. The current study determined whether these associations could be replicated in large cohorts of donors and recipients. Each SNP was tested with cohorts of patients having the same donor type (HLA-matched related, unrelated, or both) reported in the original publication, and testing was limited to the same genome (recipient or donor) and genetic model (dominant, recessive, or allelic) reported in the original study. The 21 SNPs reported in this study represent 19 genes, and the analysis encompassed 22 SNP association tests. The hazard ratio (HR) point estimates and risk ratio point estimates corresponding to odds ratios in previous studies consistently fall outside the 95% confidence intervals of HR estimates in the current study. Despite the large size of the cohorts available for the current study, the 95% confidence intervals for most HRs did not exclude 1.0. Three SNPs representing CTLA4, HPSE, and IL1R1 showed evidence of association with the risk of chronic GVHD in unrelated donor-recipient pairs from 1 cohort, but none of these associations was replicated when tested in unrelated donor-recipient pairs from an independent cohort. Two SNPs representing CCR6 and FGFR1OP showed possible associations with the risk of chronic GVHD in related donor-recipient pairs but not in unrelated donor-recipient pairs. These results remain to be tested for replication in other cohorts of related donor-recipient pairs.
Introduction
Previous studies have identified genetic variants such as single-nucleotide polymorphisms (SNPs) and other polymorphisms that influence the risk of chronic graft-versus-host disease (GVHD) after allogeneic hematopoietic cell transplantation (HCT).1-19 Many of the reported variants regulate the function of immune cells, their receptors, effector molecules, cytokines, or chemokines. Some results have suggested that the assessment of these variants before HCT might help to assess the risk of adverse outcomes for each patient, guide the clinical management of patients who are at high risk, and ultimately serve as potential biologic targets for novel therapeutics.
Previous case-control or cohort studies of SNP associations with chronic GVHD evaluated 50 to 353 individuals. No previous study has comprehensively evaluated the association of these variants in the same cohort simultaneously. In this study, we used genotyped or imputed SNP data to determine how many of the previously published associations we could replicate with substantially larger numbers of individuals from 2 cohorts.
Methods
Literature search
We performed a comprehensive PubMed search using the terms “chronic GVHD” and “polymorphism” to identify all studies published by August 2014 reporting an association of a genetic polymorphism with the odds ratio (OR) or relative risk of chronic GVHD at an α level <0.05. Studies that did not meet this threshold were not included. Reported associations of genetic deletions, insertions, microsatellites, or variable number tandem repeats with chronic GVHD were excluded because the genotyping arrays used for our study do not detect these variants.
Study cohort: FHCRC
Description.
The Fred Hutchinson Cancer Research Center (FHCRC) cohort included 3918 donor-recipient pairs of European ancestry who received allogeneic HCT from 1990 through 2011 (Table 1). European ancestry was determined by analysis of principal components. For purposes of this study, the numbers of available non-European pairs in this cohort were not sufficient for meaningful analysis. The cohort included recipients with either HLA-matched related donors (MRDs) (N = 1819) or unrelated donors (URDs) (N = 2019). The number of recipients with HLA-mismatched related donors in our cohort is not sufficient for meaningful stratified analysis. Indications for HCT included hematologic malignancy or myelodysplasia. Patients treated with either myeloablative or nonmyeloablative conditioning regimens were included in the analysis, whereas patients who received T cell–depleted grafts or were treated with rabbit antithymocyte globulin as part of the conditioning regimen were excluded from the analysis, because these interventions decrease the risk of chronic GVHD.
Baseline recipient and donor information was collected during the evaluation before HCT. URD and recipient matching for HLA-A, HLA-B, and HLA-C; DRB1; and DQB1 was confirmed by high-resolution methods in most cases. Grade 2-4 acute GVHD was diagnosed according to previously described criteria.20 Information regarding chronic GVHD and follow-up outcomes was captured prospectively by the Long-Term Follow-Up program through medical records from our outpatient clinic and from referring physicians who provided the primary care for patients. Clinical extensive chronic GVHD was diagnosed by historical criteria.21 Patients with clinical limited chronic GVHD were not considered as having chronic GVHD unless and until they developed clinical extensive disease. By National Institutes of Health (NIH) consensus criteria, this definition used in this study includes classical and overlap chronic GVHD and late acute GVHD. In the FHCRC cohort, the cumulative incidence of chronic GVHD at 2 years after HCT was 44% (95% confidence interval [CI], 42-47) among patients with HLA-MRDs and 48% (95% CI, 45-50) among patients with URDs. Methods for collection of biospecimens, genotyping, and imputation for the FHCRC cohort are summarized in the supplemental Appendix, available on the Blood Web site.
Statistical analysis of results in the FHCRC cohort.
For purposes of replicating previous results, each SNP was tested with subsets of the FHCRC cohort of patients having the same donor type (MRD, URD, or both) reported in the original publication; initial testing was limited to the same genome (recipient or donor) reported in the original study using genotypes only from the microarray platforms that passed quality control for each SNP (supplemental Table 1). The primary analysis used Cox regression models with no adjustments, treating death and recurrent malignancy as competing risks. A secondary analysis used multivariate Cox regression models with additional covariates for baseline clinical risk factors that have strong effect on the risk of chronic GVHD (HLA-matched URD vs HLA-mismatched URD vs HLA-MRD; female donor for male recipient vs other combinations; mobilized blood cell graft-versus-marrow graft; diagnosis of chronic myeloid leukemia vs other diseases; conditioning with total body irradiation vs without; and patient age).22 HLA-matching of URDs was coded according to categories shown in Table 1. The secondary analysis also included the first 4 principal components to control for population stratification.23
Each SNP was evaluated for allelic or genotypic (recessive and dominant) association as reported in the original study. For a SNP with a major allele “A” and a minor allele “a,” the recessive model tests the hypothesis that the genotype “aa” is associated with a higher or lower risk compared with the collective genotypes “AA” and “Aa” used as the reference. The dominant model tests the hypothesis that the collective genotypes “Aa” and “aa” are associated with a higher or lower risk compared with the genotype “AA” used as the reference. The allelic model tests the hypothesis that the minor allele “a” is associated with a higher or lower risk compared with the major allele “A,” and the number of copies of the minor allele is modeled as an additive effect. Because the main goal of this analysis was to replicate previously reported associations, a 2-sided P ≤ .05 was selected as the threshold of significance, despite the multiple comparisons.
To compare results of the current study with those of previous case-control studies, ORs were converted to the corresponding risk ratios after accounting for the minor allele frequency and genetic model, with the incidence of chronic GVHD set at 45%. Where necessary, risk alleles, genetic models, and ORs reported in previous studies were inverted to match the analysis in the current study. With these adjustments, the risk ratio from a previous case-control study is used to approximate the corresponding hazard ratio (HR) that might be expected in a cohort study.
Study cohort: DISCOVeRY-BMT
Description.
Recipients and donors in the Determining the Influence of Susceptibility Conveying Variants Related to One Year mortality after Unrelated Donor Allogeneic Blood or Marrow Transplant (DISCOVeRY-BMT) cohort originated from the Center for International Blood and Marrow Transplant Research (CIBMTR) and were selected for the DISCOVeRY-BMT Genome-wide Association Study.24,25 CIBMTR is collaborative research program organized by the National Marrow Donor Program/Be The Match Registry and the Medical College of Wisconsin and collects data from a voluntary working group of more than 450 transplant centers throughout the world. Participating centers contribute comprehensive baseline and longitudinal follow-up data as well as pretransplant biospecimens.
DISCOVeRY-BMT cohort 1 included 2609 patients who received a first HLA-A, HLA-B, and HLA-C, DRB1, DQB1-matched unrelated HCT transplant for treatment of acute lymphoblastic leukemia, acute myeloid leukemia, or myelodysplastic syndrome between 2000 and 2008. Patients were excluded if they had received T cell–depleted grafts or cord blood grafts, or if biorepository samples were not available from both the donor and recipient. DISCOVeRY-BMT cohort 2 included 572 patients who were selected according to the same criteria but had HCT between 2009 and 2011, together with 351 patients who received a first HLA-A, HLA-B, and HLA-C, DRB1-matched unrelated HCT for the same indications between 2000 and 2011 without assessment of HLA-DQB1 matching. For the purposes of this replication analysis, we included only individuals of European ancestry and excluded those who were treated with antithymocyte globulin as part of the conditioning regimen, yielding 1656 and 527 recipients and 1601 and 514 donors in cohorts 1 and 2, respectively. The cumulative incidence of chronic GVHD at 2 years after HCT was 43% (95% CI, 41-45) in the combined cohorts. Methods for collection of biospecimens, genotyping, and imputation for the DISCOVeRY-BMT cohort are summarized in the supplemental Appendix.
Statistical analysis of results in the DISCOVeRY-BMT cohort.
Cox proportional hazards models evaluated time to extensive chronic GVHD. Deaths from any cause and progression or recurrence of malignant disease were treated as competing risks. Multivariate models were unadjusted, including only the SNP of interest and then adjusted for the following covariates: donor sex mismatch (female donor to male recipient or not), graft type (blood, marrow), total body irradiation exposure (>900 cGy, ≤900 cGy, or none), and recipient age (continuous). Dosage data accounting for the probability of each genotype were used in all analyses of imputed data. To combine data from DISCOVeRY-BMT cohorts 1 and 2, the inverse variance weighting method was used as implemented in the R package METAL. For adjusted analyses, we report the METAL HRs, CIs, and P values under a fixed effects model because heterogeneity between cohorts was low (0 ≤ I2 < 25).26
Results
Testing of SNPs in the FHCRC cohort
The primary goal of this study was to determine whether we could replicate previously reported SNP associations with risk of chronic GVHD (Table 2). Previous studies have identified 29 informative SNPs associated with the risk of chronic GVHD according to allelic, dominant, or recessive genetic models. Three of these could not be typed or imputed from results on any of the platforms used for our study. In the current study, results are reported for only 1 of the 4 TNFSF13B SNPs reported by Clark et al5 because they have nearly perfect linkage disequilibrium, and results did not differ among them. Likewise, the IL10 SNPs rs1800872 and rs1800871 are in strong linkage disequilibrium. In the current study, results are reported only for rs1800871.
The 21 SNPs reported in this study represent 19 genes. Seven were donor SNPs representing 6 genes, and 13 were recipient SNPs representing 11 genes. The TNFA SNP is located in the major histocompatibility complex and therefore represents both the donor and recipient in HLA-identical related pairs. The analysis encompassed 22 SNP association tests because previous studies of rs1800896 showed both dominant and recessive associations.11,12
In an unadjusted analysis, results of this screen replicated results for 3 of the 22 SNP associations (Table 3). Related donor rs3093023 (CCR6) and rs2301436 (FGFR1OP) genotypes showed statistically significant dominant genetic associations with the risk of chronic GVHD (HR, 1.19; 95% CI, 1.02-1.38; P = .02; and HR, 1.23; 95% CI, 1.06-1.44; P = .01, respectively). Results of the adjusted analysis were similar. Kochi et al27 previously identified rs968334 as a functional SNP regulating the expression of CCR6, and this SNP has strong linkage disequilibrium with rs3093023. Related donor rs968334 genotypes showed a statistically significant dominant genetic association with the risk of chronic GVHD (N = 1757; HR, 1.19; 95% CI, 1.03-1.39; P = .02). Results of the adjusted analysis were similar (HR, 1.16; 95% CI 0.99-1.35; P = .06).
