Key Points
Bone marrow mesenchymal stromal cells transfer functional mitochondria to AML cells in vitro and in vivo through endocytic pathways.
This mitochondria transfer is enhanced by some chemotherapies and confers a survival advantage to leukemic blasts and leukemia initiating cells.
Abstract
Here we demonstrate that in a niche-like coculture system, cells from both primary and cultured acute myeloid leukemia (AML) sources take up functional mitochondria from murine or human bone marrow stromal cells. Using different molecular and imaging approaches, we show that AML cells can increase their mitochondrial mass up to 14%. After coculture, recipient AML cells showed a 1.5-fold increase in mitochondrial adenosine triphosphate production and were less prone to mitochondrial depolarization after chemotherapy, displaying a higher survival. This unidirectional transfer enhanced by some chemotherapeutic agents required cell–cell contacts and proceeded through an endocytic pathway. Transfer was greater in AML blasts compared with normal cord blood CD34+ cells. Finally, we demonstrate that mitochondrial transfer was observed in vivo in an NSG immunodeficient mouse xenograft model and also occurred in human leukemia initiating cells and progenitors. As mitochondrial transfer provides a clear survival advantage following chemotherapy and a higher leukemic long-term culture initiating cell potential, targeting mitochondrial transfer could represent a future therapeutic target for AML treatment.
Introduction
Acute myelogenous leukemia (AML) is a heterogeneous group of hematopoietic malignancies arising from hematopoietic stem and/or progenitor cells that display defective control of their proliferation, differentiation, and maturation. Complete remission is achieved using anthracycline and cytarabine combination therapy in ∼80% to 85% of older patients. Nevertheless, the overall outcomes for AML patients remain poor, and most patients die of relapse. The well-accepted paradigm of leukemogenesis is that leukemia arises from the transformation of a single cell and is maintained by a small population of leukemia initiating cells (LICs).1-3 It is theorized that current treatments, although highly effective against the leukemic bulk, fail to eradicate the LICs responsible for relapse.4,5 Environment-mediated drug resistance of human primary LICs is poorly described, partially due to the lack of experimental models that can incorporate both LIC stemness maintenance and the analysis of the bone marrow microenvironnement (BMME).
Interestingly, it has been observed that AML cells cocultured ex vivo with bone marrow stromal cells can upregulate BCL2 and UCP2 (uncoupling protein 2), both of which modify cellular energy metabolism and increase apoptotic threshold.6-9 Further to this, recent reports demonstrated that different cellular component of the BMME can transfer mitochondria to epithelial cells or solid cancer cell lines through hetero-cellular contacts.10-12 Although recipient cells displayed an improved metabolism, there is no clear evidence that functional mitochondria are actually transferred. It is also not known if LICs can benefit from this exchange in the context of leukemia in the BMME. We recently demonstrated that the bone marrow–derived MS-5 cell line can efficiently support primary human LICs ex vivo and contributed to LICs resistance to cytarabine over 1 week of intensive treatment.13 In this report, we present compelling evidence that supports a role for mitochondrial transfer from the bone marrow (BM) microenvironment as a mechanism that participate in the protection of leukemic cells when exposed to chemotherapy.
Materials and methods
Cells
To account for the AML heterogeneity, we studied the 8 most commonly used AML cell lines that represent a wide range of molecular abnormalities and clinical, biological, and immune-phenotypic characteristics (supplemental Table 1, available on the Blood Web site). AML cells HL-60, Kasumi-1, KG-1, MOLM-14, NB-4, SKM-1, THP-1, and U-937 or their luciferase enhanced green fluorescent protein (eGFP) lentivirus-transduced version were kindly provided by Y.C. Cells were maintained in RPMI 1640, with 10% fetal calf serum, 2 mM l-glutamine, penicillin (50 U/mL), and streptomycin (50 mg/mL). Umbilical cord blood (CB) and some AML cells were obtained after approval by the institutional review boards at the Centre Hospitalier Universitaire (Nice, France). AML samples were all collected at diagnosis. Informed consent was obtained from sampled patients. Details of the patient samples are listed in supplemental Table 2. The murine bone marrow stromal cell line MS-5 was kindly provided by Dominique Bonnet (Cancer Research UK, London, United Kingdom) and maintained in Iscove’s Modified Dulbecco’s Media 10% fetal calf serum + 2 mM l-glutamine. To generate MS-5 expressing OMI/HTRA2-mCherry, MS-5 was transduced with pBabe(puro)-Omi-mCherry construction (gift of D. Green; Memphis, TN). Mitochondria-defective ρ0 MS-5 cells were obtained after a 12-week culture in the presence of low-dose EtBr (100 ng/mL) supplemented with 1 mM pyruvate and 50 µg/mL uridine. Loss of functional mitochondria was monitored by sensitivity to carbonyl cyanide-p-trifluoromethoxyphenylhydrazone (FCCP) as previously described.14
Cocultures
Cocultures of AML cell lines with MS-5 confluent monolayer were performed in RPMI 1640. Coculture of primary samples was performed as previously described.13 Cytarabine (ARA) 3 µM, etoposide 3 µM, doxorubicin 0.3 µM, or vincristine 1 nM treatments for 72 hours were safe for the confluent MS-5 stromal cells as evidenced by 4′,6-diamidino-2-phenylindole (DAPI) staining. Staurosporine (STS) treatment was applied for 18 hours. All inhibitors were from Sigma-Aldrich. MS-5 MitoTracker loading was performed as follows: confluent MS-5 was stained for 10 minutes with 2 µM MitoTracker Green FM or 1 µM MitoTracker Red FM (Molecular Probes), washed twice, and left 72 hours to allow elimination of the unbound probe. MS-5 cells were then washed twice again before initiating cocultures with hematopoietic cells.
Imaging systems
MS-5 or MS-5 OMI/HTRA2-mCherry cells were cultured on collagen I–coated coverslips. After 72 hours of coculture, cells were fixed in 4% paraformaldehyde for 10 minutes and permeabilized for 20 minutes with saponin buffer (0.5% saponin, 5% bovine serum albumin in phosphate-buffered saline [PBS]). Fixed cells were incubated 1 hour at room temperature with anti-Tom20 (#sc11415; Santa Cruz), anti-cytochrome c (#556432; BD Biosciences), or anti-DNA (AC-30-10; Progen Biotechnik) in saponin buffer. Cells were rinsed with 0.5% saponin PBS permeating solution and incubated for 1 hour with an AlexaFluor647 anti-mouse or anti-rabbit immunoglobulin G (A31571, A31573; Life Technologies). After washing with 0.5% saponin PBS, slides were incubated for 5 minutes with 0.5 mg/mL DAPI (Sigma-Aldrich), before washing and mounting in Mowiol medium (Merck-Millipore). Confocal fluorescence images were performed on a A1R confocal laser-scanning microscope. The fluorescence emission was collected through a PL APO 60×, 1.4 NA oil objective (Nikon Instrument). Images were analyzed using NIS-Elements AR software (Nikon Instrument) or Image J (National Institutes of Health).
Flow cytometry
Flow cytometry (FCM) analyses were performed using MACSQuant or MACSQuant VYB analyzers (Miltenyi Biotec). Nonadherent cells were harvested through spinning the upside-down turned plate on an empty new collecting plate for 5 minutes at 80g. Adherent cells were harvested through trypsinization. Recovered cells were stained in Annexin binding buffer (BD Biosciences). As specified, MS-5 was counterstained with Sca-1-Pacific Blue (Biolegend) or Sca-1-phycoerythrin (PE) (BD Biosciences). Human hematopoietic cells were stained as specified with CD45-APC-Vio770, CD34-PerCP-Vio700, CD38-PE-Vio770 antibodies from Miltenyi, and CD3-allophycocyanin (APC) (BD Biosciences). All staining included AlexaFluor647-, fluorescein isothiocyanate–, PE-, or Pacific Blue–conjugated Annexin-V and or DAPI. The negative fraction was determined using appropriate isotype controls. Only viable (both DAPI- and/or Annexin-V–negative) fractions were assessed and/or sorted for all analyses. At least 2 markers were always used for leukemic cell distinction from stromal cells. For mitochondrial membrane potential (MMP) measures, harvested cells were stained with 1 µM tetramethylrhodamine, ethyl ester, perchlorate (TMRE; Sigma-Aldrich) for 30 minutes. FCM analyses were performed with FlowJo software (Tree Star).
Xenotransplantation of human leukemic cells and in vivo chemotherapy
Animals were used in accordance to a protocol approved by the Institutional Animal Care and User Ethical Committee of the UMS006 and Région Midi-Pyrénées (Approval#13-U1037-JES08). NOD/LtSz-scid IL-2Rγchainnull (NSG) mice were produced at the Genotoul Anexplo platform at Toulouse (France) using breeders obtained from The Charles River Laboratory. Adult mice (6-8 weeks old) were sublethally treated with 20 mg/kg busulfan (Busilvex; Pierre Fabre) by intraperitoneal administration 24 hours before injection of leukemic cells. AML cells were washed twice in PBS and suspended in PBS at a final concentration of 2 million cells/200 µL per mouse for intravenous injection. Two weeks after establishing MOLM-14 in NSG mice, 1 group of animals was treated with cytarabine (30 mg/kg per day by intraperitoneal administration for 5 days). Bone marrow cells were collected by flushing bones the day after the last dose of ARA. Cells were stained with human-specific APC-conjugated anti-CD19, PE-conjugated anti-CD33, APC-Cy7–conjugated anti-CD45, and PerCP-conjugated anti-murine CD45 antibodies. Primary AML engraftment was defined by the presence of a single viable CD45+CD33+CD19− population. For the functional identification of LIC and non-LIC phenotypes, samples were sorted based on the CD34 and CD38 expression and injected into NSG mice. Fractions were scored as enclosing LICs if a distinct human AML engraftment was detectable in the recipient bone marrow after 12 weeks. For samples #C3-1 and #6451, LICs were restricted to the CD34+CD38− fraction. Sample #3315 LICs were restricted to the CD34+ fraction.
Statistics
Data were analyzed for statistical significance using the Mann-Whitney unpaired 2-tailed test or the 1-way analysis of variance (ANOVA) test. Observed differences were regarded as statistically significant if the calculated 2-sided P value was <.05.
Additional methods are included in supplemental Methods.
Results
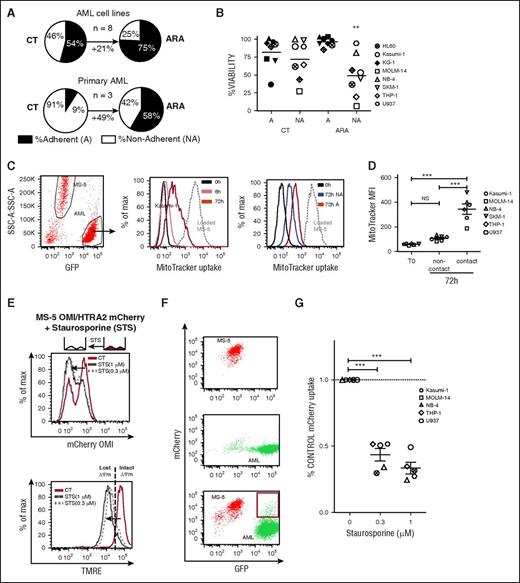
AML cells adherent to bone marrow mesenchymal cells display an improved cytarabine resistance associated with mitochondrial marker intake from stroma
Using our previously described niche-like culture system, we confirmed that chemotherapy completely skewed the nonadherent (NA) and the adherent (A) leukemic populations distribution to the benefit of the A fraction, which increased by 21% (*P < .05) and 49% (*P < .05) for AML cell lines and primary samples, respectively (Figure 1A). Annexin-V and DAPI staining revealed treatment with 3 µM ARA (not toxic for the MS-5 monolayer13 ) was only deleterious for the NA fraction, whereas A-leukemic cells remained as viable as untreated cells (Figure 1B). These data suggest that efficient cell adhesion–mediated drug-resistant mechanisms play a role in our BMME coculture model. As such, we hypothesized that mitochondrial transfer might be involved. We first developed a fluorescent approach using the MitoTracker (MT) dye to track mitochondria exchange between stromal and leukemic compartment during coculture. We previously showed that some fluorescent dyes are not well retained by cells, and this leads to unspecific dye contamination of the microenvironment.15 We checked that nonspecific dye transfer did not occur by using nontoxic doses of the MT probes after 1 hour of coculture (supplemental Figure 1A). However, after 5 hours, MT fluorescence transferred from MT-loaded MS-5 to the unstained leukemic cells with fluorescence gradually increased in a time-dependent manner from 6 to 72 hours as seen by FCM (Figure 1C) or confocal imaging (supplemental Figure 1B). MT uptake was repeatedly increased for the adherent fraction over the nonadherent one. Preventing direct cell-to-cell contact during coculture abrogated the MT transfer, thus excluding dye diffusion from stromal cells as the underlying mechanism (Figure 1D). Interestingly, the MitoTracker fluorescence exchange was unidirectional because different MT-loaded AML cell lines did not transfer their fluorescence to MS-5 (supplemental Figure 1C). Both mitochondrial potential sensitive (MitoTracker Red) and insensitive (MitoTracker Green) fluorescent probes were transferred from MS-5 to leukemic cells, suggesting an exchange of functional mitochondria rather than just mitochondrial debris. To confirm this, an mCherry-tagged version of the OMI/high temperature requirement serine protease A2 (HTRA2) was stably expressed in MS-5 cells. OMI/HTRA2, a marker of intact polarized mitochondria, is rapidly degraded following mitochondrial outer membrane permeabilization (MOMP).16 We confirmed a decrease in MS-5 OMI/HTRA2-mCherry cells fluorescence during an STS-induced MOMP (Figure 1E). After coculture, the mCherry fluorescence initially associated with MS-5 cells appeared in a subset of leukemic eGFP-positive cells analyzed by fluorescence-activated cell sorting (FACS; Figure 1F). Furthermore, the mCherry fluorescence incorporation could be alleviated in different AML cell lines by treatment with STS, suggesting that engulfed mitochondria under control conditions were intact (Figure 1G).
AML cells adherent to bone marrow mesenchymal cells have a survival selective advantage during in vitro cytarabine treatment associated with exogenous mitochondrial marker intake. (A) Pie charts showing the grand mean percentage determined from independent experiments of live adherent (A) and nonadherent cells (NA) for AML cell lines (HL60, Kasumi-1, KG-1, MOLM-14, NB4, SKM-1, THP1, and U937) or primary samples (n = 3) in CT and ARA conditions. (B) A or NA fractions of the specified AML cell lines were collected separately after 72 hours of coculture with MS-5 with or without ARA at 3 µM. Data represent the percentage of viable (Annexin-V−, DAPI−) AML cells (SSC-low, GFP+, SCA-1-PE−) for A and NA fractions in the untreated (CT) and ARA condition. n = 3 per cell line. **P < .01 by ANOVA. (C) (Left) Representative example of FCM analysis of MitoTracker (MT) uptake by Kasumi-1 AML cells (SSC-low, GFP+) cocultured for 0, 6, or 72 hours with MitoTracker Red–loaded MS-5 cells. (Middle) Gray dashed line represents the MT fluorescence intensity of MS-5 cells (SSC-high, GFP−). Solid lines represent the MT fluorescence intensity of Kasumi-1 cells at 0 hours (black) or after 6 (pink) or 72 hours (red) of coculture. (Right) MT fluorescence intensity for A (red) or NA (blue) Kasumi-1 cells. (D) eGFP-AML cell lines were cocultured with MitoTracker Red–loaded MS-5 cells in contact or in Transwell plate noncontact coculture for 72 hours. Data show the MitroTracker red grand mean of median fluorescence intensity (MFI; n = 3 per cell line) for the GFP+ gated cells. NSP > .05, ***P < .001, by ANOVA. (E) MS-5 stably expressing OMI/HTRA2-mCherry was treated with 0.3 or 1 µM STS for 18 hours. Data show mCherry fluorescence intensity (upper) or the mitochondrial membrane potential using TMRE (lower) of MS-5 OMI/HTRA2-mCherry cells in untreated (CT; red lines) and STS conditions (black dashed or dotted lines). (F) Representative FACS plots showing the mCherry and GFP fluorescence of isolated MS-5 OMI/HTRA2-mCherry (top), isolated MOLM-14 GFP (middle), and coculture (bottom). Red gate shows the mCherry fluorescence apparition for the MOLM-14 GFP+ AML cells after 72 hours of coculture. (G) Column scatter plot shows the percentage GFP+mCherry+ live cells in the GFP+ gated population of the specified AML cell lines (n = 3 per cell line) after 24 hours of coculture with MS-5 OMI/HTRA2-mCherry with the indicated dose of STS normalized to control condition.
AML cells adherent to bone marrow mesenchymal cells have a survival selective advantage during in vitro cytarabine treatment associated with exogenous mitochondrial marker intake. (A) Pie charts showing the grand mean percentage determined from independent experiments of live adherent (A) and nonadherent cells (NA) for AML cell lines (HL60, Kasumi-1, KG-1, MOLM-14, NB4, SKM-1, THP1, and U937) or primary samples (n = 3) in CT and ARA conditions. (B) A or NA fractions of the specified AML cell lines were collected separately after 72 hours of coculture with MS-5 with or without ARA at 3 µM. Data represent the percentage of viable (Annexin-V−, DAPI−) AML cells (SSC-low, GFP+, SCA-1-PE−) for A and NA fractions in the untreated (CT) and ARA condition. n = 3 per cell line. **P < .01 by ANOVA. (C) (Left) Representative example of FCM analysis of MitoTracker (MT) uptake by Kasumi-1 AML cells (SSC-low, GFP+) cocultured for 0, 6, or 72 hours with MitoTracker Red–loaded MS-5 cells. (Middle) Gray dashed line represents the MT fluorescence intensity of MS-5 cells (SSC-high, GFP−). Solid lines represent the MT fluorescence intensity of Kasumi-1 cells at 0 hours (black) or after 6 (pink) or 72 hours (red) of coculture. (Right) MT fluorescence intensity for A (red) or NA (blue) Kasumi-1 cells. (D) eGFP-AML cell lines were cocultured with MitoTracker Red–loaded MS-5 cells in contact or in Transwell plate noncontact coculture for 72 hours. Data show the MitroTracker red grand mean of median fluorescence intensity (MFI; n = 3 per cell line) for the GFP+ gated cells. NSP > .05, ***P < .001, by ANOVA. (E) MS-5 stably expressing OMI/HTRA2-mCherry was treated with 0.3 or 1 µM STS for 18 hours. Data show mCherry fluorescence intensity (upper) or the mitochondrial membrane potential using TMRE (lower) of MS-5 OMI/HTRA2-mCherry cells in untreated (CT; red lines) and STS conditions (black dashed or dotted lines). (F) Representative FACS plots showing the mCherry and GFP fluorescence of isolated MS-5 OMI/HTRA2-mCherry (top), isolated MOLM-14 GFP (middle), and coculture (bottom). Red gate shows the mCherry fluorescence apparition for the MOLM-14 GFP+ AML cells after 72 hours of coculture. (G) Column scatter plot shows the percentage GFP+mCherry+ live cells in the GFP+ gated population of the specified AML cell lines (n = 3 per cell line) after 24 hours of coculture with MS-5 OMI/HTRA2-mCherry with the indicated dose of STS normalized to control condition.
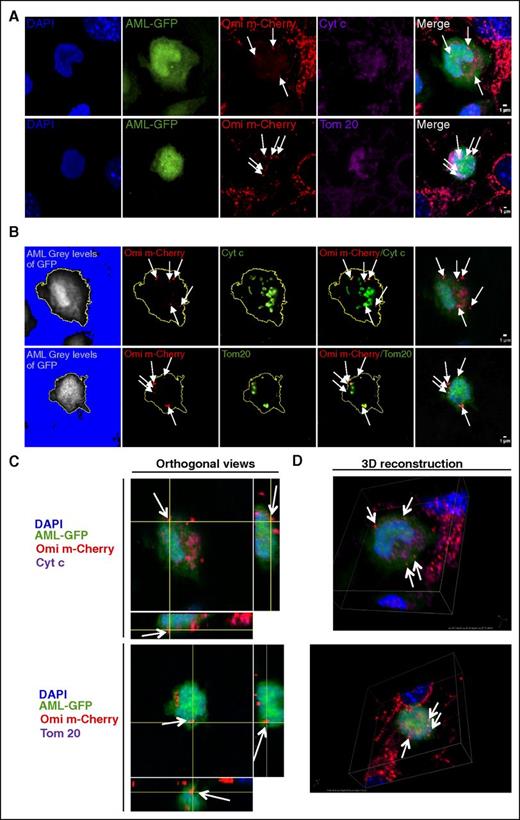
Functional mitochondria enter into AML cells
Because OMI/HTRA2 shuttles between different compartments such as the endoplasmic reticulum or the nucleus17,18 we asked whether our observation could be related to the capture of OMI/HTRA2-mCherry independently of the organelle targeted. Confocal imaging of immunofluorescently labeled cells revealed the mCherry foci seen in AML colocalized with 2 specific-mitochondria proteins, cytochrome C and anti-Tom20 (Figure 2A), demonstrating the transfer of intact mitochondria from MS-5 into AML cells. Specific slices (Figure 2B) and orthogonal views (Figure 2C) and 3D reconstruction (Figure 2D) further demonstrated that mitochondrial OMI/HTRA2-mCherry fluorescence was internalized in leukemic cells. Using nucleoid staining, we could measure a mean of 931 ± 94 and 124 ± 16 mtDNA nucleoids in MS-5 and AML cells, respectively (supplemental Figure 2A-C). Of note, we quantified a mean of 470 ± 134 mCherry spot signals per MS-5-Omi-mCherry, demonstrating that 50% of mitochondria express the fluorescent protein. Therefore, evaluation of the level of transferred mitochondria using the MS-5-Omi-mCherry niche should be corrected by a factor of 2. Using quantitative polymerase chain reaction (qPCR), we evaluated that MS-5 has n = 1631 copy of mtDNA giving a mtDNA number/nucleoid number ratio of 1.75, which is in agreement with the literature and could measure 29.7 murine mtDNA that represents 16.9 nucleoids per cell in sorted AML cells after coculture against 0.03 murine mtDNA per cell in input AML from suspension culture, which corresponds to the nonspecific background of the method (supplemental Figure 2D). As mitochondria contains an average of 1 to 3 nucleoids, we could evaluate that 5.6 to 16.9 mitochondria could be transferred from MS-5 into AML cells, which could represent at most an increase of 13.6% in the mitochondrial mass (mean transferred nucleoids = 16.9 vs mean total nucleoid = 124). Besides, we could see the preferential detection of 6 murine mitochondrially encoded mRNA (mt-Co1, mt-Co2, mt-Co3, mt-Atp6, mt-Atp8, and mt-Cytb) in human cells compared with 2 mouse nuclear mRNA (36b4, Hprt) by qPCR in Kasumi-1–sorted cells after coculture with MS-5 (supplemental Figure 3A-B). This specific detection of mitochondrial mRNA was confirmed in 3 other AML cell lines for which murine nuclear encoded mRNA Rplp0 could not be detected, whereas murine mt-Co2 was detected in human cells only after coculture (supplemental Figure 3C). The mt-Co2 sequence was not amplified in AML cells cultured in MS-5–conditioned medium (supplemental Figure 3D). Finally, a 1-kbp fragment of the murine mitochondrial genome was amplified in leukemic-sorted cells after coculture, confirming confocal results of the presence of murine genomic material in human cells and suggesting a functional heteroplasmy (supplemental Figure 3E). Overall, all these results demonstrate a cell-to-cell contact-dependent mitochondrial transfer from stromal to leukemic cells.
Functional mitochondria enter into AML cells. Confocal microscopy images of MS-5-Omi-mCherry cells cocultured with U937-GFP cells. Recorded fluorescence is indicated in the top left angle. (A) Representative cells are shown in Z-stack overlay mode. Mitochondria were evidenced using either anti-cytochrome C or anti-Tom20 antibodies. (B) Specific stack analysis. AML cell limits (yellow line) were defined by subtracting the background with ImageJ software. Single and double staining are displayed. (C) Orthogonal views and (D) 3D reconstruction images of Omi/cytochrome C and Omi/Tom20 stainings. All images: 60× enlargement; scale bar, 1 µm.
Functional mitochondria enter into AML cells. Confocal microscopy images of MS-5-Omi-mCherry cells cocultured with U937-GFP cells. Recorded fluorescence is indicated in the top left angle. (A) Representative cells are shown in Z-stack overlay mode. Mitochondria were evidenced using either anti-cytochrome C or anti-Tom20 antibodies. (B) Specific stack analysis. AML cell limits (yellow line) were defined by subtracting the background with ImageJ software. Single and double staining are displayed. (C) Orthogonal views and (D) 3D reconstruction images of Omi/cytochrome C and Omi/Tom20 stainings. All images: 60× enlargement; scale bar, 1 µm.
Mitochondrial uptake is enhanced in primary AML samples compared with normal human cord blood CD34+ cells
Using the OMI/HTRA2-mCherry fluorescence uptake, we observed that AML patient samples displayed the highest proportion of cells incorporating OMI/HTRA2-mCherry compared with normal cord blood mononuclear cells or even AML cell lines (Figure 3A). Leukemic CD34+ myeloblasts also displayed a mean 2.6 times higher fluorescence uptake of the MT probe (n = 14 samples) compared with normal cord blood mononuclear CD34+ (n = 9 samples) cells (Figure 3C), confirming that mitochondrial transfer is an enhanced mechanism for leukemic myeloblasts. Interestingly, both CD3+ T cells from leukemic and normal samples appeared unable to receive MT fluorescence from MS-5 (Figure 3B-C), suggesting that not all hematopoietic cells are recipients for mitochondrial transfer. We verified that different bone marrow–derived stromal cells such as human primary mesenchymal stem cells or human stromal cell line HS-5 could evenly transfer MT fluorescence to AML cells (supplemental Figure 4).
Mitochondria uptake is an enhanced phenomenon for primary AML sample compared with normal CB CD34+ cells and is increased in vitro and in vivo under cytarabine treatment. (A-C) Exogenous mitochondrial incorporation occurs to a greater extent for primary AML blasts compared with normal lymphoid or CB cells. (A) AML cell lines (green bars, n = 4) or AML primary (red bars, n = 4) or CB mononuclear samples (blue bars, n = 3) were cocultured with MS-5 control or MS-5 OMI/HTRA2-mCherry for 72 hours. Data show the absolute percent of mCherry-positive human cells determined by FCM. (B) AML primary or CB mononuclear samples were cultured for 72 hours with MitoTracker Green loaded MS-5. (Upper) Gating strategy for monitoring the MitoTracker green fluorescence intensity for 1 AML sample in the human live (hCD45+SCA-1−Annexin-V−DAPI−) myeloblasts (hCD34+hCD3−; red, blasts) and lymphoid (hCD34−hCD3+; green, lympho) populations. (Lower) Representative histograms plots for 1 AML patient and 1 CB. Gray dashed line represents the MitoTracker fluorescence intensity of MS-5 cells (FSC-high, SCA-1+). (C) MitoTracker MFI in the human live blasts and lymphoid populations determined for 14 AML and 9 CB primary samples. Paired t test was applied for blasts vs lympho compartments for intrasample comparison. A Mann-Whitney t test was applied for AML vs CB comparison. (D) ARA treatment increases AML uptake of functional mitochondria. AML#5957 cells (left) or AML#C3-1 cells (right) were cocultured with MitoTracker Green (MT)–loaded MS-5 or MS-5 OMI/HTRA2-mCherry cells in triplicate wells for 72 hours with the indicated dose of ARA. For each sample, data show the mean percentage (±standard deviation [SD]) of human live cell count and the mean ± SD MitroTracker MFI or the mean percentage ± SD of mCherry+ normalized to control condition for the hCD45+SCA-1−hCD34+Annexin-V−DAPI− AML cells. (E) Primary AML samples or MOLM-14 cells were injected in NSG mice. Recipient mice for MOLM-14 were treated at week 2 after injection with ARA 60 mg/kg per day over 5 days. Representative FACS analysis of hCD45 and CD33 expression before and after cell sorting of mice bone marrow at week 12 after injection of AML#C3-1. (F) Gels showing amplified DNA sequences from 4 primary AML isolated or sorted after in vivo engraftment and from isolated NSG mice bone marrow cells; hu GAPDH, human glyceraldehyde-3-phosphate dehydrogenase; mu Rplp0, mouse ribosomal protein, large, P0; mu mt-Co2, mouse mitochondrially encoded cytochrome c oxidase II. (G) Murine and human nuclear and mitochondrial gene expression profiles determined by quantitative real-time PCR from isolated NSG mice bone marrow cells, MOLM-14 isolated or sorted after in vivo engraftment from untreated (untr) or ARA-treated mice (n = 3 mice per group). 36b4, mouse nuclear encoded 60S acidic ribosomal protein P0; murine or human mt-CO2, mitochondrial encoded cytochrome c oxidase II. ΔCt corresponds to the difference between Ct of the tested specimens and the Ct of isolated human or murine cells. NSP > .05, *P < .05, ***P < .001 determined by ANOVA.
Mitochondria uptake is an enhanced phenomenon for primary AML sample compared with normal CB CD34+ cells and is increased in vitro and in vivo under cytarabine treatment. (A-C) Exogenous mitochondrial incorporation occurs to a greater extent for primary AML blasts compared with normal lymphoid or CB cells. (A) AML cell lines (green bars, n = 4) or AML primary (red bars, n = 4) or CB mononuclear samples (blue bars, n = 3) were cocultured with MS-5 control or MS-5 OMI/HTRA2-mCherry for 72 hours. Data show the absolute percent of mCherry-positive human cells determined by FCM. (B) AML primary or CB mononuclear samples were cultured for 72 hours with MitoTracker Green loaded MS-5. (Upper) Gating strategy for monitoring the MitoTracker green fluorescence intensity for 1 AML sample in the human live (hCD45+SCA-1−Annexin-V−DAPI−) myeloblasts (hCD34+hCD3−; red, blasts) and lymphoid (hCD34−hCD3+; green, lympho) populations. (Lower) Representative histograms plots for 1 AML patient and 1 CB. Gray dashed line represents the MitoTracker fluorescence intensity of MS-5 cells (FSC-high, SCA-1+). (C) MitoTracker MFI in the human live blasts and lymphoid populations determined for 14 AML and 9 CB primary samples. Paired t test was applied for blasts vs lympho compartments for intrasample comparison. A Mann-Whitney t test was applied for AML vs CB comparison. (D) ARA treatment increases AML uptake of functional mitochondria. AML#5957 cells (left) or AML#C3-1 cells (right) were cocultured with MitoTracker Green (MT)–loaded MS-5 or MS-5 OMI/HTRA2-mCherry cells in triplicate wells for 72 hours with the indicated dose of ARA. For each sample, data show the mean percentage (±standard deviation [SD]) of human live cell count and the mean ± SD MitroTracker MFI or the mean percentage ± SD of mCherry+ normalized to control condition for the hCD45+SCA-1−hCD34+Annexin-V−DAPI− AML cells. (E) Primary AML samples or MOLM-14 cells were injected in NSG mice. Recipient mice for MOLM-14 were treated at week 2 after injection with ARA 60 mg/kg per day over 5 days. Representative FACS analysis of hCD45 and CD33 expression before and after cell sorting of mice bone marrow at week 12 after injection of AML#C3-1. (F) Gels showing amplified DNA sequences from 4 primary AML isolated or sorted after in vivo engraftment and from isolated NSG mice bone marrow cells; hu GAPDH, human glyceraldehyde-3-phosphate dehydrogenase; mu Rplp0, mouse ribosomal protein, large, P0; mu mt-Co2, mouse mitochondrially encoded cytochrome c oxidase II. (G) Murine and human nuclear and mitochondrial gene expression profiles determined by quantitative real-time PCR from isolated NSG mice bone marrow cells, MOLM-14 isolated or sorted after in vivo engraftment from untreated (untr) or ARA-treated mice (n = 3 mice per group). 36b4, mouse nuclear encoded 60S acidic ribosomal protein P0; murine or human mt-CO2, mitochondrial encoded cytochrome c oxidase II. ΔCt corresponds to the difference between Ct of the tested specimens and the Ct of isolated human or murine cells. NSP > .05, *P < .05, ***P < .001 determined by ANOVA.
Mitochondrial transfer occurs in the bone marrow microenvironment and increases during in vivo chemotherapy
Treatment with cytarabine (ARA) strongly decreased viable cell counts but stimulated MT and OMI/HTRA2-mCherry transfer from the stroma to AML samples (Figure 3D). To further examine whether the mitochondria transfer observed in vitro could also occur in vivo, 4 primary AML samples were injected into immunodeficient NSG mice. Twelve weeks after, mice BM were collected and human AML cells were purified (Figure 3E). As seen in vitro, the murine Co2 RNA encoded by the murine mitochondrial DNA was PCR amplified in 4 sorted patients cells, whereas murine nuclear encoded Rlp0 could not be detected (Figure 3F). Moreover, such amplification was not seen from AML cells sorted few minutes after being mixed with NSG mice BM cells, excluding a sorted purity artifact (supplemental Figure 5). Eventually we explored the chemotherapy impact in vivo (Figure 3E). Two weeks after establishing MOLM-14 in NSG mice, 1 group of animals was treated with a known effective ARA regimen (30 mg/kg for 5 days), and human AML cells were then retrieved by cell sorting. The mouse nuclear encoded 36b4 was not detectable by qPCR in either MOLM-14 cells at input or in engrafted cells from untreated or ARA groups. On the other hand, mouse mitochondrial encoded mt-Co2 appeared in engrafted cells and was significantly increased in the ARA-treated group (Figure 3G). We did not observe any variations in the level of human mt-CO2 transcript before and after xenotransplantation. It can be concluded that the mitochondrial uptake by leukemic cells occurs physiologically in the BMME and is enhanced in vivo during chemotherapy.
Antineoplastic agent differentially stimulates the endocytosis dependent mitochondrial transfer
We questioned whether the endocytosis machinery could be involved in this organelle exchange. It appeared that endocytosis inhibitors dansylcadaverine or chlorepromazine could reduce the OMI/HTRA2-mCherry uptake of AML cells (Figure 4A) without interfering with the fluorescence level of MS-5 cells (Figure 4B). This was also observed for 1 primary AML (Figure 4C). Because the tested AML cell lines had proven to be capable of differentiation into macrophage-like cells, we wondered if our observations might be related to a macrophage activity. However, coculture environment did not modify mature monocytes/macrophages marker CD14 in contrast to PMA treatment (supplemental Figure 6A). Regarding primary samples, no differences in the proportion of CD14+CD11b+ monocytes were found between normal and AML samples before or after coculture. Beyond that, the monocytes were not maintained during the coculture (supplemental Figure 6B). These results rather ruled out a phagocytic artifact and support the engulfment of intact mitochondria by AML cells through an endocytic pathway. We continued to investigate how different clinically used chemotherapy would influence this organelle transfer. Cocultures were treated in 4 ways: with ARA, with the topoisomerase II inhibitor etoposide (ETO), with doxorubicin (DOXO), or with the microtubule-disrupting agent vincristine (VINCRI). Although those treatments were all highly efficient in killing leukemic cells, <10% of the cells of the 5 AML cell lines survived compared with the untreated condition (Figure 4D) and are capable of reinitiating cultures. ARA ETO and DOXO but not VINCRI triggered a significant increase of MT incorporation in AML (Figure 4E). MS-5-Omi-mCherry cells seeded at a low density developed many membrane protrusions that clearly contained labeled mitochondria, in particular at the levels of bulges (supplemental Figure 7A). VINCRI treatment interfered with the formation of the protrusions and mitochondria appear more localized to the central body of the cells (supplemental Figure 7B). This suggested the requirement of a dynamic cytoskeleton for the exchange to occur. The stimulating effect of ARA on the mitochondrial transfer was confirmed using the OMI/HTRA2-mCherry uptake parameter (Figure 4F). Interestingly, ARA effect was also seen when AML cells were pretreated with ARA for 24 hours before initiating the coculture, whereas no effect was observed when MT-loaded MS-5 cells were pretreated alone with ARA (Figure 4G). Macrophages were also capable to intake MT fluorescence. However, this was not modified by ARA, nor could ARA change the expression of the mature monocyte/macrophage CD14 marker on AML cells (supplemental Figure 6C). This excludes a phagocytic mechanism. These results show that some chemotherapeutic agents trigger a mitochondria transfer at the leukemic cells level.
Endocytosis-dependent mitochondrial transfer is differentially stimulated by antineoplastic agents. (A) Percentage of GFP+mCherry+ live cells in the GFP+ gated population of the specified AML cell lines after 72 hours of coculture with MS-5 OMI/HTRA2-mCherry with the indicated dose of endocytosis inhibitors dansylcadaverine (DanzylCad) or chlorepromazine (ChlorPZ), normalized to control condition. (B) Data show mCherry fluorescence intensity of MS-5 OMI/HTRA2-mCherry cells treated with the indicated dose of DanzylCad or ChlorPZ for 72 hours normalized to control untreated condition. No significant differences were observed (NS). (C) Mean percentage ± SD of mCherry+ normalized to control condition of the AML#C3-1 cells after 72 hours of coculture with MS-5 OMI/HTRA2-mCherry with the indicated dose of endocytosis inhibitors DanzylCad or ChlorPZ. (D-G) HL-60, Kasumi-1, MOLM-14, NB-4, or U937 GFP+ AML cells were cocultured for 72 hours with (D,E,G) MitoTracker Red–loaded MS-5 or (F) MS-5 OMI/HTRA2-mCherry. Cocultures were treated with cytarabine (ARA) 3 µM or etoposide 3 µM (ETO), doxorubicin 0.3 µM (DOXO), or vincristine 1 nM (VINCRI). Grand mean (±standard error of the mean [SEM]) of mean response per cell line determined from 3 to 6 independent experiments. (D) Grand mean (±SEM) of viable AML cell counts normalized to control untreated condition (CT). (E) Data shown represents the grand mean (±SEM) of MitroTracker Red MFI for the leukemic GFP+ gated cells normalized to control condition. (F) Grand mean percentage of GFP+mCherry+ live cells in the GFP+ gated population normalized to control condition. ***P < .001 in a paired t test. (G) Cocultures were treated with cytarabine 3 µM (ARA) for 72 hours or prior to coculture MS-5 only (pre-ARA MS-5) or AML cells only (pre-ARA AML) were treated with ARA for 24 hours, and then treated cells were washed 2 times with PBS. Fresh medium was added, and cocultures were performed for 72 hours. Data shown represent the grand mean ± SEM MitroTracker Red MFI for the leukemic GFP+ gated cells normalized to control conditions. (A,B,D,E,G) NSP > .05, *P < .05, **P < .01, ***P < .001, determined by ANOVA.
Endocytosis-dependent mitochondrial transfer is differentially stimulated by antineoplastic agents. (A) Percentage of GFP+mCherry+ live cells in the GFP+ gated population of the specified AML cell lines after 72 hours of coculture with MS-5 OMI/HTRA2-mCherry with the indicated dose of endocytosis inhibitors dansylcadaverine (DanzylCad) or chlorepromazine (ChlorPZ), normalized to control condition. (B) Data show mCherry fluorescence intensity of MS-5 OMI/HTRA2-mCherry cells treated with the indicated dose of DanzylCad or ChlorPZ for 72 hours normalized to control untreated condition. No significant differences were observed (NS). (C) Mean percentage ± SD of mCherry+ normalized to control condition of the AML#C3-1 cells after 72 hours of coculture with MS-5 OMI/HTRA2-mCherry with the indicated dose of endocytosis inhibitors DanzylCad or ChlorPZ. (D-G) HL-60, Kasumi-1, MOLM-14, NB-4, or U937 GFP+ AML cells were cocultured for 72 hours with (D,E,G) MitoTracker Red–loaded MS-5 or (F) MS-5 OMI/HTRA2-mCherry. Cocultures were treated with cytarabine (ARA) 3 µM or etoposide 3 µM (ETO), doxorubicin 0.3 µM (DOXO), or vincristine 1 nM (VINCRI). Grand mean (±standard error of the mean [SEM]) of mean response per cell line determined from 3 to 6 independent experiments. (D) Grand mean (±SEM) of viable AML cell counts normalized to control untreated condition (CT). (E) Data shown represents the grand mean (±SEM) of MitroTracker Red MFI for the leukemic GFP+ gated cells normalized to control condition. (F) Grand mean percentage of GFP+mCherry+ live cells in the GFP+ gated population normalized to control condition. ***P < .001 in a paired t test. (G) Cocultures were treated with cytarabine 3 µM (ARA) for 72 hours or prior to coculture MS-5 only (pre-ARA MS-5) or AML cells only (pre-ARA AML) were treated with ARA for 24 hours, and then treated cells were washed 2 times with PBS. Fresh medium was added, and cocultures were performed for 72 hours. Data shown represent the grand mean ± SEM MitroTracker Red MFI for the leukemic GFP+ gated cells normalized to control conditions. (A,B,D,E,G) NSP > .05, *P < .05, **P < .01, ***P < .001, determined by ANOVA.
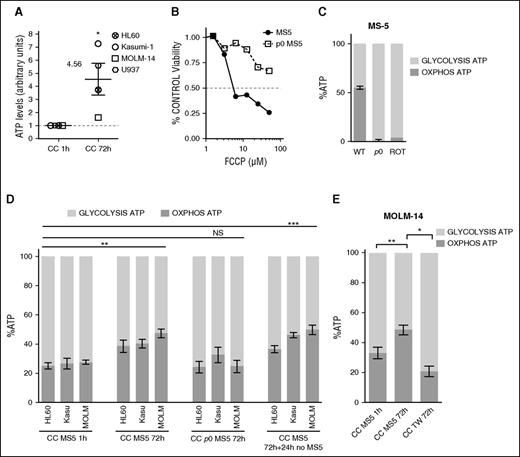
Stromal cells with functional mitochondria increase AML cells oxidative phosphorylation-derived adenosine triphosphate
We observed that AML cells cocultured for 72 hours with MS-5 displayed a 4.5-fold increase in total adenosine triphosphate (ATP; Figure 5A). To interrogate the role of stromal mitochondria in this phenomenon, we generated mitochondria-defective ρ0 MS-5 cells by long-term exposure to ethidium bromide. We confirmed the deficiency of mitochondria in ρ0 MS-5 cells by the loss of FCCP sensitivity (Figure 5B) and their inability to produce ATP from mitochondria. A similar defect in ATP production was observed after treatment of MS-5 with the respiratory chain disruptor rotenone (Figure 5C). HL-60, Kasumi-1, and MOLM-14 cell lines increased, by 13.4% to 19.8%, their oxydative phosphorylation (OXPHOS)-derived ATP production after 72 hours of coculture with MS-5 (respectively: 38.5% vs 25.1%; 40.3% vs 26.7%; and 47.4% vs 27.6%). Interestingly, this metabolic shift was maintained over 24 hours after AML cells were separated from the MS-5 cells. However, this did not happen in coculture with ρ0 MS-5 feeder cells (Figure 5D) or by preventing MS-5/AML contact (Figure 5E). Thus, stromal cells with functional mitochondria are capable of stimulating AML OXPHOS metabolism via cell contact.
AML cells in contact to stromal cells with functional mitochondria increase their oxidative phosphorylation derived ATP. (A) ATP levels normalized to cell number in specified AML cell line after 1 (control condition: CC 1h) or 72 hours of MS-5-AML cocultures (CC 72h) determined by ATP bioluminescence assay. (B-C) Mitochondrial deficient MS-5 (ρ0 MS-5) were generated by 12-week cultures using low-dose ethidium bromide. ρ0 MS-5 are insensitive to (B) FCCP and (C) have a greatly impaired ATP production by mitochondria. (C-E) Luciferase-expressing AML cells were treated with oligomycin A and iodoacetate to, respectively, block oxidative phosphorylation or glycolysis, or a combination of both inhibitors. The percentage of ATP from mitochondrial oxydative phosphorylation (OXPHOS ATP) or from glycolysis (GLYCOLYSIS ATP) is displayed. (C) Mitochondrial respiration was impaired by the respiratory chain poison rotenone (10 minutes, 10 µM). (D) Percentage of OXPHOS and GLYCOLYSIS ATP in HL-60, Kasumi-1, and MOLM-14 AML cell lines after 1- or 72-hour coculture with MS-5 (CC MS-5) or p0 MS-5 or recovered from CC MS-5 and cultured in suspension for an additional 24 hours. (E) MOLM-14 cells were culture in Transwell (0.4 µm) permeable supports to prevent cell contact. (A-C,E) The experiment is representative of 3 to 5 experiments and was performed in triplicate. (D) Mean result of 5 independent experiments (NSP > .05, *P < .1; **P < .01 ***P < .01 in a paired t test or by ANOVA).
AML cells in contact to stromal cells with functional mitochondria increase their oxidative phosphorylation derived ATP. (A) ATP levels normalized to cell number in specified AML cell line after 1 (control condition: CC 1h) or 72 hours of MS-5-AML cocultures (CC 72h) determined by ATP bioluminescence assay. (B-C) Mitochondrial deficient MS-5 (ρ0 MS-5) were generated by 12-week cultures using low-dose ethidium bromide. ρ0 MS-5 are insensitive to (B) FCCP and (C) have a greatly impaired ATP production by mitochondria. (C-E) Luciferase-expressing AML cells were treated with oligomycin A and iodoacetate to, respectively, block oxidative phosphorylation or glycolysis, or a combination of both inhibitors. The percentage of ATP from mitochondrial oxydative phosphorylation (OXPHOS ATP) or from glycolysis (GLYCOLYSIS ATP) is displayed. (C) Mitochondrial respiration was impaired by the respiratory chain poison rotenone (10 minutes, 10 µM). (D) Percentage of OXPHOS and GLYCOLYSIS ATP in HL-60, Kasumi-1, and MOLM-14 AML cell lines after 1- or 72-hour coculture with MS-5 (CC MS-5) or p0 MS-5 or recovered from CC MS-5 and cultured in suspension for an additional 24 hours. (E) MOLM-14 cells were culture in Transwell (0.4 µm) permeable supports to prevent cell contact. (A-C,E) The experiment is representative of 3 to 5 experiments and was performed in triplicate. (D) Mean result of 5 independent experiments (NSP > .05, *P < .1; **P < .01 ***P < .01 in a paired t test or by ANOVA).
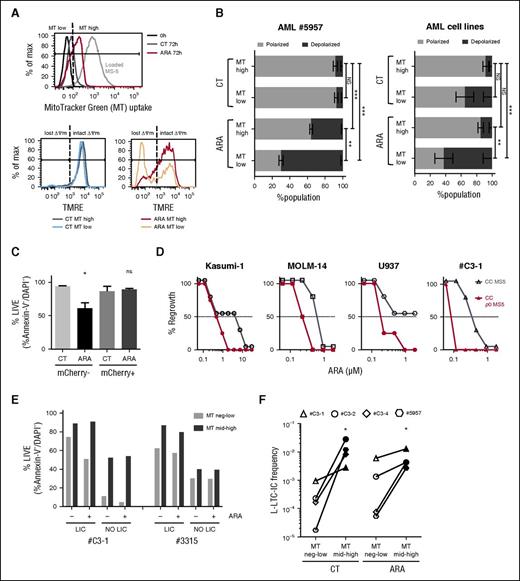
Mitochondria recipient LICs display resistance to cytarabine-induced apoptosis and have an improved replating potential
We wanted to examine whether the mitochondria uptake amplitude could influence the overall mitochondrial membrane potential (MMP) of the recipient cell. For this purpose, 1 primary AML sample and 5 AML cell lines were cocultured for 72 hours with MT-loaded MS-5, and MMP was monitored in 2 populations of leukemic cells with either low or high mitochondrial probe uptake (MT low and MT high; Figure 6A). In the untreated control condition, no MMP difference was seen within the MT low vs MT high populations for patient cells or AML cell lines. In the ARA condition, the MT high cell subset was capable of maintaining the MMP, whereas a significant MOMP (loss of ΔΨm) was quantified for MT low leukemic cells (Figure 6B). Survival benefit during chemotherapy was also observed for AML cells that uptake OMI/HTRA2-mCherry fluorescence (Figure 6C). Moreover, it appeared that 7.5- to 75-fold lower doses of ARA were sufficient to block 100% of leukemic regrowth after treatment of AML cocultured on MS-5 ρ0 stroma compared with mother stromal cells (Figure 6D). Knowing the LICs phenotype heterogeneity in AML,19-22 we prospectively functionally established for 3 samples the LICs phenotype (supplemental Figure 8A-B). Then the MT uptake was monitored for sorted LICs and non-LICs cocultured for 72 hours with MS-5 in the control condition or under ARA treatment. Fluorescence transfer was observed for both LIC and non-LIC populations (supplemental Figure 8C). We noticed that LICs always display a superior viability compared with the non-LIC subpopulation, whether untreated or in the ARA condition (Figure 6E; supplemental Figure 8D). MT uptake (MT high) was associated with a 36% mean increase in cell viability in both LICs and non-LICs in untreated or ARA conditions (P < .05 in a paired t test). Finally, we assessed, in 4 primary AML samples, the replating potential of MT low and MT high fractions sorted after coculture in the untreated or in ARA conditions. A higher frequency of the leukemic long-term culture initiating cells was calculated in MT high fractions in both untreated and ARA conditions compared with MT low fractions (Figure 6F). Taken together, these results indicate that mitochondrial donation from stromal cells is a new mechanism that contributes to the drug resistance of leukemic progenitors.
Mitochondria recipient AML cells and LICs display resistance to cytarabine-induced apoptosis and an enhanced replating potential. (A-B) FACS analysis of AML sample #5957 cells and AML cell lines cocultured for 72 hours with MitoTracker Green–loaded MS-5 with or without 3 µM cytarabine (ARA). (A) (Upper) FACS histogram analysis of MT uptake by AML #5957 cells (FSC-low, huCD45+CD34+DAPI−). The gray dashed line represents the MT fluorescence intensity of MS-5 cells (FSC-high, huCD45−). AML cells were gated for negative-low MT (MT low) and medium-high MT (MT high) populations as defined by the vertical dashed black line. (Lower) MMP using TMRE for MT low or MT high populations in the untreated (CT; lower left panel) and cytarabine conditions (ARA; lower right panel). Dashed black lines define TMRE fluorescence limit for AML cells with polarized (intact ΔΨm) and depolarized (lost ΔΨm) MMP. (B) Data shown represent the mean ± SEM percentage values of AML #5957 cells (left histogram) or for AML cell lines HL60, Kasumi-1, KG-1, THP-1, and U937 (right histogram) with polarized and depolarized MMP in the MT low and the MT high populations in CT and ARA conditions. NSP > .05, **P < .01, ***P < .001 by ANOVA. (C) HL-60, Kasumi-1, Molm-14, and U937 cells were cocultured for 72 hours with MS-5 OMI/HTRA2-mCherry with or without cytarabine 3 µM. Histogram shown represents the mean percentage of Annexin-V− and DAPI− in the mCherry− or mCherry+ cells of the GFP+ AML population. (D) Functional mitochondria in stromal cells is required to maintain regrowing potential after ARA treatment. The specified AML cells were cocultured with MS-5 or p0 MS-5 with ARA for 1 week. Then, cocultures were washed, medium was changed, and leukemic regrowth was monitored per well over 5 weeks. (E) T0-sorted LICs and non-LICs of AML samples #C3-1 and #3315 were cocultured with MT-loaded MS-5 for 72 hours with or without ARA 3 µM. Histograms represent the percentage values of Annexin-V− and DAPI− cells in the MT low or the MT high population of LIC and non-LIC in CT or ARA conditions. Refer to supplemental Figure 8A-B and to “Materials and methods” for LICs and non-LICs population definition. (F) Mitochondria recipient AML cells have a selective advantage for replating potential. AML #C3-1, #C3-2, #C3-4, and #5957 cells were cocultured for 72 hours with MT-loaded MS-5 with or without 3 µM cytarabine (ARA). MT low or MT high populations were sorted at 72 hours from CT or ARA conditions and replated in limiting dilution. Data represent leukemic long-term culture initiating cells frequencies determined at week 5 after replating. * P < .05 by paired t test.
Mitochondria recipient AML cells and LICs display resistance to cytarabine-induced apoptosis and an enhanced replating potential. (A-B) FACS analysis of AML sample #5957 cells and AML cell lines cocultured for 72 hours with MitoTracker Green–loaded MS-5 with or without 3 µM cytarabine (ARA). (A) (Upper) FACS histogram analysis of MT uptake by AML #5957 cells (FSC-low, huCD45+CD34+DAPI−). The gray dashed line represents the MT fluorescence intensity of MS-5 cells (FSC-high, huCD45−). AML cells were gated for negative-low MT (MT low) and medium-high MT (MT high) populations as defined by the vertical dashed black line. (Lower) MMP using TMRE for MT low or MT high populations in the untreated (CT; lower left panel) and cytarabine conditions (ARA; lower right panel). Dashed black lines define TMRE fluorescence limit for AML cells with polarized (intact ΔΨm) and depolarized (lost ΔΨm) MMP. (B) Data shown represent the mean ± SEM percentage values of AML #5957 cells (left histogram) or for AML cell lines HL60, Kasumi-1, KG-1, THP-1, and U937 (right histogram) with polarized and depolarized MMP in the MT low and the MT high populations in CT and ARA conditions. NSP > .05, **P < .01, ***P < .001 by ANOVA. (C) HL-60, Kasumi-1, Molm-14, and U937 cells were cocultured for 72 hours with MS-5 OMI/HTRA2-mCherry with or without cytarabine 3 µM. Histogram shown represents the mean percentage of Annexin-V− and DAPI− in the mCherry− or mCherry+ cells of the GFP+ AML population. (D) Functional mitochondria in stromal cells is required to maintain regrowing potential after ARA treatment. The specified AML cells were cocultured with MS-5 or p0 MS-5 with ARA for 1 week. Then, cocultures were washed, medium was changed, and leukemic regrowth was monitored per well over 5 weeks. (E) T0-sorted LICs and non-LICs of AML samples #C3-1 and #3315 were cocultured with MT-loaded MS-5 for 72 hours with or without ARA 3 µM. Histograms represent the percentage values of Annexin-V− and DAPI− cells in the MT low or the MT high population of LIC and non-LIC in CT or ARA conditions. Refer to supplemental Figure 8A-B and to “Materials and methods” for LICs and non-LICs population definition. (F) Mitochondria recipient AML cells have a selective advantage for replating potential. AML #C3-1, #C3-2, #C3-4, and #5957 cells were cocultured for 72 hours with MT-loaded MS-5 with or without 3 µM cytarabine (ARA). MT low or MT high populations were sorted at 72 hours from CT or ARA conditions and replated in limiting dilution. Data represent leukemic long-term culture initiating cells frequencies determined at week 5 after replating. * P < .05 by paired t test.
Discussion
In this study, we used a niche-like coculture system for AML cells to analyze the mechanisms operating cell adhesion–mediated drug resistance. We observed that an in vitro exposure to chemotherapy selects a subpopulation of leukemic cells engaged in a physical contact with stromal cells. This interaction resulted in the uptake of intact mitochondria by leukemic cells as demonstrated by the transmission of mCherry OMI/HTRA2 and colabeling with antibodies specific for the Tom20 and cytochrome C mitochondrial proteins. By confocal microscopy and species-specific qPCR, we evaluated that AML cells could receive up to 16 mitochondria representing 14% of their total mitochondrial mass (mean, 124 mitochondria per AML cell). The transfer of the organelles was associated with a 4.5-fold increase in total ATP content and a mean 50% increase in ATP produced by mitochondria. Our data are reminiscent of a previous observation10 describing the delivery of functional mitochondria by bone marrow mesenchymal stem cells to alveolar epithelial cells inducing a restoration of ATP levels in the injured recipient cells.10 Here we show that recipient AML cells can maintain their mitochondrial transmembrane potential under chemotherapy and presented a higher viability compared with nonrecipient leukemic cells. LICs are crucial to the pathophysiology of AML, in particular during resistance to treatments and disease relapse. We evidenced that LIC uptaked mitochondria from their niche, an event associated with a better survival and an increased regrowing potential. The magnitude of the mitochondrial probes uptake correlated with a better viability, suggesting that quantity of transferred mitochondrial is important. The loss of benefit observed in coculture with deficient mitochondria ρ0 MS-5 cells also strongly suggests the requirement of functionally active organelle to fully observe the cytoprotective role of the stroma. Furthermore, the metabolic change could last several hours even after disrupting the contact with the stromal cells. Several reports suggest that there are complex functional interactions between cancer cells and their cellular microenvironment with metabolic consequences for all participants. Through the Warburg effect, most cancer cells shift their ATP production from oxidative phosphorylation toward glycolysis. Although less efficient for ATP synthesis, this reprogrammation favors the synthesis of metabolic intermediates to support the production of new biomass/cancer cells.23 Also, cancer cells, through a “reverse Warburg effect,” induce stromal cells to produce oncometabolites24 to fuel their metabolism. The mitochondrial transfer we describe here, strongly associated with stressful conditions for leukemic cells, could represent an additional metabolic slavery of niche cells by leukemic cells compensating the uncoupling of leukemic mitochondria shown to be induced by mesenchymal stem cells under normoxic conditions.9
Both mitochondrial transfer and increased OX-PHOS ATP levels were shown to require cell contacts. Recent studies analyzing mitochondria exchange in other cellular systems identified some molecular players such as the gap junction protein connexin 4310 or the mitochondrial ρ-GTPase Miro1.25 We show in our model that mitochondrial uptake could undergo through endocytosis machinery in a process that remains to be better defined at a molecular level. In subconfluent cultures, we observed that MS-5 cells produce many vincristine-sensitive membrane protrusions a feature of their ability to search and find other cellular partners that could be the basis of mitochondria transfer. Our data also established that chemotherapy creates “Mayday signals” demanding for mitochondria transfer at the recipient cell level. Further investigations to molecularly define the signaling events involved in this demand may be of important clinical importance as targeting this “rescuing mechanism” would be an efficient way to improve standard chemotherapy to tackle chemoresistant AML cells. Eventually we observed that leukemic cells are more prone to mitochondrial transfer than normal CD34+ hematopoietic stem/progenitors or lymphoid CD3+ cells, thus hopping for a possible therapeutic window for a new adjuvant therapy targeting LICs to prevent clinical recurrence.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the patients who granted permission to use samples in research. The authors thank all personnel at the Institut Paoli-Calmettes Tumour Bank for access to anonymized samples and clinical data; Sylvie Bannwarth, Véronique Paquis, and Mireille Cormont Jérome Gilleron and Christophe Trojani and Paul Boileau for providing helpful material and discussions; Annabelle Mouchotte for expertise and help in ATP measurements; the Animal Care Facility staffs of the Cancer Research Center of Marseille; and Andrew Filby and Milena Elek for critical reading of the manuscript.
The Mediterranean Centre for Molecular Medicine is supported by institutional grants from INSERM. E.G. is supported by a grant from the Fondation de France. T.P., R.C., L.P., Y.C., N.V., and C.C. are supported by grants from the Institut National du Cancer and the Direction générale de l’Offre de soins for the Comprehensive Cancer Center Provence Alpes Côte d’Azur (PACA)–west. This study was supported by a grant from the Cancéropôle PACA. The microscope used in this study was funded by Conseil Général Alpes-Maritimes (appel à projets santé) and Région PACA (appel à projets plateforme).
Authorship
Contribution: R.M., J.-F.P., and E.G. designed experiments, analyzed and interpreted data, and wrote the manuscript; V.I., Y.C., and J.-E.S. helped in conception of the study and designed the experiments; E.G., R.M., V.I., M.N., J.C., D.M., D.A., E.S., R.C., and L.P. performed research and analyzed data; T.P., Y.C., N.V., C.C., C.R., and J.-E.S. provided vital cells and patient materials and data; and J.-E.S. and V.I. edited the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Emmanuel Griessinger, Inflammation, Cancer & Cancer Stem Cells, INSERM U1065, C3M, Bâtiment Universitaire Archimed, 151 Route de Ginestière, BP 2 3194, 06204 Nice Cedex 3, France; e-mail: emmanuel.griessinger@unice.fr; or Jean-François Peyron, Inflammation, Cancer & Cancer Stem Cells, INSERM U1065, C3M, Bâtiment Universitaire Archimed, 151 Route de Ginestière, BP 2 3194, 06204 Nice Cedex 3, France; e-mail: jean-francois.peyron@unice.fr.
References
Author notes
J.-F.P. and E.G. contributed equally to this work.

![Figure 3. Mitochondria uptake is an enhanced phenomenon for primary AML sample compared with normal CB CD34+ cells and is increased in vitro and in vivo under cytarabine treatment. (A-C) Exogenous mitochondrial incorporation occurs to a greater extent for primary AML blasts compared with normal lymphoid or CB cells. (A) AML cell lines (green bars, n = 4) or AML primary (red bars, n = 4) or CB mononuclear samples (blue bars, n = 3) were cocultured with MS-5 control or MS-5 OMI/HTRA2-mCherry for 72 hours. Data show the absolute percent of mCherry-positive human cells determined by FCM. (B) AML primary or CB mononuclear samples were cultured for 72 hours with MitoTracker Green loaded MS-5. (Upper) Gating strategy for monitoring the MitoTracker green fluorescence intensity for 1 AML sample in the human live (hCD45+SCA-1−Annexin-V−DAPI−) myeloblasts (hCD34+hCD3−; red, blasts) and lymphoid (hCD34−hCD3+; green, lympho) populations. (Lower) Representative histograms plots for 1 AML patient and 1 CB. Gray dashed line represents the MitoTracker fluorescence intensity of MS-5 cells (FSC-high, SCA-1+). (C) MitoTracker MFI in the human live blasts and lymphoid populations determined for 14 AML and 9 CB primary samples. Paired t test was applied for blasts vs lympho compartments for intrasample comparison. A Mann-Whitney t test was applied for AML vs CB comparison. (D) ARA treatment increases AML uptake of functional mitochondria. AML#5957 cells (left) or AML#C3-1 cells (right) were cocultured with MitoTracker Green (MT)–loaded MS-5 or MS-5 OMI/HTRA2-mCherry cells in triplicate wells for 72 hours with the indicated dose of ARA. For each sample, data show the mean percentage (±standard deviation [SD]) of human live cell count and the mean ± SD MitroTracker MFI or the mean percentage ± SD of mCherry+ normalized to control condition for the hCD45+SCA-1−hCD34+Annexin-V−DAPI− AML cells. (E) Primary AML samples or MOLM-14 cells were injected in NSG mice. Recipient mice for MOLM-14 were treated at week 2 after injection with ARA 60 mg/kg per day over 5 days. Representative FACS analysis of hCD45 and CD33 expression before and after cell sorting of mice bone marrow at week 12 after injection of AML#C3-1. (F) Gels showing amplified DNA sequences from 4 primary AML isolated or sorted after in vivo engraftment and from isolated NSG mice bone marrow cells; hu GAPDH, human glyceraldehyde-3-phosphate dehydrogenase; mu Rplp0, mouse ribosomal protein, large, P0; mu mt-Co2, mouse mitochondrially encoded cytochrome c oxidase II. (G) Murine and human nuclear and mitochondrial gene expression profiles determined by quantitative real-time PCR from isolated NSG mice bone marrow cells, MOLM-14 isolated or sorted after in vivo engraftment from untreated (untr) or ARA-treated mice (n = 3 mice per group). 36b4, mouse nuclear encoded 60S acidic ribosomal protein P0; murine or human mt-CO2, mitochondrial encoded cytochrome c oxidase II. ΔCt corresponds to the difference between Ct of the tested specimens and the Ct of isolated human or murine cells. NSP > .05, *P < .05, ***P < .001 determined by ANOVA.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/128/2/10.1182_blood-2015-07-655860/4/m_253f3.jpeg?Expires=1769086907&Signature=DRZwUmBOawL6IXvFZyfshPCao6oKyXXmI1Ozt5WI9XX54Xj-FyQNISgF4B117c24-4ouKcxxabCbrQEgYXqF210ZDdtAnkIeJJ-K8TB~3h~K07z~o5OQsOJPQSSZ3qWA4AhLp3MLLB1Umbwg6eZOZE8Cav-XrEwE-PWrNgPXEWgKEUbNQvBdb~rDm1k6yuvWXBE39znwNSqkBlcDOgKRgryD9Mn9PhqRVCwBa0iBp-y1Ap1fkAaBfmq1zBjMBPcevOXRU2oDW6Z9VUuJXtJ~r~oJlDCduJJs7~9PUM6LBip5Z1sSWsaSiw4I1eID11BM9muMjeDcbGS~Ks0~kySM~Q__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 4. Endocytosis-dependent mitochondrial transfer is differentially stimulated by antineoplastic agents. (A) Percentage of GFP+mCherry+ live cells in the GFP+ gated population of the specified AML cell lines after 72 hours of coculture with MS-5 OMI/HTRA2-mCherry with the indicated dose of endocytosis inhibitors dansylcadaverine (DanzylCad) or chlorepromazine (ChlorPZ), normalized to control condition. (B) Data show mCherry fluorescence intensity of MS-5 OMI/HTRA2-mCherry cells treated with the indicated dose of DanzylCad or ChlorPZ for 72 hours normalized to control untreated condition. No significant differences were observed (NS). (C) Mean percentage ± SD of mCherry+ normalized to control condition of the AML#C3-1 cells after 72 hours of coculture with MS-5 OMI/HTRA2-mCherry with the indicated dose of endocytosis inhibitors DanzylCad or ChlorPZ. (D-G) HL-60, Kasumi-1, MOLM-14, NB-4, or U937 GFP+ AML cells were cocultured for 72 hours with (D,E,G) MitoTracker Red–loaded MS-5 or (F) MS-5 OMI/HTRA2-mCherry. Cocultures were treated with cytarabine (ARA) 3 µM or etoposide 3 µM (ETO), doxorubicin 0.3 µM (DOXO), or vincristine 1 nM (VINCRI). Grand mean (±standard error of the mean [SEM]) of mean response per cell line determined from 3 to 6 independent experiments. (D) Grand mean (±SEM) of viable AML cell counts normalized to control untreated condition (CT). (E) Data shown represents the grand mean (±SEM) of MitroTracker Red MFI for the leukemic GFP+ gated cells normalized to control condition. (F) Grand mean percentage of GFP+mCherry+ live cells in the GFP+ gated population normalized to control condition. ***P < .001 in a paired t test. (G) Cocultures were treated with cytarabine 3 µM (ARA) for 72 hours or prior to coculture MS-5 only (pre-ARA MS-5) or AML cells only (pre-ARA AML) were treated with ARA for 24 hours, and then treated cells were washed 2 times with PBS. Fresh medium was added, and cocultures were performed for 72 hours. Data shown represent the grand mean ± SEM MitroTracker Red MFI for the leukemic GFP+ gated cells normalized to control conditions. (A,B,D,E,G) NSP > .05, *P < .05, **P < .01, ***P < .001, determined by ANOVA.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/128/2/10.1182_blood-2015-07-655860/4/m_253f4.jpeg?Expires=1769086907&Signature=eiRgi09MSqWIXG0hRPwGtWvuXxexGszmwdRJ6-W2fvlvUrh3Scj6uptkzaCWCQYZLubms~JEUch4RyTz8JZQsKXPtVEYxElK9mRYHmPTOLzLkObQ5bRrPOk7aBWvZLraysnsjCfm2smYBYls8uUT7PuzF5rzPx-ZoXh29XXTuRxS3xRIbfIw-lslFYcsiqbKqzIKUZXvjCHylb6QFDfxg4aspzj55y6W7Qw~0z8F9RUXHqzRMs3u0Ybtq2MbK9dLZJJZmHr97GAHdajkh94fKdB8Fq68VfeJhm4l1sdq32A1n74awaHPSHPaFUVXabnEcWXhOJWX9M-r7V-i7IdJqQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)