Key Points
Investigation of the iron-restrictive effect of minihepcidin peptides in the treatment of β-thalassemia and polycythemia vera.
Abstract
In β-thalassemia and polycythemia vera (PV), disordered erythropoiesis triggers severe pathophysiological manifestations. β-Thalassemia is characterized by ineffective erythropoiesis, reduced production of erythrocytes, anemia, and iron overload and PV by erythrocytosis and thrombosis. Minihepcidins are hepcidin agonists that have been previously shown to prevent iron overload in murine models of hemochromatosis and induce iron-restricted erythropoiesis at higher doses. Here, we show that in young Hbbth3/+ mice, which serve as a model of untransfused β-thalassemia, minihepcidin ameliorates ineffective erythropoiesis, anemia, and iron overload. In older mice with untransfused β-thalassemia, minihepcidin improves erythropoiesis and does not alter the beneficial effect of the iron chelator deferiprone on iron overload. In PV mice that express the orthologous JAK2 mutation causing human PV, administration of minihepcidin significantly reduces splenomegaly and normalizes hematocrit levels. These studies indicate that drug-like minihepcidins have a potential as future therapeutics for untransfused β-thalassemia and PV.
Introduction
In β-thalassemia, mutations in the β-globin gene lead to a relative excess of α-globin, which, together with heme, forms hemichromes, molecules with toxic potential to trigger reactive oxygen species (ROS), apoptosis of erythroid progenitors, and short-lived erythrocytes.1-3 This results in anemia, chronically high levels of erythropoietin, and ineffective erythropoiesis—overproduction of erythroid progenitors that fail to generate mature erythrocytes.4 Even when transfusions are not required, the manifestations of the disease can be severe, including chronic anemia and splenomegaly as well as pulmonary hypertension and an increased risk of thrombosis.4 Anemia and ineffective erythropoiesis also cause increased iron absorption mediated by duodenal hypoxia and erythroid-mediated suppression of the iron regulator hepcidin.5-10 The resulting iron overload adds to morbidity by causing cirrhosis, risk of cardiomyopathy, and hepatocarcinoma and exacerbating erythroid cell damage, apoptosis, and ineffective erythropoiesis.1 Even untransfused patients with β-thalassemia may require iron chelation to avoid the morbidity and mortality associated with severe iron overload.1 Using a mouse model of untransfused β-thalassemia (Hbbth3/+), we previously showed that anemia, ineffective erythropoiesis, and iron overload were ameliorated by increasing hepcidin and causing iron restriction, yielding diminished hemichrome formation and decreased erythroid cell damage.2,3 Another disease in which iron restriction is known to be beneficial is polycythemia vera (PV). PV is a myeloproliferative disorder triggered by activating mutations in JAK2, an important kinase in the principal signaling pathway of the erythropoietin (EPO) receptor.11-15 Most of the clinical manifestations of PV are caused by an increased erythrocyte count, leading to an increased risk of pulmonary hypertension and thrombosis. The mainstay of therapy for PV is therapeutic phlebotomy, the goal of which is to reduce the hematocrit (HCT) level to ≤45% to minimize the risk of thrombosis.16 Because phlebotomy does not influence the underlying defect in bone marrow (BM) red blood cell (RBC) production, the effect of phlebotomy is transient, until patients become iron deficient. As alternative approaches, evidence suggests that systemic iron deficiency or erythroid-targeted iron restriction could be effective in PV patients via inhibition of erythropoietin signaling downstream of JAK2.11-15 Minihepcidins are small engineered peptides that reproduce the iron-restrictive effects of the hormone hepcidin.17,18 In a mouse model of severe hemochromatosis (Hamp-KO), administration of a minihepcidin prevented iron overload and caused partial redistribution of iron from the liver to the spleen.17,18 Minihepcidins caused dose-dependent inhibition of intestinal iron absorption and, at higher doses, restricted the iron supply to erythropoiesis. Based on these observations, this study investigates whether minihepcidin ameliorates anemia and iron overload in β-thalassemic mice and prevents erythrocytosis in PV mice.
Methods
Animal models
All animals (C57BL/6 background) were bred at the mouse facility of Weill Cornell Medical College and Children’s Hospital of Philadelphia. The mice received ad libitum access to water and normal chow that contained 200 ppm iron. We used Hbbth3/+ mice (The Jackson Laboratory) as a model of β-thalassemia intermedia. An animal model of PV was obtained by crossing mice carrying a Jak2V617F conditional knockin allele with mice expressing Cre recombinase under the control of VAV regulatory elements (VAV-Cre).11 Jak2V617F/+ VAV-Cre double-transgenic mice developed a PV-like phenotype, which was transplantable, as previously demonstrated.11 To determine if treatment with minihepcidin could improve hypoxia in our mouse model of β-thalassemia, the hypoxia reporter mouse, oxygen-dependent degradation luciferase (ODD-Luc), was crossed with Hbbth3/+ and in vivo luminescence imaging was performed at the end point.5 All animals were treated with either vehicle control or minihepcidin (M004 or M009) by subcutaneous (SC) injections twice a week for the indicated duration. Hbbth3/+ animals were also exposed to the oral iron chelator deferiprone (DFP) alone or in combination with minihepcidin. Animals were sacrificed 3.5 days after the final dose for analyses. Blood samples were analyzed as previously described.3
Drug preparation
The minihepcidins M004 and M009 were prepared fresh for each administration. Each drug was formulated in ethanol 100%, SL-220 (SUNBRIGHT) and water at desired concentration. The ethanol concentration was 10% of the final volume injected. Minihepcidins were administrated twice weekly for 6 weeks, and data were collected 3.5 days after the last injection. The iron chelator deferiprone (Sigma-Aldrich) was added into the drinking water at a concentration of 1.25 mg/mL.
Measurement of tissue iron content and serum iron parameters
Serum parameters (iron, transferrin saturation [TSAT]) were measured using the Integra 800 Automated Clinical Analyzer (Roche Diagnostics) or the Iron/TIBC Reagent Set (BioPacific Diagnostic). Serum EPO was analyzed using the Mouse Erythropoietin Quantikine ELISA Kit (R&D Systems) following the manufacturer’s instructions. The level of serum hepcidin was determined using the Hepcidin-Murine-Compete kit (Intrinsic LifeSciences) following manufacturer’s instructions. Tissue iron content for Hbbth3/+ was assayed at the Diagnostic Center for Population and Animal Health at Michigan State University (Lansing, MI).
Fluorescence-activated cell sorter analysis
Erythropoiesis and ROS in BM and spleen cells were analyzed as already described.2 For all analyses, cells were sorted using a FACSCalibur instrument (BD Biosciences) and the results analyzed with FlowJo software (Tree Star).
Analyses of α- and β-globin chains on RBC membranes
To visualize membrane-bound globins (hemichrome), we used urea gel electrophoresis (TAU gel), which separates α- and β-globin subunits using denaturing conditions. The same number of erythrocytes was processed for each sample that was loaded onto the gel (150 × 106).2
Measurement of RBC lifespan
RBCs of Hbbth3/+ mice are characterized by shortened lifespan compared with wild-type (WT) mice. To evaluate whether treatment with minihepcidin could improve this feature, we performed lifespan analysis of peripheral RBCs using flow cytometry as previously described.3
Quantitative polymerase chain reaction
Total RNA from livers was purified using the PureLink RNA Mini Kit (Ambion, Life Technologies) and analyzed with the SuperScript III Platinum SYBR Green One-Step qRT-PCR Kit (Invitrogen, Life Technologies). Messenger RNA concentrations of the target genes were normalized by a housekeeping gene (GAPDH).
In vivo imaging
ODD-Luc-Hbbth3/+ mice were treated for 6 weeks with biweekly injections of M004 or vehicle. At the end point, in vivo imaging was performed. Animals received luciferin substrate by IV injection. Images were captured using the Xenogen IVIS Optical Imaging System (Xenogen). The system specifically and quantitatively images the expression of the reporter luciferase (Luc) gene in mice.
Erythroferrone quantification by ELISA
A sandwich enzyme-linked immunosorbent assay (ELISA) using mouse monoclonal antibodies against mouse erythroferrone (Erfe) was performed as described by Kautz et al.19
Statistics
Data are presented as mean ± standard deviation (SD). When multiple comparisons were needed, statistical analysis was performed using one-way analysis of variance (ANOVA) with Tukey multiple comparison adjustment. For comparisons between 2 groups, we used a 2-tailed Mann-Whitney U test. All data were analyzed using GraphPad Prism version 6 (Microsoft GraphPad Software, La Jolla, CA).
Study approval
All animal studies were conducted under protocols approved by the Institutional Animal Care and Use Committee of Weill Cornell Medical College and Children’s Hospital of Philadelphia.
Results
Pharmacokinetics of minihepcidin peptides
Studies were performed with 2 minihepcidin peptides, designated M004 and M009. M004 is a potent analog of the 9 N-terminal amino acids of hepcidin. M004 reduces cell-surface expression of ferroportin with a 50% effective concentration of 9.7 nM and causes >80% reduction in serum iron 24 hours after SC administration of a dose of 7.5 mg/kg in rats. M004 was discontinued in favor of M009, which was more stable in aqueous solution and therefore easier to administer. M009 is a prodrug that is converted to M004 following systemic administration. Chemically, M009 is the same as M004, except for the addition of an S-methyl group on Cys7, and because the iron-restrictive effect of M009 is determined by the generation of M004, we consider them to be interchangeable treatments. Administration of M009 at a dose of 7.5 mg/kg generates high levels of M004 and a >80% reduction in serum iron that was sustained for at least 48 hours (supplemental Figure 1, available on the Blood Web site).
Administration of minihepcidin ameliorated ineffective erythropoiesis, anemia, splenomegaly, and iron overload in young Hbbth3/+ mice
Two-to 3-month-old Hbbth3/+ mice are anemic and manifest organ iron overload but do not require RBCs transfusions.20-22 In order to test whether minihepcidin could improve both iron overload and anemia in young animals, M004 was administered to 2-month-old Hbbth3/+ male mice. Initial dose-finding studies were conducted using a daily intraperitoneal injection for 2 to 6 weeks of 0.25 to 5 mg/kg per day. In these studies, a dose-dependent decrease in TSAT was observed, consistent with the results observed in WT animals. The dose-dependent effect of minihepcidin-induced iron restriction on ineffective erythropoiesis in the Hbbth3/+ mouse was biphasic. At the low end of the tested range (0.25 to 1.25 mg/kg per day), we observed normalization of the ratio between mature and immature erythroid cells (CD44+-TER119+). This was associated with a 15% increase in RBC number and a marked reduction in reticulocyte count (62%) and spleen size (43%) when compared with Hbbth3/+ animals that received the vehicle (supplemental Figure 2A-B; supplemental Tables 1 and 2). In contrast, at doses of 2.5 to 5 mg/kg per day, severe iron restriction (as low as TSAT = 6%; supplemental Figure 2C and supplemental Table 3) was detected, leading to iron-restricted erythropoiesis and exacerbation of anemia (hemoglobin [Hb] reduction as low as 2.6 g/dL) (supplemental Figure 2D; supplemental Table 4). At these doses, accumulation of immature erythroid precursors was observed by flow cytometry studies, reflecting the expected erythroid maturation block caused by severe iron restriction of erythropoiesis (supplemental Figure 2E, population 1). Prussian blue staining demonstrated the increased sequestration of iron in splenic macrophages and Kupffer cells (supplemental Figure 2F) as expected after the administration of a hepcidin mimetic. The biphasic effect of minihepcidin can be attributed to the fact that at very high doses, the salutary effect of minihepcidin on thalassemic erythropoiesis is outweighed by the inhibitory effect of very severe iron restriction.
Pharmacokinetic data (supplemental Figure 1) indicated that the circulating concentration of active minihepcidin peptides remained high 24 hours after administration and that daily administration would result in accumulation of peptide, potentially leading to severe iron restriction. We therefore explored whether less frequent dosing could achieve sustained improvement in anemia and normalization of tissue iron with diminished risk of iron-restricted erythropoiesis. The dose of 2.625 mg/kg administered subcutaneously twice weekly was selected to generate blood concentrations similar to daily dosing of 0.63 to 1.25 mg/kg per day. The longer dose interval in these studies was chosen to permit the iron-restrictive effect of each dose to dissipate before the next injection and thus avoid severely iron-restricted erythropoiesis.
