In this issue of Blood, Lopes da Silva and Cutler clarify a long-standing issue of the polarity of release of the multimeric adhesive glycoprotein von Willebrand factor (VWF).1
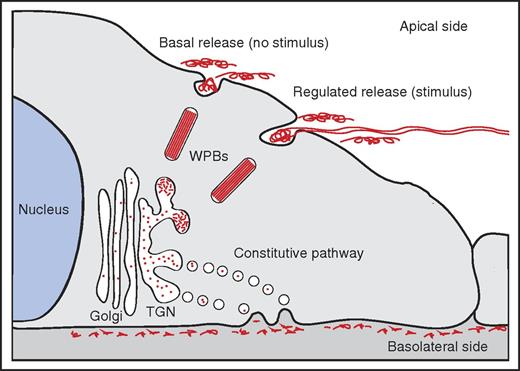
Polarity of VWF release. In the TGN, a small proportion of newly synthesized VWF is selected for entry into WPBs. WPB release primarily occurs at the apical side of the cell, either in the absence of a stimulus (basal release) or in the presence of a stimulus (regulated release). Apically released VWF is composed of high-molecular weight (HMW) multimers; regulated release of VWF has been shown to result in the formation of VWF fibers on the surface of endothelial cells. Constitutive released VWF, containing LMW multimers, is targeted to the basolateral side of endothelial cells.
Polarity of VWF release. In the TGN, a small proportion of newly synthesized VWF is selected for entry into WPBs. WPB release primarily occurs at the apical side of the cell, either in the absence of a stimulus (basal release) or in the presence of a stimulus (regulated release). Apically released VWF is composed of high-molecular weight (HMW) multimers; regulated release of VWF has been shown to result in the formation of VWF fibers on the surface of endothelial cells. Constitutive released VWF, containing LMW multimers, is targeted to the basolateral side of endothelial cells.
Release of VWF at the apical side results in the appearance of VWF multimers in the vascular lumen. Under-flow apical release of VWF is generally linked to the assembly of web-like structures or fibers that efficiently catch platelets, leukocytes, and red blood cells from the circulation.2,3 In contrast, basolateral release promotes accumulation of VWF in the subendothelial matrix and may promote platelet adhesion following vascular injury.
The biosynthesis of VWF in endothelial cells has been described in considerable detail.4 At the trans-Golgi network (TGN), VWF finds itself at a crossroad: part of the newly synthesized VWF is packaged into elongated secretory storage organelles designated Weibel-Palade bodies (WPBs). Within these organelles, VWF is organized in tightly coiled, tubular structures that result from condensation of VWF multimers through its amino-terminal D domains at low pH.5,6 Following stimulation of endothelial cells with agonists such as histamine or vasopressin, WPBs release their content into the vascular lumen. During this process VWF tubules unfurl and rapidly form spider-web–like fibers that adhere to the vessel wall.2,3
The remainder of the newly synthesized VWF enters the “constitutive” secretory pathway in which short-lived TGN-derived vesicles containing low-molecular-weight (LMW) VWF multimers fuse with the plasma membrane. Earlier studies by Sporn et al had suggested that only a relatively small proportion of newly synthesized VWF enters the storage pathway and that the vast majority is released via the “constitutive” secretory pathway.7 Release of WPBs has also been shown to occur spontaneously in the absence of an external stimulus,8 which has been defined as “basal release” in analogy to the “basal secretion” described in neuro-endocrine cells in the early 1990s.9 A systematic study dissecting the individual contributions of all the routes through which VWF can be secreted, the multimeric nature of the content, and the polarity of release had yet to be performed.
In their report in Blood, Lopes da Silva and Cutler have meticulously taken apart the different contributions of the constitutive, basal, and regulated components of VWF secretion on both sides of the endothelial cell. Convincingly, they show that constitutively released VWF is directed toward the basolateral side of endothelial cells, whereas basal release of WPBs (ie, in the absence of a stimulus) primarily occurs on the apical side of the cell (see figure). Regulated release of WPBs in response to stimuli such as histamine or thrombin also occurs primarily at the apical side of endothelial cells. Complementary multimer analysis shows that apically released VWF originating from basal or regulated released VWF is composed of HMW multimers, whereas constitutively basolaterally released VWF is enriched in LMW VWF multimers. Interestingly, they found that the adaptor protein 1 (AP-1) complex, which the Cutler group has previously found on nascent WPBs,10 functions as a gatekeeper for the entry into the basal/regulated compartment.
Altogether, the data of Lopes da Silva and Cutler reveal that AP-1–mediated entry into WPBs is a pivotal “career” step for VWF molecules because it allows them to move up the ranks and assemble into HMW multimers. VWF dimers or smaller multimers that fail to make it to this elite level contained within WPBs need to quickly move “out” of the endothelial cell via the constitutive pathway. The pool of HMW multimers contained within WPBs is moving “up,” and gets time and resources to further mature and optimize its polymeric size and to condense into tubule-like structures. Eventually, these fully matured WPBs are released from endothelial cells in either the presence (regulated pathway) or absence (basal release) of a stimulus at the apical side (see figure). This shows that the “up or out” policy, familiar to many of those working in academia or fast-paced areas in the private sector, is also adopted by endothelial cells to ensure adequate delivery of biologically active HMW VWF polymers to the vascular lumen.
Conflict-of-interest disclosure: The authors declare no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal