Key Points
RA/arsenic induces proteasomal degradation of mutant NPM1, yielding AML growth arrest and apoptosis.
RA/arsenic treatment restored nucleolar localization of NPM1 and significantly reduced bone marrow blasts in NPM1 mutant AML patients.
Abstract
Nucleophosmin-1 (NPM1) is the most frequently mutated gene in acute myeloid leukemia (AML). Addition of retinoic acid (RA) to chemotherapy was proposed to improve survival of some of these patients. Here, we found that RA or arsenic trioxide synergistically induce proteasomal degradation of mutant NPM1 in AML cell lines or primary samples, leading to differentiation and apoptosis. NPM1 mutation not only delocalizes NPM1 from the nucleolus, but it also disorganizes promyelocytic leukemia (PML) nuclear bodies. Combined RA/arsenic treatment significantly reduced bone marrow blasts in 3 patients and restored the subnuclear localization of both NPM1 and PML. These findings could explain the proposed benefit of adding RA to chemotherapy in NPM1 mutant AMLs, and warrant a broader clinical evaluation of regimen comprising a RA/arsenic combination.
Introduction
Acute myeloid leukemia (AML) is a genetically heterogeneous disease with a highly variable prognosis and an overall high mortality rate. The 5-year overall survival of adult AML patients is less than 50%, and only 20% of elderly patients survive over 2 years.1 Cytogenetic alterations classify AML into 3 risk-based categories: favorable, intermediate, and unfavorable.2 Patients with normal karyotype belong to the intermediate risk category and their prognosis is determined by specific genetic alterations, particularly Nucleophosmin-1 (NPM1) mutation and FMS-like tyrosine kinase 3 (FLT3) internal tandem duplication (ITD).3
NPM1 is an essential gene4 encoding a nucleolar shuttling protein5 with multiple functions, including stabilization of the p14Arf tumor suppressor protein, regulation of ribosome biogenesis, control of centrosome duplication, and p53 activation.4,5 In mutant NPM1 proteins, critical tryptophan residues in the C-terminus are lost and a de novo nuclear export signal is created. This leads to accumulation of mutant NPM1, together with normal NPM1, in the cytoplasm of leukemic cells rather than in their nucleolus. NPM1 mutations drive leukemogenesis, as hematopoietic disorders were observed in transgenics or knock-in mice.6-11 Some studies suggested that addition of retinoic acid (RA) to conventional chemotherapy improves survival, selectively in AML patients harboring the NPM1 mutation in the absence of FLT3-ITD.12 Other clinical studies reported negative results.13,14 Overall, there is no consensus yet as to whether the addition of RA to chemotherapy improves the outcome of patients with NPM1 mutant AML. Moreover, the basis for the proposed clinical response to RA remains obscure.
Arsenic trioxide (arsenic) and RA are very effective treatments for acute promyelocytic leukemia (APL), a distinct AML subtype characterized by the expression of the promyelocytic leukemia (PML) RA receptor α (RARA) fusion protein.15,16 PML/RARA delocalizes PML, a protein implicated in control of p53-driven senescence. PML nuclear bodies (NB) are implicated in both APL pathogenesis and therapy response.17 Both RA and arsenic induce degradation of PML/RARA through distinct pathways. Their combination cures APL in mice17-19 and patients.20-22
Here, we demonstrate that arsenic and RA synergistically induce proteasome-mediated degradation of mutant NPM1, resulting in differentiation, growth arrest, and apoptosis, selectively in AML cells harboring a NPM1 mutation. Expression of NPM1 mutant protein was associated with altered formation of PML NBs. The RA/arsenic combination significantly reduced leukemic blasts in the bone marrow (BM) of 3 NPM1-mutant AML patients and restored nucleolar localization of NPM1 and PML NBs both ex vivo and in vivo. These findings raise an unexpected parallelism between APL and NPM1 mutant AMLs, provide a basis for its proposed RA sensitivity and warrant a broader evaluation of therapeutic regimen comprising a RA/arsenic combination, particularly, in elderly patients.