Two-month-old Hbbth3/+ males were treated for 6 weeks, twice weekly, with M004 or vehicle control by SC injections. Administration of M004 was associated with an increase in RBCs to levels observed in WT animals (Figure 1A) and an increase in Hb concentration of >2 g/dL (Figure 1B). Reduced spleen weight (−52%; Figure 1C) and reticulocyte count (−60%; Figure 1D) reflected improved erythropoietic efficiency, which was further corroborated by flow cytometry studies. Minihepcidin treatment reduced hemichrome and ROS formation (Figure 1E-F) and improved RBC morphology and lifespan (Figure 1G-H). Hematological improvement was likely caused by enhanced erythroid cell survival resulting from decreased oxidative damage. Total organ iron accumulation was decreased by 50% in the liver and 46% in the kidney (Figure 1I-J). Splenic iron concentration increased by 24%, reflecting retention of iron in splenic macrophages as a result of increased hepcidin activity (Figure 1K). However, total iron levels in the spleen were decreased by 42% (Figure 1L).
The iron-restrictive effect of minihepcidin peptide M004 improved ineffective erythropoiesis in a mouse model of thalassemia intermedia. Administration of M004 (2.625 mg/kg) in 2-month-old Hbbth3/+ animals twice a week for 6 weeks resulted in increased RBC count (A), improved anemia (as shown by increased Hb levels) (B), reduced splenomegaly (C), and reticulocyte count (D). Hemichrome formation was markedly reduced, as indicated by the reduced intensity of the α precipitates in animals treated with minihepcidin compared with control group (TAU gel) (E). ROS formation was also significantly reduced (F). To measure ROS levels, the erythroid compartment was at first analyzed by staining BM and spleen cells with CD44 and Ter119 antibodies that allowed the separation of erythroid cells into 5 distinct populations. The cells were then stained for ROS detection using the indicator CM-H2DCFDA. Here, we show a representative example of the ROS-positive cells (for control and treated groups) in the fraction that represents mature RBCs (F). RBC morphology (shown by Giemsa staining of peripheral blood smear) was markedly improved by M004 administration (G) and lifespan normalized (H). As a result of decreased erythroid iron uptake, total organ iron content was significantly reduced in liver (I) and kidney (J). Perls’ Prussian blue staining on liver, spleen, and kidney sections of animals receiving vehicle or M004 shows that parenchymal iron is reduced (liver, kidney) while retention of iron in the splenic macrophage is increased (K). Images were captured using a Leica DM4000B upright scope paired with a Spot RT/SE Slider camera (10×/numerical aperture 0.40 objective) and then acquired using the Spot 5.1 software. Perls’ Prussian blue staining in spleen sections revealed increased iron retention, but when organ weight was taken in consideration, the spleen iron concentration was statistically reduced in M004-treated vs vehicle-treated animals (L). Results are presented as mean ± SD; ***P < .001, **P < .01, and *P < .05, 2-tailed Mann-Whitney U test. WT data are displayed as a reference guide but are not included in the between-group test comparisons. st, standard.
The iron-restrictive effect of minihepcidin peptide M004 improved ineffective erythropoiesis in a mouse model of thalassemia intermedia. Administration of M004 (2.625 mg/kg) in 2-month-old Hbbth3/+ animals twice a week for 6 weeks resulted in increased RBC count (A), improved anemia (as shown by increased Hb levels) (B), reduced splenomegaly (C), and reticulocyte count (D). Hemichrome formation was markedly reduced, as indicated by the reduced intensity of the α precipitates in animals treated with minihepcidin compared with control group (TAU gel) (E). ROS formation was also significantly reduced (F). To measure ROS levels, the erythroid compartment was at first analyzed by staining BM and spleen cells with CD44 and Ter119 antibodies that allowed the separation of erythroid cells into 5 distinct populations. The cells were then stained for ROS detection using the indicator CM-H2DCFDA. Here, we show a representative example of the ROS-positive cells (for control and treated groups) in the fraction that represents mature RBCs (F). RBC morphology (shown by Giemsa staining of peripheral blood smear) was markedly improved by M004 administration (G) and lifespan normalized (H). As a result of decreased erythroid iron uptake, total organ iron content was significantly reduced in liver (I) and kidney (J). Perls’ Prussian blue staining on liver, spleen, and kidney sections of animals receiving vehicle or M004 shows that parenchymal iron is reduced (liver, kidney) while retention of iron in the splenic macrophage is increased (K). Images were captured using a Leica DM4000B upright scope paired with a Spot RT/SE Slider camera (10×/numerical aperture 0.40 objective) and then acquired using the Spot 5.1 software. Perls’ Prussian blue staining in spleen sections revealed increased iron retention, but when organ weight was taken in consideration, the spleen iron concentration was statistically reduced in M004-treated vs vehicle-treated animals (L). Results are presented as mean ± SD; ***P < .001, **P < .01, and *P < .05, 2-tailed Mann-Whitney U test. WT data are displayed as a reference guide but are not included in the between-group test comparisons. st, standard.
Minihepcidin ameliorated hypoxia in Hbbth3/+ mice
Hbbth3/+ mice were crossed with the hypoxia reporter mouse, ODD-Luc.5 In vivo luminescence imaging of these animals showed that mice affected by β-thalassemia are systemically hypoxic as a result of inadequate tissue oxygenation (hypoxia).5 ODD-Luc-Hbbth3/+ mice exhibit increased luminescence (indication of increased hypoxia) mostly in the abdomen and peripheral areas (such as the paws and tails) when compared with nonthalassemic mice.
We postulated that minihepcidin administration could reduce tissue hypoxia by improving tissue oxygenation as a result of increased Hb levels. ODD-Luc-Hbbth3/+ mice were treated for 6 weeks with 2.625 mg/kg M004 using biweekly SC injections. As shown previously, thalassemic mice treated with minihepcidin exhibit increased erythrocyte number and Hb concentration (Figure 2A-B), decreased reticulocytosis, and reduced splenomegaly (Figure 2C-D). Ineffective erythropoiesis was also ameliorated, as shown by flow cytometry analysis (Figure 2E). At the end point, the animals received IV injection of a luciferin substrate followed by in vivo imaging. Analysis of the results revealed a significant reduction in luminescence mostly in the abdomen of the mice that received minihepcidin compared with control mice (Figure 2F), indicating reduced hypoxia due to improved tissue oxygenation.
Minihepcidin peptide ameliorated hypoxia in animals affected by non–transfusion-dependent thalassemia.Hbbth3/+ mice were crossed with the hypoxia reporter mouse ODD-Luc and treated with M004 (2.625 mg/kg) twice a week for 6 weeks. As expected, minihepcidin administration increased RBC numbers (A) and Hb level (B) and reduced reticulocyte count (C) and splenomegaly (D). Improved erythropoietic efficiency was confirmed by flow cytometry analysis of erythroid precursors (as shown in the CD44-FSC [forward scatter] plots) (E). Reduction in hypoxia was evaluated by in vivo imaging. Reduction in luminescence was detected in the abdomen of animals receiving minihepcidin compared with the vehicle-treated one, indicating reduction in hypoxia (F). Results are presented as mean ± SD; *P < .05, 2-tailed Mann-Whitney U test. WT data are displayed as a reference guide but are not included in the between-group test comparisons.
Minihepcidin peptide ameliorated hypoxia in animals affected by non–transfusion-dependent thalassemia.Hbbth3/+ mice were crossed with the hypoxia reporter mouse ODD-Luc and treated with M004 (2.625 mg/kg) twice a week for 6 weeks. As expected, minihepcidin administration increased RBC numbers (A) and Hb level (B) and reduced reticulocyte count (C) and splenomegaly (D). Improved erythropoietic efficiency was confirmed by flow cytometry analysis of erythroid precursors (as shown in the CD44-FSC [forward scatter] plots) (E). Reduction in hypoxia was evaluated by in vivo imaging. Reduction in luminescence was detected in the abdomen of animals receiving minihepcidin compared with the vehicle-treated one, indicating reduction in hypoxia (F). Results are presented as mean ± SD; *P < .05, 2-tailed Mann-Whitney U test. WT data are displayed as a reference guide but are not included in the between-group test comparisons.
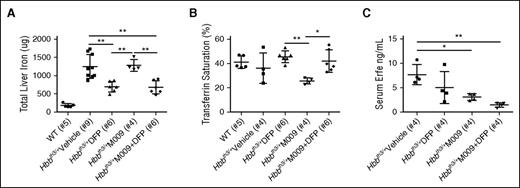
Beneficial effects on iron overload and erythropoiesis in old Hbbth3/+ mice after combination therapy of M009 and the iron chelator deferiprone
In clinical settings, the treatment of β-thalassemia in patients with severe iron overload would likely employ a combination of minihepcidin with oral chelation therapy. We therefore conducted studies in 4- to 5-month-old male Hbbth3/+ mice with established iron overload to evaluate whether the concurrent use of the oral iron chelator DFP and minihepcidin would cause any therapeutic interactions. M009, which generates M004 in circulation, was administered subcutaneously at the same dose used in previous studies with M004 (2.625 mg/kg). We first analyzed the effect of a single injection of M009. This was sufficient to reduce serum hepcidin up to 36 hours (supplemental Figure 3A), but it had no effect on serum Erfe concentrations (not shown). In this setting, the endogenous hepcidin concentration reached baseline levels at 48 to 72 hours.
M009 or vehicle control was then administered by SC injections biweekly for 6 weeks. Mice were stratified to receive free access to regular water or water containing DFP (1.25 mg/mL). At end-point analysis, iron-overloaded animals treated with DFP alone showed significantly decreased liver iron content (Figure 3A). This was accompanied by a trend toward increased TSAT (Figure 3B) and serum iron (supplemental Figure 3B), suggesting that DFP by itself does not restrict iron delivery to the erythron. The combination of DFP and M009 produced the same reduction in liver iron content as seen with DFP alone. Levels of serum iron (supplemental Figure 3B) and TSAT (Figure 3B) in animals that received M009 alone were reduced compared with the DFP-treated animals and controls, but no differences were detected when the 2 drugs were administrated simultaneously. No differences in serum hepcidin concentrations (supplemental Figure 3C), hepcidin messenger RNA expression (not shown), or expression of other iron-related genes (supplemental Figure 3D-F) were observed between groups. However, ELISA assays of serum Erfe indicated a significant reduction of this factor (Figure 3C).
Minihepcidin M009 did not impair the ability of DFP to reduce iron overload in a mouse model of non–transfusion-dependent thalassemia. DFP alone (added into the drinking water at concentration of 1.25 mg/mL) caused a significant reduction in liver iron content, whereas the combination of DFP with minihepcidin M009 (used at a dose of 2.625 mg/kg) did not impair the ability of DFP to reduce organ iron content when compared with vehicle-treated animals (A). Animals treated with DFP showed a trend toward increased TSAT that was instead decreased in animals treated with M009 (B). ELISA assay showed reduction of serum Erfe levels (C). Results represent mean ± SD; **P < .01, *P < .05. Analysis was performed using one-way ANOVA with Tukey multiple comparison adjustment. WT data are displayed as a reference guide but are not included in the between-group test comparisons.
Minihepcidin M009 did not impair the ability of DFP to reduce iron overload in a mouse model of non–transfusion-dependent thalassemia. DFP alone (added into the drinking water at concentration of 1.25 mg/mL) caused a significant reduction in liver iron content, whereas the combination of DFP with minihepcidin M009 (used at a dose of 2.625 mg/kg) did not impair the ability of DFP to reduce organ iron content when compared with vehicle-treated animals (A). Animals treated with DFP showed a trend toward increased TSAT that was instead decreased in animals treated with M009 (B). ELISA assay showed reduction of serum Erfe levels (C). Results represent mean ± SD; **P < .01, *P < .05. Analysis was performed using one-way ANOVA with Tukey multiple comparison adjustment. WT data are displayed as a reference guide but are not included in the between-group test comparisons.
Combination of minihepcidin and the iron chelator DFP improved anemia and reversed splenomegaly in older Hbbth3/+ mice
Treatment with the minihepcidin M009 alone or in combination with DFP produced the same profile of hematological changes described following treatment with M004, resulting in a significant increase in circulating Hb (Figure 4A), RBC count (Figure 4B), and HCT level (Figure 4C). No differences in hematological parameters were observed between DFP-treated and vehicle-treated mice (Figure 4A-C). This indicates that treatment with the iron chelator alone was not sufficient to improve erythropoiesis, despite reduced organ iron content.23 Reticulocyte count and spleen size were both reduced with M009 and M009 + DFP treatment, reflecting improved erythropoietic efficiency (Figure 4D-E). Flow cytometry studies of BM and spleen erythroid populations from minihepcidin-treated animals demonstrated an increase in the relative proportion of mature erythroid cells (Figure 2E) and reduced levels of ROS (Figure 4F). Parameters of RBC damage (red cell distribution width) (Figure 4G), hemichrome formation (Figure 4H), and erythrocyte morphology (Figure 4I) were improved. We previously demonstrated that Hbbth3/+ animals show increased EPO concentrations in response to anemia.20,24 Overexpression of EPO leads to increased phosphorylation of the Jak2 kinase and overexpression of the genes downstream of the Jak2-Stat5 pathway involved in cell proliferation and survival.20,24,25 As minihepcidin improved erythropoiesis and ameliorated anemia, EPO concentrations declined (Figure 4J).
Minihepcidin peptide alone or in combination with an iron chelator improved anemia and splenomegaly in animals with β-thalassemia. Treatment with DFP alone (1.25 mg/mL of drinking water) was not associated with changes in hematological parameters such as Hb level (A), RBC number (B), and HCT concentration (C). Combination with minihepcidin M009 (2.625 mg/kg) led to significant improvement in anemia when compared with vehicle treatment, as indicated by increased Hb level, RBC number, and HCT concentration (A-C). Reticulocyte count (D) and spleen size (E) were both significantly reduced when animals received minihepcidin alone or in association with DFP, reflecting improved erythropoietic efficiency. Flow cytometry studies of BM and spleen erythroid populations from thalassemic animals that received minihepcidin alone or in combination with DFP demonstrated reduced levels of ROS formation (F). As in Figure 1F, we show here a representative example of ROS-positive cells (control and treated groups) in the fraction that represents mature RBCs. Parameters of erythrocyte damage such as red cell distribution width (RDW; G), hemichrome formation (as shown with TAU gel; H), and red cell morphology (Giemsa staining of peripheral blood smear; I) were improved in animals treated with minihepcidin alone or in combination with DFP (when compared with control groups and the standard [st] in panel H). Erythropoietin levels (Epo) were reduced in animals receiving M009 as a consequence of improved anemia (J). Results represent mean ± SD; ****P < .0001, ***P < .001, **P < .01, and *P < .05. Analysis was performed using one-way ANOVA with Tukey multiple comparison adjustment. WT data are displayed as a reference guide but are not included in the between-group test comparisons.
Minihepcidin peptide alone or in combination with an iron chelator improved anemia and splenomegaly in animals with β-thalassemia. Treatment with DFP alone (1.25 mg/mL of drinking water) was not associated with changes in hematological parameters such as Hb level (A), RBC number (B), and HCT concentration (C). Combination with minihepcidin M009 (2.625 mg/kg) led to significant improvement in anemia when compared with vehicle treatment, as indicated by increased Hb level, RBC number, and HCT concentration (A-C). Reticulocyte count (D) and spleen size (E) were both significantly reduced when animals received minihepcidin alone or in association with DFP, reflecting improved erythropoietic efficiency. Flow cytometry studies of BM and spleen erythroid populations from thalassemic animals that received minihepcidin alone or in combination with DFP demonstrated reduced levels of ROS formation (F). As in Figure 1F, we show here a representative example of ROS-positive cells (control and treated groups) in the fraction that represents mature RBCs. Parameters of erythrocyte damage such as red cell distribution width (RDW; G), hemichrome formation (as shown with TAU gel; H), and red cell morphology (Giemsa staining of peripheral blood smear; I) were improved in animals treated with minihepcidin alone or in combination with DFP (when compared with control groups and the standard [st] in panel H). Erythropoietin levels (Epo) were reduced in animals receiving M009 as a consequence of improved anemia (J). Results represent mean ± SD; ****P < .0001, ***P < .001, **P < .01, and *P < .05. Analysis was performed using one-way ANOVA with Tukey multiple comparison adjustment. WT data are displayed as a reference guide but are not included in the between-group test comparisons.
M009 was also used to treat 4-month-old females Hbbth3/+ and showed a trend similar to that observed in males. We observed improved liver iron content, hematological parameters, and erythroid maturation, mostly in the spleen, as well as a reduction in splenomegaly (supplemental Figure 4A-F).
Administration of minihepcidin normalized HCT levels and resolved splenomegaly in a mouse model of PV
The progeny of floxed Jak2V617F/+ mice crossed with a mouse hemizygous for the Vav-cre transgene expressed in hematopoietic cells can develop PV, and this phenotype can be easily propagated by engrafting Jak2V617F/+-Vav-cre BM cells into WT C57BL/6 mice.26 Using these mice, we tested the hypothesis that minihepcidin-induced iron restriction could work as medical phlebotomy by reversing erythrocytosis and normalizing the HCT level in murine PV. To this end, we studied the effects of markedly higher doses of M009 than were previously used in studies in the Hbbth3/+ mice to ensure that a high level of iron restriction (serum iron reduction of >80% throughout the interval between doses) was present throughout the study.
We treated PV mice with vehicle or M009 (10 and 15 mg/kg) administered by biweekly SC injections for 3 weeks, with end-point analysis performed 3.5 days after the last injection. Treatment was initiated 1 month after BM transplantation, when the PV phenotype had been fully established. At 3 weeks, treatment showed a dose-dependent reduction (or normalization) in the HCT level, RBC count, and Hb concentration in animals receiving minihepcidin when compared with mice exposed to the vehicle (Figure 5A-C). Splenomegaly was reduced (Figure 5D), and flow cytometric profiles of erythroid precursors improved (Figure 5E). Flow cytometry analysis indicated that iron restriction mediated by minihepcidin led to a reduction of erythroid progenitor cells when compared with vehicle (fraction 1 to 4). No significant changes in mean corpuscular hemoglobin were seen. We did not observe differences in liver iron content in treated animals vs controls, but a significant increase in splenic iron levels was detected at both doses (supplemental Figure 5A). These findings were confirmed by Perls’ Prussian blue staining in liver and spleen sections (supplemental Figure 5B), showing increased iron retention by macrophages.
Beneficial effect of minihepcidin peptide in a mouse model of PV. Mice affected by PV (Jak2V617F/+ VAV-Cre double transgenic) were generated by transplantation of PV BM cells into lethally irradiated C57Bl/6 recipients. Four weeks post-BMT, when the phenotype was fully established, mice received SC injections of minihepcidin M009 (10 or 15 mg/kg) or vehicle twice a week for 3 weeks. At end point, hematological parameters such as HCT (A), RBC (B), and Hb (C), normally very high in PV mice, were significantly reduced (in a dose-dependent manner). Splenomegaly was also reduced (D). BM and spleen erythroid profiles were normalized (E), reflecting improved erythropoietic activity (as shown in the CD44-FSC [forward scatter] plots). Results represent mean ± SD; ****P < .0001, ***P < .001, **P < .01, and *P < .05. Analysis was performed using one-way ANOVA with Tukey multiple comparison adjustment.
Beneficial effect of minihepcidin peptide in a mouse model of PV. Mice affected by PV (Jak2V617F/+ VAV-Cre double transgenic) were generated by transplantation of PV BM cells into lethally irradiated C57Bl/6 recipients. Four weeks post-BMT, when the phenotype was fully established, mice received SC injections of minihepcidin M009 (10 or 15 mg/kg) or vehicle twice a week for 3 weeks. At end point, hematological parameters such as HCT (A), RBC (B), and Hb (C), normally very high in PV mice, were significantly reduced (in a dose-dependent manner). Splenomegaly was also reduced (D). BM and spleen erythroid profiles were normalized (E), reflecting improved erythropoietic activity (as shown in the CD44-FSC [forward scatter] plots). Results represent mean ± SD; ****P < .0001, ***P < .001, **P < .01, and *P < .05. Analysis was performed using one-way ANOVA with Tukey multiple comparison adjustment.
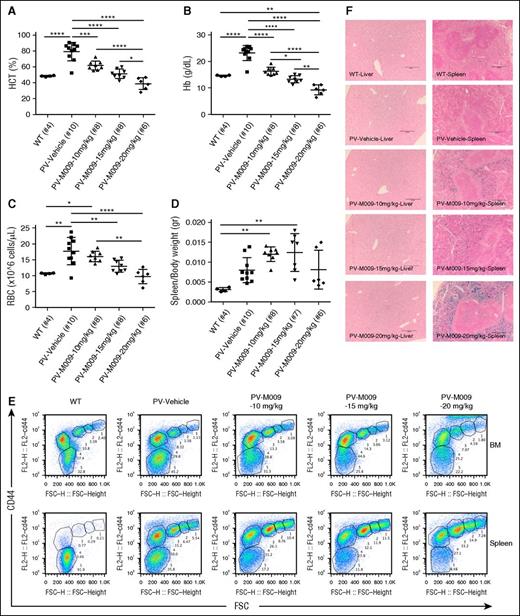
Prolonged administration of minihepcidin normalizes HCT levels but can cause iron-restricted erythropoiesis in a mouse model of PV
To investigate the effect of minihepcidin at similar or higher doses and for longer periods, we performed studies using M009 at a dose range of 10 to 20 mg/kg administered by twice-weekly SC injections for 6 weeks. At the end of the treatment period, we observed decreased HCT levels (Figure 6A) due to a large reduction in erythrocytosis in M009-treated animals. Circulating Hb concentrations and RBC numbers (Figure 6B-C) were also reduced in a dose-dependent fashion to the same or lower level than seen in healthy untreated WT littermates. However, we also observed a dose-dependent development of iron-restricted erythropoiesis by splenomegaly (Figure 6D) and expansion of early erythroid progenitors by flow cytometry studies (Figure 6E). These observations indicate that in PV minihepcidin administration needs to be titrated to reduce erythrocytosis while minimizing the negative effects of erythrocyte iron deficiency. As seen in animals treated for 3 weeks, administration of minihepcidin led to increased iron deposition in splenic macrophages, while no major changes were observed in liver sections (Figure 6F).
Prolonged treatment of PV mice with minihepcidin can cause iron-restricted erythropoiesis. PV mice (Jak2V617F/+ VAV-Cre double transgenic) generated by BM transplantation (as seen before) start receiving M009 or vehicle by SC injections 4 weeks post-BM transplant (at a dose of 10, 15, or 20 mg/kg) twice a week for 6 weeks. Hematological parameters such as HCT (A), Hb (B), and RBC (C) were normalized in a dose-dependent fashion. However, this prolonged treatment was also associated with the development of iron-restricted erythropoiesis. This was indicated by increased splenomegaly (D) and erythroid maturation block shown by flow cytometry studies (E). Prussian blue staining on liver and spleen sections highlights increased iron deposition in the spleen, but not changes in liver iron content (F). Images were captured using a Leica DM4000B upright scope paired with a Spot RT/SE Slider camera (10×/numerical aperture 0.40 objective) and then acquired using the Spot 5.1 software. Results represent mean ± SD; ****P < .0001, ***P < .001, **P < .01, and *P < .05. Analysis was performed using one-way ANOVA with Tukey multiple comparison adjustment.
Prolonged treatment of PV mice with minihepcidin can cause iron-restricted erythropoiesis. PV mice (Jak2V617F/+ VAV-Cre double transgenic) generated by BM transplantation (as seen before) start receiving M009 or vehicle by SC injections 4 weeks post-BM transplant (at a dose of 10, 15, or 20 mg/kg) twice a week for 6 weeks. Hematological parameters such as HCT (A), Hb (B), and RBC (C) were normalized in a dose-dependent fashion. However, this prolonged treatment was also associated with the development of iron-restricted erythropoiesis. This was indicated by increased splenomegaly (D) and erythroid maturation block shown by flow cytometry studies (E). Prussian blue staining on liver and spleen sections highlights increased iron deposition in the spleen, but not changes in liver iron content (F). Images were captured using a Leica DM4000B upright scope paired with a Spot RT/SE Slider camera (10×/numerical aperture 0.40 objective) and then acquired using the Spot 5.1 software. Results represent mean ± SD; ****P < .0001, ***P < .001, **P < .01, and *P < .05. Analysis was performed using one-way ANOVA with Tukey multiple comparison adjustment.
