In this issue of Blood, Casu et al report on results from their studies on potential therapeutic options to restrict iron as a treatment of 2 differing blood disorders: the anemia and iron overload associated with β-thalassemia, and the erythrocytosis associated with polycythemia vera (PV). They examine the use of derivatives of hepcidin, a 25-amino acid peptide secreted by the liver, which is widely accepted as the “iron stores” regulator.1
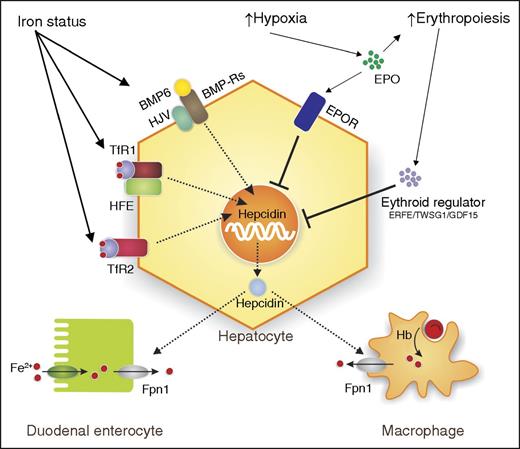
Regulation of hepcidin. Schema illustrating some of the complex regulation of hepcidin by iron status, hypoxia, and erythropoietic demand. Increased iron status induces hepcidin through: bone morphogenetic protein 6 (BMP6), bone morphogenetic protein receptors (BMP-Rs), hemojuvelin (HJV), hemochromatosis protein (HFE), and transferrin receptor 1 (TfR1) and TfR2. Hypoxia and increased erythropoiesis work through: erythropoietin (EPO), erythropoietin receptor (EPOR), and the erythropoietic regulators, erythroferrone (ERFE), growth differentiation factor 15 (GDF15), and twisted gastrulation BMP signaling modulator 1 (TWSG1), to inhibit hepcidin expression. Iron absorbed by duodenal enterocytes and recycled from Hb from senescent red blood cells (RBCs) by macrophages is transported into the circulation by ferroportin 1 (Fpn1). Hepcidin secreted by the hepatocyte binds to Fpn1, causing its internalization and degradation, thereby limiting iron transport. Hb, hemoglobin.
Regulation of hepcidin. Schema illustrating some of the complex regulation of hepcidin by iron status, hypoxia, and erythropoietic demand. Increased iron status induces hepcidin through: bone morphogenetic protein 6 (BMP6), bone morphogenetic protein receptors (BMP-Rs), hemojuvelin (HJV), hemochromatosis protein (HFE), and transferrin receptor 1 (TfR1) and TfR2. Hypoxia and increased erythropoiesis work through: erythropoietin (EPO), erythropoietin receptor (EPOR), and the erythropoietic regulators, erythroferrone (ERFE), growth differentiation factor 15 (GDF15), and twisted gastrulation BMP signaling modulator 1 (TWSG1), to inhibit hepcidin expression. Iron absorbed by duodenal enterocytes and recycled from Hb from senescent red blood cells (RBCs) by macrophages is transported into the circulation by ferroportin 1 (Fpn1). Hepcidin secreted by the hepatocyte binds to Fpn1, causing its internalization and degradation, thereby limiting iron transport. Hb, hemoglobin.
Ferroportin is the multitransmembrane iron exporter which is involved in iron absorption in the duodenum and iron recycling/export in other organs including the spleen and liver.2 Hepcidin plays a central role in the regulation of iron homeostasis as a negative regulator of iron transport: it binds to ferroportin, and induces its internalization and eventual degradation, thus reducing iron egress into the circulation. Hepcidin is itself regulated by many factors including iron levels, erythropoietic demand, and inflammation3 (see figure). Most cases of dysregulated iron metabolism are linked to aberrant expression of hepcidin; this is a hallmark of the iron overload disorder hereditary hemochromatosis. Here, the misregulation of hepcidin due to mutations in regulatory proteins such as HFE, TfR2, and HJV results in low hepcidin levels despite increased body iron levels.
This exquisite regulation of iron homeostasis by a single peptide provides opportunities to influence iron levels in the body in many clinical conditions. Induction of hepcidin or exogenous administration of hepcidin may be of therapeutic value in many iron-related disorders. As proof of concept, transgenic overexpression of hepcidin prevents iron overload in an animal model of hemochromatosis,4 and reduces iron loading and improves anemia in β-thalassemia.5
Minihepcidins, mimetics of the hepcidin peptide with improved pharmacological properties, have been reported to have therapeutic potential in animal studies for treatment of severe iron overload.6,7 In β-thalassemia and PV, it has been postulated that iron restriction may be of therapeutic value. The rationale here is that restricting iron through the use of hepcidin mimetics would be therapeutic in these blood disorders. To address this, Casu et al administered minihepcidins, comprising the first 9 amino acids of the mature hepcidin peptide, to animal models of β-thalassemia and PV.
β-thalassemia, an autosomal recessively inherited blood disorder, is characterized by ineffective erythropoiesis and anemia due to mutations in, or deficiency of, the β-globin gene.8 Depending on the severity of the disorder, treatments include blood transfusions to correct the anemia, and eventually iron chelation to correct the resulting transfusional iron overload. The β-thalassemia intermedia mice used here, heterozygous β1/β2 globin gene deletion mice (Hbbth3/+), display anemia, hepatic iron loading, and increased erythropoietin levels.
PV, a blood cancer, is characterized by excessive production of RBCs by the bone marrow. PV is caused by activation of the JAK-STAT pathway and activating mutations in JAK.9 The authors use a transgenic mouse model of PV (Jak2V617F/+-Vav-iCre) in which the Jak2 mutation is expressed in cells of the hematopoietic system. The PV phenotype is then propagated in wild-type mice through bone marrow transplant for extended studies.
In β-thalassemic mice, administration of low levels of minihepcidins resulted in decreased serum iron and increased mature RBCs; however, at higher doses, severe iron restriction was observed with worsening of the anemia. Longer dose intervals between minihepcidins resolved this; β-thalassemia mice treated with minihepcidin had reduced liver and kidney iron loading and improvements in hematological indices as reflected by improved morphology and lifespan of RBCs, increased number of RBCs, and Hb. The concomitant increase in tissue macrophage iron was an expected effect of hepcidin mimetics, and thus reduced ferroportin. The authors postulated that hematological improvements were likely due to reduced oxidative damage.
To further explore the mechanisms involved, the authors used β-thalassemic mice crossed with a hypoxia-reporter mouse, the oxygen-dependent degradation (ODD-luciferase). These novel transgenic mice allowed an in vivo and real-time analysis of the level of oxygenation in tissues through imaging of luminescence. Hypoxia or inadequate oxygenation is a feature of β-thalassemia. Administration of minihepcidins resulted in increased Hb levels and improved tissue oxygenation as reflected in increased luminescence in the abdomen.
If this therapeutic option is ultimately transferred to the clinic it is likely that β-thalassemia patients would be treated with an iron chelator and minihepcidins. Casu et al administered both minihepcidins and deferiprone, an oral iron chelator, to older β-thalassemia mice, which have established hepatic iron loading. Deferiprone-treated mice had decreased liver iron and increasing serum iron indices but no improvement in hematological parameters, suggesting that chelation alone was not sufficient to improve erythropoiesis despite reduced liver iron.
Mice treated with both deferiprone and minihepcidin also had lower hepatic iron levels but no differences in serum iron indices. However, treatment with minihepcidin alone or in combination with iron chelator resulted in significant hematological improvement: increased Hb, RBC count, and hematocrit, improved anemia, and reversed splenomegaly. Minihepcidin treatment resulted in a decreased erythropoietin concentration, associated with improved erythropoiesis, and reduction in anemia and reactive oxygen species (ROS).
In PV mice, iron restriction due to administration of minihepcidins for 3 weeks resulted in a reduction in splenomegaly, erythrocytosis, circulating Hb and erythroid progenitor cells; this was accompanied by an increase in splenic iron but no change in hepatic iron. However, prolonged administration of minihepcidin for 6 weeks, while resulting in normalization of hematopoiesis, also resulted in splenomegaly and development of iron-restricted erythropoiesis. In PV, minihepcidin treatments must be titrated to decrease any negative effects of extended iron restriction. What is not addressed in this study is the role of minihepcidins in the new targeted therapeutics for PV, for example, Jak2 inhibitors, which are currently being developed.
Although the consequences of hepcidin and its mimetics on iron restriction have been described previously, these exciting studies by Casu et al suggest that minihepcidin treatment in β-thalassemia may establish a new point from which erythropoiesis can be re-initiated, with decreased ROS and formation of membrane-bound globins leading to improved erythropoiesis. Minihepcidin peptides thus appear to be potential therapeutics for a number of erythropoietic disorders.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal