Key Points
Anti-CD45 RIT may replace TBI and simplify BMT-preparative regimens.
Anti-CD45 RIT and haploidentical BMT, without TBI, prolongs survival in a murine leukemia model.
Abstract
Many patients with hematologic malignancies cannot tolerate hematopoietic cell transplantation (HCT), whereas others may not have a compatible human leukocyte antigen–matched donor. To overcome these limitations, we optimized a conditioning regimen employing anti-CD45 radioimmunotherapy (RIT) replacing total body irradiation (TBI) before haploidentical HCT in a murine model. Mice received 200 to 400 μCi 90Y-anti-CD45 antibody (30F11), with or without fludarabine (5 days starting day –8), with cyclophosphamide (CY; days –2 and +2) for graft-versus-host disease prophylaxis, and 1.5 × 107 haploidentical donor bone marrow cells (day 0). Haploidentical bone marrow transplantation (BMT) with 300 μCi 90Y-anti-CD45 RIT and CY, without TBI or fludarabine, led to mixed chimeras with 81.3 ± 10.6% mean donor origin CD8+ cells detected 1 month after BMT, and remained stable (85.5 ± 11% mean donor origin CD8+ cells) 6 months after haploidentical BMT. High chimerism levels were induced across multiple hematopoietic lineages 28 days after haploidentical BMT with 69.3 ± 14.1%, 75.6 ± 20.2%, and 88.5 ± 11.8% CD3+ T cells, B220+ B cells, and CD11b+ myeloid cells, respectively. Fifty percent of SJL leukemia-bearing mice treated with 400 μCi 90Y-DOTA-30F11, CY, and haploidentical BMT were cured and lived >200 days. Mice treated with 200 μCi 90Y-DOTA-30F11 had a median overall survival of 73 days, while untreated leukemic mice had a median overall survival of 34 days (P < .001, Mantel-Cox test). RIT-mediated haploidentical BMT without TBI may increase treatment options for aggressive hematologic malignancies.
Introduction
Allogeneic hematopoietic cell transplantation (HCT) is the only curative therapy for many patients with advanced acute myeloid leukemia (AML). Unfortunately, many patients relapse even after myeloablative HCT, and ablative conditioning regimens are associated with significant toxicities. It is therefore important to reduce the relapse risk without substantially increasing the toxicity of the HCT conditioning regimen for advanced leukemia patients. It also remains critical to extend the option of HCT to patients who do not have a readily available HLA-matched donor, especially patients from ethnic minority groups.1,2 The procurement of stem cells from unrelated donors requires a median of 4 months, during which time patients may relapse and/or lose options for, or develop complications precluding HCT.3 Furthermore, National Marrow Donor Program registry data suggest that potential minority donors, who are registered at lower numbers, are likely to have high attrition rates (60% vs 40% for Caucasian donors).4-6 Almost all patients have a related donor identical for 1 HLA haplotype (haploidentical), mismatched at HLA-A, -B, or -DR of the unshared haplotype. Given that all patients share 1 HLA haplotype with each biologic parent, child, and half of their siblings, an eligible HLA-haploidentical donor can be identified rapidly in nearly all cases, with 1 institution identifying an average of 2.7 HLA-haploidentical donors per patient.7
Early haploidentical bone marrow (BM) transplantation (BMT) trials for patients with relapsed/refractory myeloid malignancies were challenged by high-rates of graft-versus-host disease (GVHD), nonrelapse mortality (NRM), and relapse.8-12 Therefore, developing a safe and effective approach to HCT using HLA-haploidentical donors, in which the graft-versus-leukemia effect may be expected to be augmented, remains an important goal.11,13 Efforts to decrease relapse after HCT have largely focused on intensification of cytoreductive therapy, by increasing doses of total body irradiation (TBI) or chemotherapy. Randomized leukemia trials have shown that relapse can, in fact, be reduced by increasing the TBI dose.14,15 However, the NRM observed in these studies increased with the higher TBI doses, leading to no net improvement in overall survival.16
Two significant advances offer the potential to reduce relapse with minimal toxicity and improve HCT outcomes. First, radiolabeled monoclonal antibodies (Abs) can target high doses of radiation to hematolymphoid tissues with at least 2- to 3-fold more radiation delivered to BM, and at least 5-fold more to the spleen compared with normal organs.17-20 Second, by carefully manipulating both pre- and post-HCT immunosuppression, engraftment of allogeneic cells, including haploidentical BM, can be achieved reliably using low-dose preparative regimens. Specifically, a combination of cyclophosphamide (CY) administered before and after infusion of T cell–replete BM allografts from haploidentical donors has been shown to reliably facilitate engraftment.21-24 We have exploited these advancements to investigate the targeted delivery of radiation to CD45-expressing hematologic tissues using a 90Y-anti-CD45 radiolabeled Ab in lieu of TBI before haploidentical BMT. In addition, we sought to minimize toxicity associated with HCT by reducing chemotherapy requirements, while still allowing stable engraftment from haploidentical donor cells. We demonstrate here that 90Y-anti-CD45 radioimmunotherapy (RIT) combined with pre- and posttransplant CY in the absence of TBI or fludarabine (FLU) before haploidentical BMT can facilitate high levels of haploidentical BM engraftment and improve survival using a murine leukemia model.