Discussion
In β-thalassemia and anemia, increased EPO levels and expanded but ineffective erythropoiesis result in lowered hepcidin levels, increased intestinal iron absorption, and TSAT.3,6,8,21,27 In turn, excess iron contributes to the development and worsening of ineffective erythropoiesis and reduced erythroblast maturation.1 Here, we show that minihepcidins can break this maladaptive, vicious circle and thus establish a new set point for BM erythropoiesis, ameliorating ineffective erythropoiesis and decreasing erythroid cell damage. Remarkably, the RBC count and lifespan are restored to those observed in WT mice. We surmise that the corrective effect begins with a reduction in hemichrome and ROS formation, leading to subsequent amelioration of erythroid damage and increased effective erythropoiesis. We expect that in a clinical setting, these biological effects may result in other beneficial changes not explored in our mouse model. For instance, improved RBC morphology may reduce stickiness, peripheral RBC destruction, and thrombotic risk. Decreased spleen size could reduce the need for surgical splenectomy. An improvement in anemia reduces hypoxia, which, together with decreased destruction of RBCs, may prevent or delay lethal complications such as pulmonary hypertension. Preventing iron accumulation addresses a critical mechanism of the disease and should avoid or delay the development of cirrhosis and liver cancer.
Here, we also conducted studies in the Hbbth3/+ mouse to evaluate whether the concurrent use of the oral iron chelator DFP and minihepcidin would lead to therapeutic interaction. Our data show that minihepcidin and DFP retain their positive effects on erythropoiesis and iron overload. Based on these observations, we anticipate that in untransfused β-thalassemic patients with moderate or severe iron overload, treatment with minihepcidin could improve erythropoiesis, whereas oral chelation therapy would still be beneficial in reversing established iron overload.
Interestingly, chelation alone failed to cause iron restriction or improve hematological parameters, as also seen in previous studies.2,28 In DFP-treated mice, despite a decrease in liver iron concentration, TSAT and serum iron levels were not decreased. However, when DFP was combined with minihepcidin, we observed the same beneficial effects on ineffective erythropoiesis, RBC lifespan, and anemia, even though levels of serum iron and TSAT were not decreased compared with solvent-treated mice. The potential mechanisms by which this may occur are speculative. Perhaps as DFP releases iron from the liver, it delivers some iron to serum transferrin via a shuttling effect. However, when minihepcidin is administered together with DFP, the overall flow of iron for erythropoiesis might be reduced, as some iron is diverted to macrophages (supplemental Figure 6). Additional studies will be needed to test this model. Nevertheless, the lack of an impact of concurrent minihepcidin therapy on the tissue iron–depleting properties of DFP indicates that the 2 modalities may have additive or synergistic therapeutic effects. In addition, these data indicate that chelator-induced iron excretion can be accomplished despite increased macrophage iron sequestration in minihepcidin-treated mice. These results also bode well for the design of clinical trials in which the therapeutic effects of minihepcidin should be testable without interference with chelator therapy, the current standard of care. In short-term experiments, endogenous hepcidin levels were reduced, reaching baseline levels at 48 to 72 hours (supplemental Figure 3A). This, together with the fact that endogenous hepcidin levels were not increased at the end of the 6-week study (supplemental Figure 3C) while serum Erfe levels were decreased, points to the multiple regulators of hepcidin, where the net effect of decreasing both Erfe and systemic iron levels resulted in unchanged hepcidin levels. Altogether these observations document the amelioration of ineffective erythropoiesis as indicated by a better balance between immature and mature erythroid cells in the BM and spleen and more RBCs in circulation but less serum EPO (Figures 2E, 4B, and 4J).
Our data also indicate that minihepcidin may be beneficial in the treatment of PV by limiting iron availability to erythroid precursors, thereby inhibiting JAK2-stimulated erythropoiesis and reducing RBC production. Controlling HCT is of critical importance in PV management. Here, the ability of minihepcidin to rapidly impose iron-restricted erythropoiesis contrasts with intermittent phlebotomy, where the removal of RBCs is rapidly compensated, at least until enough RBC mass is removed to cause iron deficiency. These results suggest that minihepcidin therapy could be used as a “medical phlebotomy” to provide continuous control of accelerated erythropoiesis in the treatment of PV. Because of the association between high HCT levels and poor outcomes, the rapid and continuous control of HCT levels using minihepcidin could be highly clinically meaningful if clinical trials show that it can be done safely.
In conclusion, the drug-like minihepcidin peptides offer a new potential pharmacotherapeutic approach to achieve therapeutic iron restriction in the treatment of β-thalassemia, PV, and perhaps other less common disorders associated with abnormal erythropoiesis.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by Merganser Biotech and grants from the National Institute of Diabetes and Digestive and Kidney Diseases and National Heart, Lung, and Blood Institute of the National Institutes of Health: R01 DK095112 and R01 DK090554 (S.R.), DK095201 (Y.M.S.), R01 DK090554 (T.G. and E.N.), R01 DK107309 (E.N.), R01 DK107670 (Y.G.), R01 DK095112 (R.E.F., S.R., and Y.G.), and K08 HL105682 (Y.G.). C.C. is supported by the Cooley's Anemia Foundation, and P.R.O. is supported by The Child Reach Foundation.
Authorship
Contribution: C.C., P.R.O., H.C., Y.G., P.P., and E.V.V. performed research and analyzed the results; V.N. provided support with statistical analysis; Y.G., R.E.F., Y.M.S., E.N., and T.G. discussed the experiments and reviewed the paper; and S.R., C.C., and B.M. designed research, analyzed results, and wrote the paper.
Conflict-of-interest disclosure: B.M. has stock in Merganser Biotech. S.R. has restricted stocks in Merganser Biotech. S.R. is a consultant for Novartis Pharmaceuticals, Bayer Healthcare, Merganser Biotech and Keryx Pharmaceuticals. S.R. is a member of scientific advisory board of Merganser Biotech and Ionis Pharmaceuticals. T.G. and E.N. are consultants and stockholders of Intrinsic LifeSciences, Merganser Biotech, and Silarus Therapeutics. The remaining authors declare no competing financial interests.
Correspondence: Stefano Rivella, Children’s Hospital of Philadelphia, Abramson Research Center, 3615 Civic Center Blvd, Room 316B, Philadelphia, PA 19104; e-mail: rivellas@email.chop.edu.


![Figure 2. Minihepcidin peptide ameliorated hypoxia in animals affected by non–transfusion-dependent thalassemia. Hbbth3/+ mice were crossed with the hypoxia reporter mouse ODD-Luc and treated with M004 (2.625 mg/kg) twice a week for 6 weeks. As expected, minihepcidin administration increased RBC numbers (A) and Hb level (B) and reduced reticulocyte count (C) and splenomegaly (D). Improved erythropoietic efficiency was confirmed by flow cytometry analysis of erythroid precursors (as shown in the CD44-FSC [forward scatter] plots) (E). Reduction in hypoxia was evaluated by in vivo imaging. Reduction in luminescence was detected in the abdomen of animals receiving minihepcidin compared with the vehicle-treated one, indicating reduction in hypoxia (F). Results are presented as mean ± SD; *P < .05, 2-tailed Mann-Whitney U test. WT data are displayed as a reference guide but are not included in the between-group test comparisons.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/128/2/10.1182_blood-2015-10-676742/4/m_265f2.jpeg?Expires=1765149192&Signature=Ru8zkQ2i19i0Q5JYlhTwE8hyMf97TFpGZ7Nf2SVcYrbFpkkqN-QIfxRUZj7Sg3K4COb91pFBAIAr5jtEWJUx-NT6r3c3s45cAEANaiJxKEAkgIpiVtb~5P0KUWnLvXyqQetjIs3NK-oVN9P4tsIhYmSwpIdEZnTS3QviQr4xxbiCzo8gZfZEViK8uz87-2s3LjM5rVoH0JNbTf0Sl5M7rHtiCgfM1A991e7sXWwt~KGI2FDSe42TTH55T3thXVYrXsAFXT1mqQPv9Y9FX0F~9V4f-6BXpL1TXfBVW~Sbe3g3BgouqWSMyZW5rBGYVMreE-z41I1H6iE5HAqOKtuwfA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 4. Minihepcidin peptide alone or in combination with an iron chelator improved anemia and splenomegaly in animals with β-thalassemia. Treatment with DFP alone (1.25 mg/mL of drinking water) was not associated with changes in hematological parameters such as Hb level (A), RBC number (B), and HCT concentration (C). Combination with minihepcidin M009 (2.625 mg/kg) led to significant improvement in anemia when compared with vehicle treatment, as indicated by increased Hb level, RBC number, and HCT concentration (A-C). Reticulocyte count (D) and spleen size (E) were both significantly reduced when animals received minihepcidin alone or in association with DFP, reflecting improved erythropoietic efficiency. Flow cytometry studies of BM and spleen erythroid populations from thalassemic animals that received minihepcidin alone or in combination with DFP demonstrated reduced levels of ROS formation (F). As in Figure 1F, we show here a representative example of ROS-positive cells (control and treated groups) in the fraction that represents mature RBCs. Parameters of erythrocyte damage such as red cell distribution width (RDW; G), hemichrome formation (as shown with TAU gel; H), and red cell morphology (Giemsa staining of peripheral blood smear; I) were improved in animals treated with minihepcidin alone or in combination with DFP (when compared with control groups and the standard [st] in panel H). Erythropoietin levels (Epo) were reduced in animals receiving M009 as a consequence of improved anemia (J). Results represent mean ± SD; ****P < .0001, ***P < .001, **P < .01, and *P < .05. Analysis was performed using one-way ANOVA with Tukey multiple comparison adjustment. WT data are displayed as a reference guide but are not included in the between-group test comparisons.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/128/2/10.1182_blood-2015-10-676742/4/m_265f4.jpeg?Expires=1765149192&Signature=r7sXRluPOmc1zxNybJcbf-xjDPwdnanGjBsEKgG-2su1TSyCVnTLKqHcRyBeFkBKn44-cuzvhWT2VBhWrScyu1S0DtV8a59wD~vsHF9S0IXRg4ESGgh5M8MbID5aNVBaNAdCDFH~kP0F85e86SCiHNx1Wr44Hkx1l-D7X72bE5o5PW3kM6ET4-CkCkVwdj-qurnkX9uYuI4eN1Zi6wKkb42Yj6kc30K4sBsW2-5KTb5uaoabv9NSQB7Op0X6eH3JYM4KRNGpb0Cnry8vAPyTMlksB0-SgqwXaYZXxVM7SgDCz5uAobWDA13O2HjHFEVFDLzf6oeH4c3ZKN6P6dPkBw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 5. Beneficial effect of minihepcidin peptide in a mouse model of PV. Mice affected by PV (Jak2V617F/+ VAV-Cre double transgenic) were generated by transplantation of PV BM cells into lethally irradiated C57Bl/6 recipients. Four weeks post-BMT, when the phenotype was fully established, mice received SC injections of minihepcidin M009 (10 or 15 mg/kg) or vehicle twice a week for 3 weeks. At end point, hematological parameters such as HCT (A), RBC (B), and Hb (C), normally very high in PV mice, were significantly reduced (in a dose-dependent manner). Splenomegaly was also reduced (D). BM and spleen erythroid profiles were normalized (E), reflecting improved erythropoietic activity (as shown in the CD44-FSC [forward scatter] plots). Results represent mean ± SD; ****P < .0001, ***P < .001, **P < .01, and *P < .05. Analysis was performed using one-way ANOVA with Tukey multiple comparison adjustment.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/128/2/10.1182_blood-2015-10-676742/4/m_265f5.jpeg?Expires=1765149192&Signature=TbwQDYbC6UW6H~ftPlZvZCJ815sKrQiwf2iT-pZgabfTAWFEM17FeqDG1Nuc46CfdoY6k080fQvLMAQ2q5jFaySO2H3uhrsZ3rxr9b4SusG~yiab0ZbzMe-IykOVc9KRwA36d6HDTla9hSBHRvgphYv8Bne~ZDSzv3Gc3B6TVF5VAA3OTxfvz6MrbgOuo2afa8WThPpFcHXvmsrcLwE3GyEXAInUcjn55vGIxl93ajxAdDKeGZQA0fzrQ8wUAlssT6AGHGwJAc-527G2QRu8EgvTOvE1gw7nljXyq-waU36H9urcx293vNfe37vYk~Zcv~TbgdlzlLcrQkWCVEka9Q__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)