For exploratory purposes, donor CCR6 rs3093023 and rs968334 genotypes and donor FGFR1OP rs2301436 genotypes were tested for dominant genetic associations with the risk of chronic GVHD in unrelated recipients (N = 2002, 1998, and 2011, respectively). Results showed no statistically significant association in the unadjusted analyses (HR, 1.07; 95% CI, 0.93-1.22; P = .36; HR, 1.07; 95% CI, 0.94-1.23; P = .30; and HR 1.04; 95% CI 0.90-1.19; P = .62, respectively). Results of the adjusted analyses were similar (data not shown). Tests for homogeneity showed no significant differences between the respective HRs for related and unrelated HCT.
URD rs3087243 (CTLA4) genotypes showed statistically significant recessive genetic association with the risk of chronic GVHD (HR, 0.84; 95% CI, 0.72-0.99; P = .04). Results of the adjusted analysis were similar. Related donor rs3087243 genotypes, however, did not show a statistically significant recessive genetic association (HR, 0.95; 95% CI, 0.80-1.13; P = .57). A test for homogeneity showed no significant difference between the respective HRs for related and unrelated HCT.
Previous studies evaluated 6 SNP associations with chronic GVHD in a combined cohort of related and unrelated graft recipients. Because the balance between related and unrelated grafts varied considerably in these studies, we tested these SNPs with stratification for type of graft (supplemental Table 3). Unrelated patient HSPE rs4693608 genotypes showed a statistically significant dominant genetic association with risk of chronic GVHD (HR, 1.26; 95% CI, 1.06-1.51; P = .01). Results of the adjusted analysis were similar. Related patient HSPE rs4693608 genotypes, however, did not show an association with the risk of chronic GVHD (HR, 0.98; 95% CI, 0.81-1.19; P = .86), and a test for homogeneity suggested a significant difference between the respective HRs for related and unrelated HCT (P = .05). URD IL1R1 rs3917225 genotypes showed a statistically significant dominant genetic association with the risk of chronic GVHD (HR, 1.17; 95% CI, 1.02-1.35; P = .03). Results of the adjusted analysis were similar. Related donor IL1R1 rs3917225 genotypes, however, did not show an association with the risk of chronic GVHD (HR, 0.96; 95% CI, 0.82-1.11; P = .55). The other 4 SNPs previously associated with chronic GVHD in mixed related and unrelated HCT cohorts showed no statistically significant associations with chronic GVHD in separate analyses of related and unrelated HCT (supplemental Table 3).
Comparison of current FHCRC results with previously published results
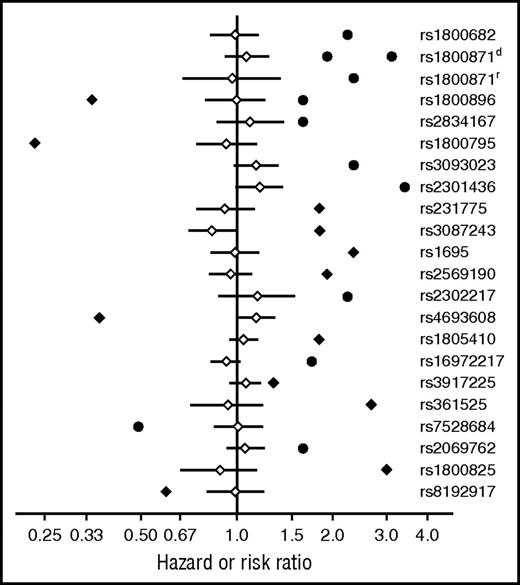
As shown in Figure 1, the HR point estimates and risk ratio point estimates corresponding to ORs in previous studies consistently fall outside the 95% CIs for HRs in the current study of the FHCRC cohort. HRs for SNPs showing a possible association with chronic GVHD in the current study are much closer to 1.0 than those in previous studies.
HR point estimates and risk ratio point estimates corresponding to ORs in previous studies consistently fall outside the 95% CIs of HR estimates in the current study. Adjusted HR point estimates (♢) and 95% CIs (―) from the FHCRC cohort are shown together with HR point estimates (♦) or risk ratio estimates corresponding to ORs (●) in previous studies. Results for rs1800871d and rs1800871r, respectively, represent dominant and recessive genetic models.
HR point estimates and risk ratio point estimates corresponding to ORs in previous studies consistently fall outside the 95% CIs of HR estimates in the current study. Adjusted HR point estimates (♢) and 95% CIs (―) from the FHCRC cohort are shown together with HR point estimates (♦) or risk ratio estimates corresponding to ORs (●) in previous studies. Results for rs1800871d and rs1800871r, respectively, represent dominant and recessive genetic models.
Further testing of selected SNPs in the DISCOVeRY-BMT cohorts
In the FHCRC cohort, SNPs representing CTLA4, HPSE, and IL1R1 showed evidence of association in URD HCT but not in MRD HCT, whereas SNPs representing CCR6 and FGFR1OP showed evidence of association with the risk of chronic GVHD in MRD HCT but not in URD HCT. These 5 SNPs were tested for association with chronic GVHD in a cohort of 2183 URD-recipient pairs from the combined DISCOVeRY-BMT cohorts, according to the same recipient or donor genome and dominant, recessive, or allelic genetic model reported in the original study. No statistically significant associations were observed in this analysis (supplemental Table 4). No large cohort of related donor-recipient pairs with genotyping data is available for replication of current results from testing SNPs representing CCR6 and FGFR1OP in the FHCRC cohort.
Global survey of candidate SNPs
We also screened the set of 21 SNPs for association with chronic GVHD in both the entire FHCRC cohort and in the related and unrelated subsets, testing donor and recipient genotypes with allelic, dominant, and recessive models, using the first 4 principal components as covariates. For this purpose, we set a threshold of interest at P < .001 to account for multiple comparisons. No SNP association met this level of statistical significance.
Discussion
Only 2 of the candidate SNPs tested in this study (CCR6, FGFR1OP) remain as possibly associated with the risk of chronic GVHD. These associations were replicated in related donor-recipient pairs but not in URD-recipient pairs from the FHCRC, and the results have yet to be tested in other cohorts of related donor-recipient pairs. An overriding effect of HLA-DP mismatching or more extensive mismatching for minor antigens could explain why genetic associations observed with MRD in our study could not be replicated with URD, but such a mechanism could not explain the similarly frequent cases where genetic associations observed in URD (eg, CTLA4, HPSE, ILR1) were not observed with MRD. Because we have no demonstrable biological explanation for the lack of correspondence between results with MRD and URD, the evidence for a true association of these SNPs with chronic GVHD remains indeterminate. The HRs for all tested associations were much closer to 1.0 than reported in previous studies. Similar reductions in HRs or ORs toward 1.0 across several studies have been observed in the analysis of SNP associations with other diseases and may have a variety of explanations.28-30 A global survey of candidate SNPs did not yield any new findings that would meet the threshold of statistical significance adjusted for multiple comparisons.
The inability to replicate previous results in the current study is reminiscent of a previous study that replicated only 1 of 16 SNPs previously reported to be associated with acute GVHD.31 At least 2 explanations should be considered in accounting for our inability to replicate previous results in the current study. Four SNPs, respectively representing FCRL3, IL2, CCL5, and GZMB, were previously tested in Asian cohorts only. Population stratification could account for the lack of association of these SNPs with chronic GVHD in the FHCRC European ancestry cohort. The T allele of rs1800871 in IL10, however, was previously associated with an increased risk of chronic GVHD both in a European ancestry cohort11 and in an Asian cohort.8 Therefore, population stratification cannot explain why this SNP was not associated with the risk of chronic GVHD in the current study.
As a second explanation, it is possible that unknown heterogeneity in the pathogenesis of chronic GVHD and stratification of genetic risk factors might mask associations that would be apparent in specific subsets of patients.32 Although our conclusions are valid for the FHCRC and DISCOVeRY-BMT cohorts, they might not reflect results in cohorts of patients selected or treated in ways that differ from our cohort. Finally, changes in the criteria for the diagnosis or lack of precision in making the diagnosis of chronic GVHD during the past decade could have contributed to our inability to replicate previous results.33 Criteria for the diagnosis of chronic GVHD were not defined in 4 of the previous studies.1-3,15 Two studies used historical criteria for the diagnosis of extensive chronic GVHD,9,18 and only 1 used the 2004 NIH criteria for chronic GVHD.5 The remaining studies used historical or modified criteria for the diagnosis of limited or extensive chronic GVHD.21 In the current study, we used historical or modified criteria for the diagnosis of extensive chronic GVHD, which include classical and overlap chronic GVHD together with late acute GVHD according to NIH criteria. The cumulative incidence frequencies of chronic GVHD were closely similar between the FHCRC and DISCOVeRY-BMT cohorts. Criteria for the diagnosis in the current study did not include patients with limited chronic GVHD. In the FHCRC cohort, however, <5% of the patients had limited chronic GVHD that never met criteria for extensive chronic GVHD. Therefore, it is unlikely that the use of clinical extensive chronic GVHD as the diagnostic criterion accounts for our inability to replicate previous results.
Previous studies evaluated between 50 and 353 individuals, whereas 18 of the 22 SNPs evaluated in the FHCRC cohort were tested in 1200 to 3737 individuals, and the 5 SNPs evaluated in the DISCOVeRY-BMT cohorts were tested in 2115 to 2183 individuals. HRs with nominal statistical significance (P < .05) in the FHCRC cohort were in the range from 1.15 to 1.25. These HRs are consistent with those reported in other studies of immune-mediated diseases.34-37 Point estimates for hazard and risk ratios from previous studies consistently fell outside the 95% CIs for HR estimates in the current study, demonstrating that the inability to replicate previous results in the current study cannot be explained by insufficient statistical power.
We were able to test SNPs representing CTLA4, HPSE, and IL1R1 for association with chronic GVHD in a large cohort of unrelated pairs from the DISCOVeRY-BMT cohorts. In this cohort, any GVHD persisting after day 100 is considered chronic GVHD, similar to the definition used for the FHCRC cohort in this study. The results did not support any association of these SNPs with the risk of chronic GVHD. In the current study, the minor allele frequencies of SNPs representing CCR6 and FGFR10P spanned a narrow and highly favorable range from 0.42 to 0.47. If the incidence of chronic GVHD incidence is 0.45, a cohort of 1000 to 2500 patients would be needed to provide at least 80% power to verify HRs within the 1.15 to 1.23 range observed for these SNPs in our study. We conclude that a definitive analysis of the chronic GVHD associations of the CCR6 and FGFR1OP SNPs reported here will depend on further studies in sufficiently powered large cohorts with chronic GVHD diagnosed by criteria similar to those used in the current study.
Despite our best efforts, we were unable to replicate the results of previous studies and, as such, our results offer no support for biologically plausible inferences resulting from those studies. In the absence of an obvious explanation for the inability to replicate previous results, we would conclude that most, but not necessarily all, of the previous results were false positives. We acknowledge that our study could have false-negative results, but, if so, it is likely that many true effects are too small to be detected, despite the large size of our cohort.
Our results have important implications for future genetic association studies of chronic GVHD. The current study used the historical definition of chronic GVHD to match the diagnostic criteria used in most previous studies. Future studies could benefit by using the more refined criteria for diagnosing chronic GVHD developed by the NIH Consensus Development Project. Previous studies evaluated associations for only a small numbers of SNPs. Future studies would benefit from expanding the number of SNPs to be evaluated. Genomewide surveys offer broad opportunity for discovery but incur a very heavy statistical penalty for multiple comparisons. A curated set of candidate SNPs that are known to alter immune function could be used to elucidate specific biological mechanisms, as suggested by a recent study of SNPs associated with sclerosis in patients with NIH-defined chronic GVHD.32 In designing such studies, careful consideration must be given to the number of SNPs and genetic models that can be tested while accounting appropriately for multiple comparisons, the minimum minor allele frequency, the anticipated effect size, the available numbers of patients and donors, and the need to replicate any new discoveries in an independent cohort.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Jenna Gravley for outstanding assistance in maintaining the biospecimen repository at the Fred Hutchinson Cancer Research Center (FHCRC) and verifying the identity of samples used in this study, and the FHCRC Genomics Shared Resource Laboratory (Jeff Delrow, Director) for the genotyping of FHCRC cohort II.
Work at the FHCRC was supported by the National Institutes of Health, National Heart, Lung, Blood Institute (NHLBI; grants R01-HL105914 and R01-HL87690) and the National Cancer Institute, Department of Health and Human Services (grant P30-CA015704). The DISCOVeRY-BMT study is funded by the National Institutes of Health, NHLBI (grant R01 HL102278). The RPCI Biostatistics and Bioinformatics Shared Resource is partially funded by the National Institutes of Health/National Cancer Institute (NCI) (grant P30CA016056). The Center for International Blood and Marrow Transplant Research is supported by the NCI, NHLBI, and the National Institute of Allergy and Infectious Diseases (Public Health Service grant/cooperative agreement 5U24-CA076518); the NHLBI and NCI (grant/cooperative agreement 5U10HL069294); the Health Resources and Services Administration (contract HHSH250201200016C); the Office of Naval Research (grants N00014-14-1-0028 and N00014-15-1-0848); grants from Alexion and Amgen, Inc.; an anonymous donation to the Medical College of Wisconsin; and by the Be the Match Foundation, Bristol Myers Squibb Oncology, Celgene Corporation, Chimerix, Inc., Fred Hutchinson Cancer Research Center, Gamida Cell Ltd, Genentech Inc, Genzyme Corporation, Gilead Sciences Inc, Health Research Inc, Roswell Park Cancer Institute, HistoGenetics Inc, Incyte Corporation, Jazz Pharmaceuticals Inc, Jeff Gordon Children’s Foundation, The Leukemia & Lymphoma Society, The Medical College of Wisconsin, Merck & Co Inc, Mesoblast, Millennium, The Takeda Oncology Co, Miltenyi Biotec Inc, National Marrow Donor Program, Neovii Biotech NA Inc, Novartis Pharmaceuticals Corporation, Onyx Pharmaceuticals, Optum Healthcare Solutions Inc, Otsuka America Pharmaceutical Inc, Otsuka Pharmaceutical Co Ltd–Japan, Oxford Immunotec, Perkin Elmer Inc, Pharmacyclics, Sanofi US, Seattle Genetics, Sigma-τ Pharmaceuticals, Spectrum Pharmaceuticals Inc, St. Baldrick’s Foundation, Sunesis Pharmaceuticals Inc, Swedish Orphan Biovitrum Inc, Telomere Diagnostics Inc, TerumoBCT, Therakos Inc, University of Minnesota, and 7Wellpoint, Inc.
The views expressed in this article do not reflect the official policy or position of the National Institutes of Health, the Department of the Navy, the Department of Defense, Health Resources and Services Administration, or any other agency of the US Government.
Authorship
Contribution: P.J.M., J.A.H., L.P.Z., B.E.S., E.H.W., M.B., S.H., L.Y., L.S.-C., and T.H. designed the study. E.H.W., M.E.D.F., S.J.L., P.A.C., S.S., X.Z., and M.P. provided data and samples. W.F., L.P.Z., D.S., X.S., L. Pooler, and C.A.H. provided genotyping and imputation. W.F., D.M.L., L. Preus, X.Z., L.S.-C., and T.H. provided data quality control and coding. B.E.S., L.Y., Q.H., S.L., and L.S.-C. provided statistical analysis. P.J.M., J.A.H., L.Y., S.J.L., P.M., L.S.-C., and T.H. interpreted results. P.J.M., J.A.H., T.H., and L.S.-C. wrote the manuscript. Funding was awarded to J.A.H., P.J.M., B.E.S., L.S.-C., and T.H. All authors critically revised the manuscript for important intellectual content and approved the manuscript for publication.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Paul J. Martin, Fred Hutchinson Cancer Research Center, P.O. Box 19024, Seattle, WA, 98109-1024; e-mail: pmartin@fredhutch.org.