Methods
Mice
Female B6SJLF1/J mice 8 to 12 weeks old possessing the H-2Db haplotype (Jackson Laboratories, Bar Harbor, ME) were used as recipient animals. Male CB6F1/J mice 8 to 16 weeks old, expressing the H-2Dd haplotype, (Jackson Laboratories), were used as haploidentical BM donors. Mice were housed at the Fred Hutchinson Cancer Research Center animal care facility (Seattle, WA) in a pathogen-free environment under protocols approved by the Fred Hutchinson Cancer Research Center Institutional Animal Care and Use Committee. After RIT administration, mice were placed on a diet containing Uniprim antibiotic (irradiated, 4100 ppm from Harlan Laboratories, Indianapolis, IN). Murine myeloid leukemia cells were produced by serial passage through SJL/J mice, stored in a cryogenic freezing medium, then thawed and prepared for injection as previously described.25
Antibodies, conjugation, and radiolabeling
30F11, a rat immunoglobulin G2b (IgG2b) Ab specific for murine CD45, was produced and assayed for biological function as previously described.25 Polyclonal rat IgG Ab (Sigma Aldrich, St. Louis, MO) was used as a negative control as previously described.26 1,4,7,10-tetraazacyclododecane-N,N′,N″,N′″-tetra-acetic acid (DOTA) conjugation and subsequent radiolabeling of Abs with 90Y (90Y; Perkin Elmer, Waltham MA), were performed as previously described.26 Radiolabeling efficiencies were >90% as determined by instant thin layer chromatography, and all labeled preparations were purified over a PD10 column (BioRad, Hercules CA). Radiolabeled DOTA-Ab conjugates had a final assayed radiochemical purity of >99%.
Haploidentical BMT and assessment of chimerism
Groups of 5 or 10 leukemia-free recipient B6SJLF1/J mice were treated either with or without 5 daily IV doses of FLU (100 mg/kg per day) starting on day –8 before BMT. Recipient mice were also treated with or without CY 200 mg/kg per day by intraperitoneal (IP) injections on days –2 and +227 and with or without 200 to 400 µCi of 90Y-labeled anti-murine CD45 Ab IV (100 μg/dose DOTA-30F11) on day –3 or –4. Recipient mice received 1.5 × 107 haploidentical CB6F1/J BM cells on day 0. Long bones from haploidentical donor mice were resected, trimmed of adherent tissues, and homogenized with a mortar and pestle. Cells were then washed 3 times in nonsupplemented RPMI medium, centrifuged at 1000 × g for 5 minutes and passed through a 40-µm Nylon Cell Strainer (BD Falcon, Franklin Lakes, NJ) with each wash. Cells were resuspended in phosphate-buffered saline, and 1.5 × 107 cells in 200 µL were slowly injected IV into recipient mice.
Peripheral blood (∼100 µL) was obtained from transplanted mice by phlebotomy from the retroorbital plexus to initially assess the percent of donor origin CD8+ cells (H-2Dd haplotype) from total CD8+ cells at day 28, then monthly for 6 months after BMT. Briefly, erythrocytes from 50 µL of peripheral blood from each mouse were lysed in a 96-well, round-bottom plate per the manufacturer’s protocol using PharmLyse Buffer from BD Pharmingen (San Diego, CA). Cells were then stained with CD8+-specific biotinylated rat anti-mouse CD8a (clone 53-6.7), followed by PerCP-streptavidin and/or phycoerythrin-R–conjugated mouse anti-mouse H-2Dd, all from BD Pharmingen. Stained cells were analyzed on a Guava EasyCyte Mini Flow Cytometer (Millipore, Billerica, MA) with a minimum of 150 000 events analyzed. Analysis of CD3+, B220+, and CD11b+ peripheral blood for multilineage chimerism studies using multiparametric flow cytometric (MFC) was processed as described previously. Blood was incubated with peridinin chlorophyll protein complex–conjugated rat anti-mouse CD45R/B220, phycoerythrin R–conjugated hamster anti-mouse CD3e, allophycocyanin-conjugated rat anti-mouse CD11b, and fluorescein isothiocyanate (FITC)-conjugated mouse anti-mouse H-2Dd (all from BD Pharmingen). Stained cells were analyzed on a BD Canto 2 flow cytometer (Becton Dickinson and Company, Franklin Lakes, NJ) with a goal of 100 000 events. A 1:1 mixture of peripheral blood from nontransplanted B6SJLF1/J and from donor CB6F1 mice was stained in a similar manner to define CD8+, CD3+, B220+, CD11b+, and H-2Dd threshold gating parameters. Chimerism data were expressed as the percentage of donor-derived CD8+, CD3+, B220+, or CD11b+ cells from total cells from the peripheral blood of recipient mice.
Anti-CD45 RIT and haploidentical BMT in a disseminated leukemia model
B6SJLF1/J mice received 1 × 105 SJL leukemia cells via tail vein injection on day –5 and were then injected with 0.67 nmol of DOTA-30F11 labeled with 200 or 400 µCi of 90Y, or injected with 0.67 nmol of DOTA-rat IgG labeled with 400 µCi of 90Y on day –3 or –4. In therapy experiments with leukemic mice, all transplanted mice were also given CY (200 mg/kg per day) on days –2 and +2, delivered IP, followed by 1.5 × 107 haploidentical BM cells IV on day 0. Mice were monitored daily for appearance, condition, and changes in body weight. Mice were euthanized if they became moribund or lost >30% of their initial body weight from either progressive leukemia or toxicity. Survival curves were plotted with GraphPad Prism (San Diego, CA) and compared using the Mantel-Cox test. Differences in groups were reported only when statistically significant and justified by Bonferroni-corrected threshold for multiple comparisons.
Results
Anti-CD45 RIT without TBI before haploidentical BMT
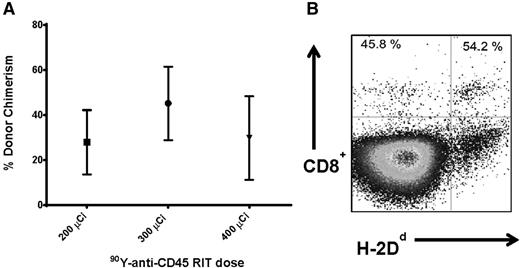
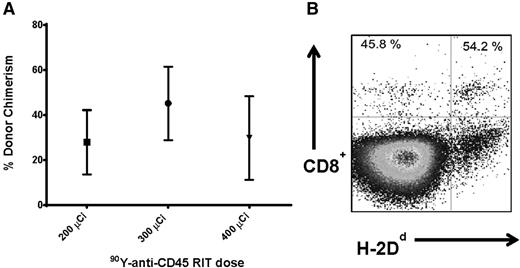
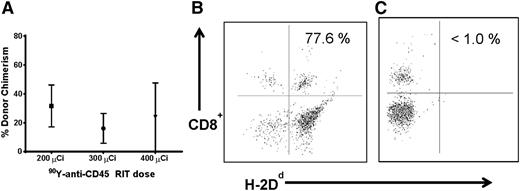
We first investigated whether targeted anti-CD45 RIT could eliminate the need for TBI before haploidentical BMT to minimize toxicity associated with external beam radiotherapy. Groups of 5 female B6SJL/F1 recipient mice (H-2Db haplotype) received 100 mg/kg per day FLU for 5 days before treatment with 200 to 400 μCi 90Y-DOTA-30F11 on day –3, followed by 1.5 × 107 haploidentical BM cells on day 0. Peripheral blood from transplanted recipient mice was obtained 28 days after BMT and assessed for the percentage of donor origin CD8+ cells (H-2Dd haplotype). MFC analysis was used to assess haploidentical engraftment after BMT with RIT and FLU in the absence of TBI. Mice treated with 200, 300, and 400 μCi 90Y-DOTA-30F11 and FLU had mean levels of donor origin H-2Dd CD8+ cells in the blood at day 28 of 27.8 ± 31.9%, 45.2 ± 36.6%, and 29.8 ± 32.2% (mean ± standard deviation), respectively (Figure 1A). The highest dose of 90Y-DOTA-30F11 investigated was excessively toxic; 2 of the 5 mice in the 400 μCi 90Y-DOTA-30F11 plus FLU group required euthanasia before day 28 because of excessive weight loss and lethargy. The surviving mice treated with the highest dose of 400 μCi 90Y-DOTA-30F11 and FLU did not exhibit a significantly different chimerism level from other groups, with a mean of 29.8 ± 32.2% donor origin CD8+ cells at day 28 (Figure 1A).
Chimerism following haploidentical BMT with a conditioning regimen of 90Y-anti-CD45 RIT and FLU. (A) Mean CD8+ donor chimerism in peripheral blood 28 days after haploidentical BMT in mice given FLU × 5 days (100 mg/kg per day IP on days −8 to −4) and 200, 300, or 400 µCi 90Y-DOTA-30F11 on day −3 followed by 1.5 × 107 haploidentical BM cells on day 0. (B) MFC analysis of peripheral blood 28 days after haploidentical BMT in a B6SJLF1/J transplanted mouse pretreated with FLU 100 mg/kg per day × 5 days and 200 µCi 90Y-DOTA-30F11, depicting the percentage of CD8+ cells expressing the H-2Dd haplotype.
Chimerism following haploidentical BMT with a conditioning regimen of 90Y-anti-CD45 RIT and FLU. (A) Mean CD8+ donor chimerism in peripheral blood 28 days after haploidentical BMT in mice given FLU × 5 days (100 mg/kg per day IP on days −8 to −4) and 200, 300, or 400 µCi 90Y-DOTA-30F11 on day −3 followed by 1.5 × 107 haploidentical BM cells on day 0. (B) MFC analysis of peripheral blood 28 days after haploidentical BMT in a B6SJLF1/J transplanted mouse pretreated with FLU 100 mg/kg per day × 5 days and 200 µCi 90Y-DOTA-30F11, depicting the percentage of CD8+ cells expressing the H-2Dd haplotype.
Anti-CD45 RIT without FLU or TBI before haploidentical BMT
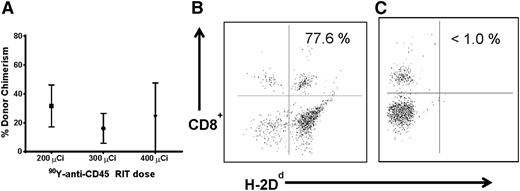
We then sought to further minimize conditioning requirements by investigating donor hematopoietic cell engraftment using anti-CD45 RIT without FLU or TBI. Five female B6SJL/F1 recipient mice per group were treated with 200, 300, or 400 μCi 90Y-DOTA-30F11 followed by delivery of 1.5 × 107 haploidentical BM cells. Mice treated with anti-CD45 RIT without TBI or FLU demonstrated hematopoietic engraftment from haploidentical donors. Mice treated with 200, 300, and 400 μCi 90Y-DOTA-30F11 alone exhibited 31.6 ± 32.5%, 16.1 ± 23%, and 23.8 ± 41.2% mean donor origin CD8+ cells at day 28 after BMT, respectively (Figure 2). Overall chimerism levels were similar to those seen in mice treated with FLU in combination with anti-CD45 RIT (mean of donor origin CD8+ cells, 27.8%-45.2% [Figure 1]). Conversely, mice that received FLU 100 mg/kg per day for 5 days before BMT without RIT or TBI did not show significant haploidentical engraftment because mice in this group had <1.0% donor origin CD8+ cells 28 days after BMT (Figure 2C). These studies suggest that anti-CD45 RIT can be sufficiently immunosuppressive to allow for engraftment from haploidentical donors in the absence of chemotherapy and/or TBI in this murine model.
Chimerism following haploidentical BMT with a conditioning regimen of 90Y-anti-CD45 RIT without FLU. (A) Mean CD8+ donor chimerism in peripheral blood 28 days after haploidentical BMT in mice pretreated with 200, 300, or 400 µCi 90Y-DOTA-30F11 on day −3, without FLU. MFC analysis of peripheral blood 28 days after haploidentical BMT in a B6SJLF1/J mouse pretreated with (B) FLU × 5 days (100 mg/kg per day IP on days −8 to −4) and 400 µCi 90Y-DOTA-30F11 on day −3 or (C) FLU × 5 days without anti-CD45 RIT, both transplanted with 1.5 × 107 haploidentical BM cells on day 0, depicting the percentage of CD8+ cells expressing the H-2Dd haplotype.
Chimerism following haploidentical BMT with a conditioning regimen of 90Y-anti-CD45 RIT without FLU. (A) Mean CD8+ donor chimerism in peripheral blood 28 days after haploidentical BMT in mice pretreated with 200, 300, or 400 µCi 90Y-DOTA-30F11 on day −3, without FLU. MFC analysis of peripheral blood 28 days after haploidentical BMT in a B6SJLF1/J mouse pretreated with (B) FLU × 5 days (100 mg/kg per day IP on days −8 to −4) and 400 µCi 90Y-DOTA-30F11 on day −3 or (C) FLU × 5 days without anti-CD45 RIT, both transplanted with 1.5 × 107 haploidentical BM cells on day 0, depicting the percentage of CD8+ cells expressing the H-2Dd haplotype.
Anti-CD45 RIT with pre- and posttransplant CY
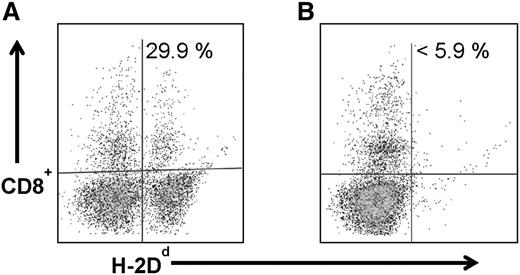
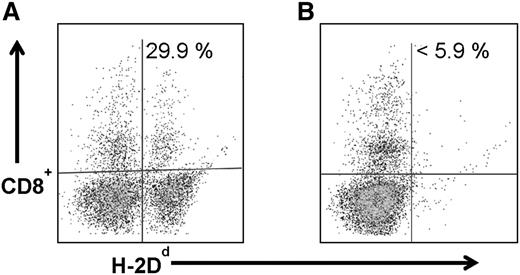
Because anti-CD45 RIT without TBI or FLU generated adequate but not complete engraftment of haploidentical donor cells, we investigated whether chimerism levels might be improved by adding CY to the regimen, particularly because the haploidentical BMT platform includes pre- and posttransplant CY. In earlier haploidentical BMT murine studies, the addition of pretransplant CY improved chimerism levels.27 Therefore, groups of 5 B6SJL/F1 recipient mice were treated with 300 μCi 90Y-DOTA-30F11 on day −3 before infusion of 1.5 × 107 donor haploidentical BM cells on day 0 followed by CY (200 mg/kg per day) delivered days −2 and +2. In these experiments, anti-CD45 RIT combined with CY given before and after haploidentical BMT resulted in haploidentical donor cell engraftment, with mean donor origin CD8+ cells of 18.4 ± 13.7% 28 days after transplant. Importantly, CY alone given before and after BMT without anti-CD45 RIT or TBI, was not sufficient for haploidentical donor cell engraftment, as these mice all exhibited <5.9% donor origin CD8+ cells in peripheral blood (Figure 3B).
Chimerism following haploidentical BMT with a conditioning regimen of 90Y-anti-CD45 RIT with pre- and post-BMT CY. MFC analysis of peripheral blood 28 days after haploidentical BMT in a B6SJLF1/J transplanted mouse that received 1.5 × 107 haploidentical BM cells on day 0 and (A) 300 µCi 90Y-DOTA-30F11 on day −3 and CY (200 mg/kg per day on days −2 and +2) or (B) CY alone without RIT, depicting the percentage of CD8+ cells expressing the H-2Dd haplotype.
Chimerism following haploidentical BMT with a conditioning regimen of 90Y-anti-CD45 RIT with pre- and post-BMT CY. MFC analysis of peripheral blood 28 days after haploidentical BMT in a B6SJLF1/J transplanted mouse that received 1.5 × 107 haploidentical BM cells on day 0 and (A) 300 µCi 90Y-DOTA-30F11 on day −3 and CY (200 mg/kg per day on days −2 and +2) or (B) CY alone without RIT, depicting the percentage of CD8+ cells expressing the H-2Dd haplotype.
Combinations of FLU and CY with anti-CD45 RIT for haploidentical BMT
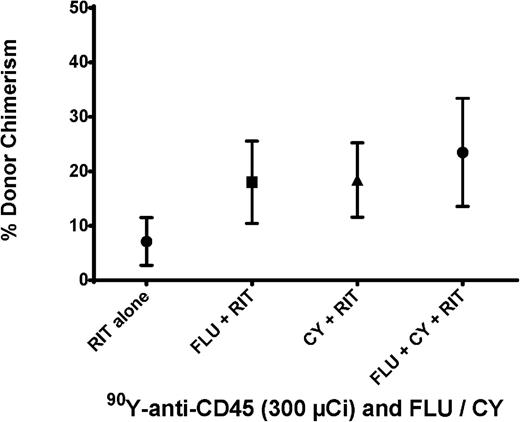
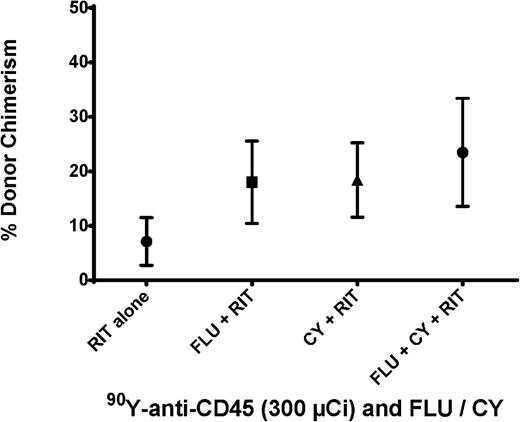
To further optimize the preparative regimen for haploidentical BMT, we investigated the impact of FLU and CY combinations with anti-CD45 RIT (in the absence of TBI) on engraftment. Groups of 5 female B6SJL/F1 recipient mice were treated with FLU (100 mg/kg per day) on days –8 to –4 and CY (200 mg/kg per day) on days –2 and +2 in conjunction with 300 μCi 90Y-DOTA-30F11 on day –3 before haploidentical BMT (day 0). Consistent engraftment of haploidentical donor cells was observed with mean chimerism levels of 23.5 ± 19.9% donor CD8+ cells detected 28 days after BMT (Figure 4). These chimerism levels were similar in all groups receiving combinations of chemotherapy with 300 μCi 90Y-DOTA-30F11; the groups pretreated with CY plus RIT and FLU plus RIT had mean levels of donor origin CD8+ cells of 18.4 ± 13.7% and 18 ± 16.9%, respectively. Because similar chimerism levels were observed in all groups, we selected the regimen of CY and RIT without FLU as the preparative regimen for subsequent therapeutic studies. In addition, weights were similar for mice treated with different combinations of 90Y-anti-CD45 RIT, CY, and FLU, suggesting no significant difference in toxicity. The preparative regimen of CY and RIT without FLU would not only facilitate engraftment, but also afford posttransplant GVHD prophylaxis.
Chimerism following haploidentical BMT with a conditioning regimen of 90Y-anti-CD45 RIT, FLU, and/or CY. Mean percentage of CD8+ cells expressing the H-2Dd haplotype in peripheral blood from transplanted mice 28 days after BMT. Recipient mice were treated with 300 µCi 90Y-DOTA-30F11 on day −3 alone, with FLU × 5 days (100 mg/kg per day on days −8 to −4), with CY (200 mg/kg per day on days −2 and +2), or with both FLU and CY.
Chimerism following haploidentical BMT with a conditioning regimen of 90Y-anti-CD45 RIT, FLU, and/or CY. Mean percentage of CD8+ cells expressing the H-2Dd haplotype in peripheral blood from transplanted mice 28 days after BMT. Recipient mice were treated with 300 µCi 90Y-DOTA-30F11 on day −3 alone, with FLU × 5 days (100 mg/kg per day on days −8 to −4), with CY (200 mg/kg per day on days −2 and +2), or with both FLU and CY.
Anti-CD45 RIT with CY and haploidentical BMT as treatment of leukemia
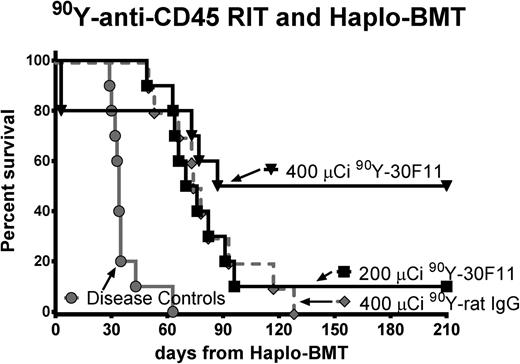
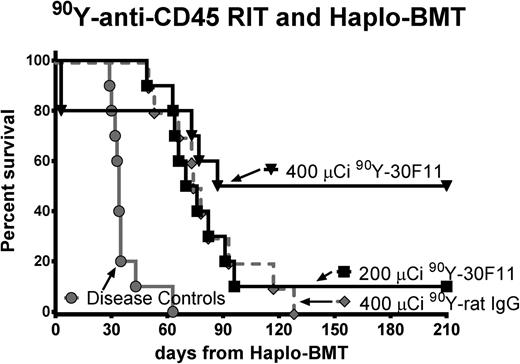
We also performed therapeutic studies in leukemic mice using anti-CD45 RIT in lieu of TBI and FLU to assess the efficacy of this regimen in the setting of haploidentical BMT. Groups of 10 B6SJL/F1 mice were injected with 1 × 105 leukemia SJL cells on day –5, and then 200 μCi or 400 μCi 90Y-DOTA-30F11 was given 3 or 4 days before BMT, with CY (200 mg/kg per day) delivered on days –2 and +2. Five of 10 (50%) mice treated with 400 μCi of 90Y-DOTA-30F11 survived more than 200 days, compared with only 1 of 10 (10%) mice given 200 μCi of 90Y-DOTA-30F11 (Figure 5). Mice given 200 μCi 90Y-DOTA-30F11 survived a median of 73 days compared with untreated leukemic control mice with a median overall survival of 34 days (P < .001, Mantel-Cox testing). None of the mice treated with nontargeting control Ab (400 μCi 90Y-DOTA-rat IgG) was a long-term survivor. Two mice from the 400 μCi 90Y-DOTA-30F11 group died of toxicity on day +3, apparently from internal bleeding in the setting of thrombocytopenia, as observed at necropsy.
Overall survival using 90Y-anti-CD45 RIT and haploidentical BMT in leukemia-bearing mice. Survival of SJL leukemia-bearing mice treated with pre- and posttransplant CY (200 mg/kg per day on days −2 and +2), (black box) 200 or (black inverse triangle) 400 µCi of 90Y-DOTA-30F11, or (gray diamond) 400 µCi 90Y-DOTA-rat IgG on day −3 or −4, and transplanted on day 0 with 1.5 × 107 haploidentical BM cells. Untreated leukemia-bearing mice (gray circle) served as controls.
Overall survival using 90Y-anti-CD45 RIT and haploidentical BMT in leukemia-bearing mice. Survival of SJL leukemia-bearing mice treated with pre- and posttransplant CY (200 mg/kg per day on days −2 and +2), (black box) 200 or (black inverse triangle) 400 µCi of 90Y-DOTA-30F11, or (gray diamond) 400 µCi 90Y-DOTA-rat IgG on day −3 or −4, and transplanted on day 0 with 1.5 × 107 haploidentical BM cells. Untreated leukemia-bearing mice (gray circle) served as controls.
Long-term engraftment of donor origin CD8+ haploidentical cells
To assess long-term engraftment from haploidentical donors, peripheral blood chimerism levels from separate cohorts of mice that received anti-CD45 RIT and CY were assayed monthly for 6 months after haploidentical BMT. Because chimerism levels were in the 18% to 45% range 28 days after BMT, it was hypothesized that circulating residual radioactivity from the RIT administered 3 days before the BMT may have compromised hematopoietic engraftment. Thus, to minimize nonspecific irradiation of rescue BM cells, the interval between RIT and BMT was increased to 4 days. Groups of 5 B6SJL/F1 recipient mice were given 300 μCi 90Y-DOTA-30F11 on day –4, followed by CY (200 mg/kg per day) administered by IP injections on days –2 and +2, and 1.5 × 107 haploidentical BM cells administered by tail vein injections on day 0. Chimerism levels among these mice ranged from 73.3% to 93.9% (see supplemental Figure 1, available on the Blood Web site), with an average of 81.3 ± 10.6% donor origin CD8+ cells detected in peripheral blood at day 28 after BMT (Table 1). Peripheral blood mean donor chimerism levels in recipient mice remained stable for at least 6 months posttransplant, with an average donor chimerism level of 85.5 ± 11.0% in CD8+ T cells at 6 months after BMT (Table 1).
Engraftment of donor origin CD3+, B220+, and CD11b+ hematopoietic cells
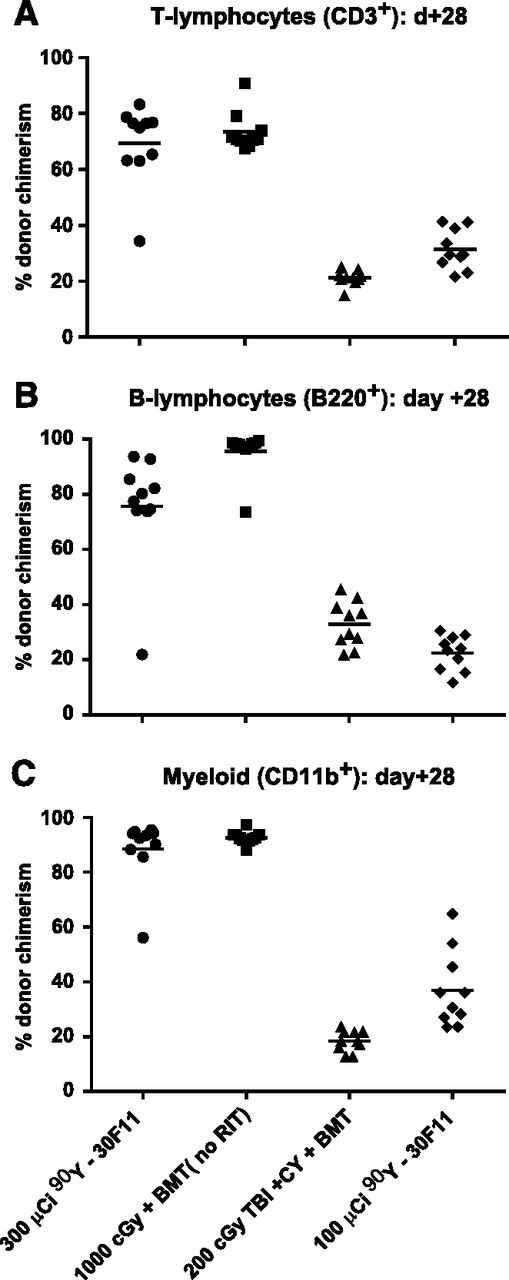
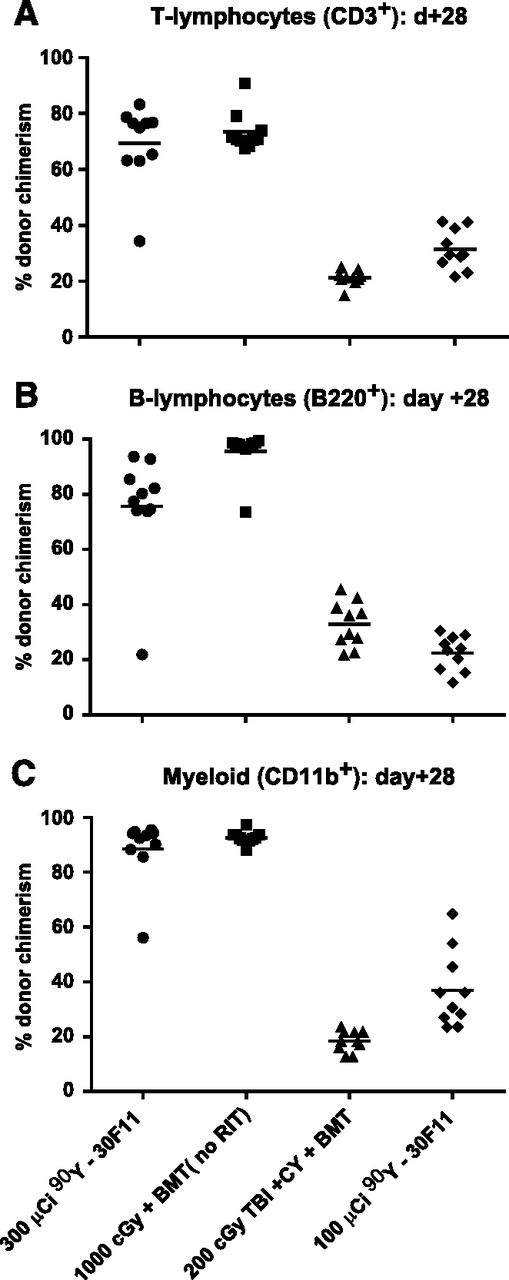
To confirm that hematopoietic engraftment from haploidentical donors was not limited to infused CD8+ cells from T cell–replete BM, MFC analysis for donor-derived T cells (CD3+), B cells (B220+), and myeloid cells (CD11b+) was performed on peripheral blood from a separate cohort of transplanted mice. Mice treated with 300 μCi 90Y-anti-CD45 RIT before haploidentical BMT had CD3+, B220+, and CD11b+ donor chimerism levels 28 days after haploidentical BMT of 69.3 ± 14.1%, 75.6 ± 20.2%, and 88.5 ± 11.8%, respectively (Figure 6). These chimerism levels were comparable to CD3+, B220+, and CD11b+ donor chimerism achieved with myeloablative 1000 cGy TBI (73.34 ± 6.9%, 95.6 ± 7.8%, and 92.7 ± 2.3%, respectively) and were more favorable than chimerism achieved via 200 cGy TBI (21.2 ± 2.7%, 32.9 ± 8.2%, and 18.5 ± 3.8%, respectively; supplemental Table 1).
Multilineage chimerism following haploidentical BMT with a conditioning regimen of 90Y-anti-CD45 RIT, TBI, and/or CY. Individual and mean percentages of (A) CD3+ (T cells), (B) B220+ (B cells), or (C) CD11b+ (myeloid cells) expressing the H-2Dd haplotype in peripheral blood from transplanted mice 28 days after BMT. Recipient mice were treated with 100 or 300 µCi 90Y-DOTA-30F11 on day −4 or 200 or 1000 cGy TBI on day 0 and with CY (200 mg/kg per day on days −2 and +2).
Multilineage chimerism following haploidentical BMT with a conditioning regimen of 90Y-anti-CD45 RIT, TBI, and/or CY. Individual and mean percentages of (A) CD3+ (T cells), (B) B220+ (B cells), or (C) CD11b+ (myeloid cells) expressing the H-2Dd haplotype in peripheral blood from transplanted mice 28 days after BMT. Recipient mice were treated with 100 or 300 µCi 90Y-DOTA-30F11 on day −4 or 200 or 1000 cGy TBI on day 0 and with CY (200 mg/kg per day on days −2 and +2).
Discussion
Early clinical attempts using haploidentical HCT were hampered by delayed engraftment, high graft rejection rates, high NRM, and a high incidence of GVHD using T cell–replete grafts.10-12,28 Subsequently, T cell–depleted grafts were investigated and shown to confer lower GVHD rates, but were compromised by high risks of graft failure and/or relapse.29,30 Successful engraftment with full donor chimerism and low rates of GVHD was later demonstrated using a “mega-dose” of T cell–depleted peripheral blood stem cells (>10 × 106 CD34+ cells/kg body weight) after a myeloablative, TBI-based, preparative regimen.31-33 Other advancements have addressed the increased risk of fatal infections from T cell–depleted grafts and delayed immune reconstitution by infusing pathogen-specific T cells34-36 or sequentially infusing CD4+CD25+ regulatory T cells followed by conventional CD4+CD25- regulatory T cells.37,38 GVHD rates have also been addressed by T-cell depletion ex vivo via positive or negative selection of CD34+ cells.39 Additionally, by preferentially infusing γδ T cells, felt not to be involved in GVHD but required for antileukemic effect,40 via negative selection of αβ T cells, good outcomes with low rates of grade III-IV GVHD have been reported.39,41,42 More recent haploidentical transplantation efforts have added high-dose CY after HCT to deplete alloreactive T cells in vivo, achieving almost complete donor chimerism by 1 month after transplantation in the majority of human recipients.21,43 Nevertheless, many of these advancements have relied on delivery of at least minimal doses of TBI as part of nonmyeloablative haploidentical HCT. In this study, we sought to minimize systemic chemotherapy and TBI requirements as a means to minimize toxicity associated with myeloablative HCT, allow for haploidentical donor engraftment, and maximize outcomes of myeloid leukemia in a syngeneic mouse model.
These experiments provide evidence that 90Y-anti-CD45 RIT, when combined with CY before and after BMT in the absence of TBI or FLU, resulted in persistent hematopoietic engraftment from haploidentical donors. High chimerism levels were seen across multiple lineages assayed (cytotoxic T cells [CD8+], total T cells [CD3+], B cells [B220+], and myeloid cells [CD11b+]). Moreover, chimerism achieved with anti-CD45 RIT approach was more favorable than chimerism levels obtained with other standard low-intensity approaches such as 2 Gy TBI. In addition, mice transplanted with 90Y-anti-CD45 RIT with pre- and posttransplant CY did not exhibit significant weight loss to suggest excessive toxicity or gut GVHD, although dedicated toxicity and GVHD studies were not performed. These results further support the replacement of TBI and FLU before BMT with targeted anti-CD45 RIT because this potentially curative preparative regimen yielded long-term survivors in this syngeneic murine leukemia model. Moreover, the elimination of TBI and FLU from this preparative regimen may allow for a less toxic HCT strategy with lower relapse rates resulting from delivery of targeted radiation to leukemic cells. The augmented cytoreduction of leukemia provided by anti-CD45 RIT should help improve relapse rates, which in some initial haploidentical BMT with posttransplant CY studies were 45% to 51% at 1 year.21,24 Finally, these murine studies showed that high levels of donor origin chimerism can be achieved without TBI or FLU, relying instead on 90Y-anti-CD45 RIT and CY alone. In particular, we have demonstrated that haploidentical chimerism was persistent and stable in this model, with >80% donor origin CD8+ cells up to 6 months after BMT (Table 1).
The level of haploidentical hematopoietic engraftment achieved with this anti-CD45 RIT approach appears to be consistent with prior murine haploidentical BMT models using chemotherapy and TBI. Using multiple mouse strains, Luznik and colleagues were able to achieve durable mixed hematopoietic chimerism in haploidentical mice treated with 5 days of pretransplantation FLU (100 mg/kg per day on days –6 to –2), low-dose TBI (200 cGy on day –1), and peritransplantation CY (200 mg/kg per day on days –3 and +3).27 The levels of donor chimerism were proportional to the TBI dose and the BM cell dose. Depending on whether FLU and/or CY were used as pre-HCT treatments, donor chimerism levels ranged from 52% to 88%, but dramatically fell to <5% when TBI was not used as part of the preparative regimen, suggesting that the majority of the immunosuppressive effect was derived from TBI. We observed similar levels of durable engraftment using delivery of targeted radiation via anti-CD45 RIT in our studies; when FLU or CY alone was used without TBI or RIT, virtually no donor CD8+ cells were observed 28 days after haploidentical BMT.
Other preparative regimens have explored non-TBI methods to facilitate engraftment from haploidentical donors. Various combinations of FLU and/or CY before haploidentical HCT have been evaluated and compared with results employing lethal and sublethal doses of TBI.44 However, this approach required dosing of FLU and/or CY for at least 10 days and resulted in relatively low mean levels of donor chimerism (∼11%). Longer therapy with FLU/CY for up to 27 days did not yield significantly higher levels of donor chimerism. Conversely, the preparative approach for haploidentical BMT using anti-CD45 RIT was able to facilitate high, persistent levels of donor engraftment without extended dosing of FLU or systemic TBI.
More recent attempts to promote durable engraftment following haploidentical HCT have incorporated sirolimus and posttransplant CY, albeit still in conjunction with low-dose TBI.45 Lymphoid chimerism levels of nearly 40%, and myeloid chimerism levels of nearly 60%, were achieved in haploidentical recipient mice 1 month after HCT by initiating sirolimus instead of cyclosporine before HCT with posttransplant CY. Interestingly, chimerism levels waned slowly over time to half of the initial chimerism levels for both myeloid (∼30%) and lymphoid (∼20%) cells 1 year after HCT. All experiments were performed with TBI, so it is not possible to predict the outcomes if conditioning was limited to sirolimus and posttransplant CY without TBI. In the current studies, chimerism levels were stable and did not wane over 6 months.
Although our studies used the anti-CD45 RIT-based preparative regimen before haploidentical BMT as curative therapy in a preclinical model of hematologic malignancies, this non-TBI preparative strategy, in theory, might be equally appropriate for nonmalignant indications in which no fully HLA-matched donors are available and the goal of minimal toxicity is highly desired. One example is sickle cell disease, in which allogeneic HCT is currently the only curative treatment, with a reported cure rate of >90%, yet few eligible individuals with sickle cell disease proceed to HCT.46-49 Other inherited disorders that can benefit from HCT include severe combined immunodeficiency disorders 50,51 and metabolic disorders.52,53
In summary, 90Y-anti-CD45 RIT combined with peritransplant CY, without TBI or FLU, facilitated persistent, stable hematopoietic engraftment from haploidentical donors. Currently, there are clinical trials exploring RIT as adjunct to HCT, but these studies suggest that replacing TBI with anti-CD45 RIT and minimal chemotherapy may be feasible and should be explored in clinical trials as a means to further minimize transplant-related toxicity. The appeal of haploidentical BMT will likely increase because transplant outcomes from peripherally mobilized stem cells appear to be equivalent to outcomes from BM-derived stem cells.54-56 Although this preparative regimen prolonged survival in a syngeneic murine leukemia model, this approach may also be considered for nonmalignant diseases in which alternative HCT donors are needed.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by the National Institutes of Health (grants R01 CA138720, R01 CA109663, R01 CA076287, R01 CA136639, R01 CA172582, P01 CA044991, K24 CA184039 from the National Cancer Institute, and UL1RR025014 from the National Center for Advancing Translational Sciences) and awards from the Lymphoma Research Foundation (O.W.P and J.M.P), Damon Runyon Cancer Foundation (J.M.P.), Leukemia and Lymphoma Society (O.W.P., A.K.G., and J.J.O.), the American Society of Blood and Marrow Transplantation (J.J.O.), the Frederick Kullman Memorial Fund (J.M.P.), and the Penny E. Petersen Endowed Chair (O.W.P) funded by James and Sherry Raisbeck.
Authorship
Contribution: J.J.O., A.K., and J.M.P. designed research; J.J.O., A.K., M.D.H., E.R.B., and J.M.P. performed the research; E.R.B., D.K.H., D.S.W., and M.D.H. contributed vital new reagents or analytical tools; J.J.O. and A.K. collected data; J.J.O., A.K., E.R.B., T.A.G., S.H.L.F., R.M., D.S.W., M.D.H., D.J.G., A.K.G., P.O., B.M.S., E.J.F., L.L., O.W.P., and J.M.P. analyzed and interpreted data; and J.J.O., A.K., and J.M.P. wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Johnnie J. Orozco, Fred Hutchinson Cancer Research Center, 1100 Fairview Ave North, MS D3-190, Seattle, WA 98109-1024; e-mail: jorozco@fredhutch.org.