Methods
Patients, cells, and treatments
KG1a (ATCC CCL246.1), ML-2 (DSM ACC15), THP-1 (DSM ATCC 16), MOLM-13 (DSM ACC 554), and HEL (DSM ACC 11) AML cell lines (gift from F. Mazurier) with wild-type (WT) NPM1, were grown in RPMI-1640 medium containing 10% fetal bovine serum (FBS) and antibiotics. OCI-AML3 (DSM ACC582) AML cells (gift from D. Bouscary) and IMS-M2 (gift from B. Falini) harboring the NPM1 mutation without FLT3-ITD were grown in minimum essential medium–α containing 20% FBS and antibiotics. Cells were seeded at a density of 2 × 105/mL. Primary AML cells from the BM of 3 patients (patients 1-3) were extracted following Ficoll separation and cultured in minimum essential medium–α supplemented with 20% FBS and antibiotics. These samples were collected after approval by the Institutional Review Board and after patients provided informed consent in accordance with the Declaration of Helsinki. Arsenic was used at 0.1 or 1 μM, RA at 0.3 or 1 μM, and the proteasome inhibitor PS-341 at 10 nM. Cell growth was assessed using the CellTiter 96 cell proliferation assay kit (Promega Corp., Madison, WI) or by trypan blue dye assay. Five elderly AML patients (patients 4-8) with NPM1 mutation, who were judged unfit for chemotherapy, received compassionate RA (Vesanoid Roche) (45 mg/m2 per day by mouth) and arsenic trioxide (Trisenox, Teva) intravenously (0.1 mg/kg per day).
Fluorescence-activated cell sorter analysis
Annexin V staining
Phosphatidyl-serine exposure in treated AML cells was assessed using Annexin V-fluorescein isothiocyanate (Sigma-Aldrich). For terminal deoxynucleotidyltransferase-mediated dUTP nick end labeling (TUNEL) assay, fluorescein-conjugated dUTP incorporated in nucleotide polymers was detected and quantified using flow cytometry. For both annexin and TUNEL assays, approximately 10 000 cells per sample were acquired and analyzed using CellQuest software. For CD11b staining, mouse anti-human CD11b-APC/Cy7 antibody (BD Pharmingen) was used to detect and quantify CD11b cell surface differentiation marker. There were 10 000 events per sample acquired and BD fluorescence-activated cell sorter Diva software was used for analysis.
Immunoblot analysis
Cells were solubilized at 4°C in lysis buffer. There were 50 µg of proteins loaded onto a 12% sodium dodecyl sulfate–polyacrylamide gel, subjected to electrophoresis and transferred onto nitrocellulose membranes. Blots were incubated with specific antibodies and washed, and proteins were visualized using the enhanced chemiluminescence system (Santa Cruz, Germany). The following antibodies were used: monoclonal anti- NPM1 recognizing both WT and mutated NPM1 (WT + c) (Abnova, Abcam), monoclonal anti-p53 (Santa Cruz), monoclonal anti-phospho-p53 (P-p53) (Cell Signaling), monoclonal anti-actin (Santa Cruz), monoclonal anti-glyceraldehyde-3-phosphate dehydrogenase (Abnova), polyclonal anti-mutated NPM1 (NPM1c) (Abnova), polyclonal anti-Fibrillarin (Abcam), polyclonal anti-p21(Santa Cruz), and polyclonal anti-poly-ADP-ribose polymerase (PARP) (Santa Cruz).
Immunofluorescence and confocal microscopy
OCI-AML3 and THP-1 cells were cytospun onto glass slides (5 minutes, 800 rpm) and fixed with methanol at −20°C. Immunofluorescence assays were performed using primary antibodies against NPM1, the nucleolar marker Fibrillarin, SUMO-1, or PML. Images were acquired by confocal microscopy using a Zeiss LSM 710 confocal microscope (Zeiss, Oberkochen, Germany) with a Plan Apochromat 63/1.4 numeric aperture oil-immersion objective, using Zen 2009 (Carl Zeiss). We have used a blinded method of counting NPM1, PML, and SUMO-1 bodies. Z-sections derived from 100 cells per condition were coded by one investigator and counted by another investigator for the presence of regular or faint PML bodies.
Synergy studies and statistical analysis
Proliferation experiments on cell lines were repeated at least 3 times. Data are reported as the mean ± standard error. Computerized combination index (CI) was generated automatically using CompuSyn software based on the CI-isobol method of Chou et al.23 The CI was used to assess synergistic effects (CI <1), additive effects (CI = 1), or antagonistic effects (CI >1). Two statistical tests were performed to validate significance: the t test and the one-way analysis of variance test.
Results
RA or arsenic induce differentiation, growth arrest, and apoptosis in NPM1 mutated AMLs only
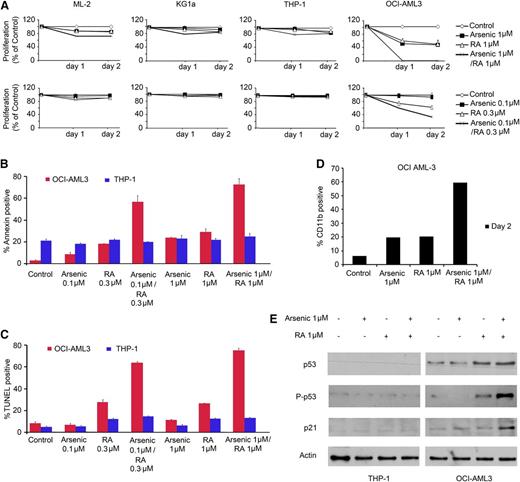
AML cell lines with mutated NPM1 (OCI-AML3, IMS-M2) or WT NPM1 (KG1a, ML-2, THP-1, MOLM-13, HEL) were treated with RA and/or arsenic up to 48 hours. OCI-AML3 cells were much more sensitive to RA than IMS-M2, KG1a, ML-2 THP-1, MOLM-13, and HEL cells for cell cycle arrest and/or cell death (Figure 1A; supplemental Figure 1A, available on the Blood Web site). OCI-AML3, and IMS-M2 cells were also considerably more sensitive to arsenic than control cells (Figure 1A; supplemental Figure 1A). Synergy studies, analyzed using a computerized CI23 revealed a strong synergistic effect of arsenic and RA for growth arrest in OCI-AML3 cells at 24 hours (CI = 0.46 and 0.035, for low- or high-arsenic concentration, respectively) (Figure 1A). Increases in annexin-V, TUNEL positivity, and PARP cleavage were only observed in OCI-AML3 cells treated with arsenic or RA (Figure 1B-C; supplemental Figure 2). Thus, this combination synergized for induction of apoptosis, reaching 75% after 48 hours of treatment, exclusively in OCI-AML3 cells (Figure 1B-C; supplemental Figure 2). Furthermore, we observed a major induction of CD11b expression upon RA/arsenic treatment of OCI AML-3 and, to a lesser extent in IMS-M2, pointing to partial differentiation (Figure 1D; supplementary Figure 1A). Importantly, in OCI-AML3 cells, RA, or the RA/arsenic combination selectively upregulated p53, its active phosphorylated form and its downstream effector p21 (Figure 1E), likely explaining RA-induced cell cycle arrest and apoptosis (supplemental Figure 2).
RA and arsenic induce growth inhibition and apoptosis in NPM1 mutated AML cell line. (A) AML cell lines with normal NPM1 (ML-2, KG1a, and THP-1) or mutated NPM1 (OCI-AML3 and IMS-M2) were treated with arsenic (1 μM or 0.1 μM), RA (1 μM or 0.3 µM) or a combination of both. Cell growth (percent of control) was assayed in triplicate wells. The results represent the average of at least 3 independent experiments. (B) Annexin V staining of THP-1 or OCI-AML3 cells treated for 48 hours as described. (C) TUNEL assay of THP-1 or OCI-AML3 cells treated for 48 hours as described. The results are the average of 3 independent experiments. (D) Flow cytometry analysis using CD11b differentiation marker on OCI-AML3 treated for 48 hours. (E) Western blot analysis for p53, P-p53, p21, or actin in THP-1 and OCI-AML3 cells treated for 48 hours as described.
RA and arsenic induce growth inhibition and apoptosis in NPM1 mutated AML cell line. (A) AML cell lines with normal NPM1 (ML-2, KG1a, and THP-1) or mutated NPM1 (OCI-AML3 and IMS-M2) were treated with arsenic (1 μM or 0.1 μM), RA (1 μM or 0.3 µM) or a combination of both. Cell growth (percent of control) was assayed in triplicate wells. The results represent the average of at least 3 independent experiments. (B) Annexin V staining of THP-1 or OCI-AML3 cells treated for 48 hours as described. (C) TUNEL assay of THP-1 or OCI-AML3 cells treated for 48 hours as described. The results are the average of 3 independent experiments. (D) Flow cytometry analysis using CD11b differentiation marker on OCI-AML3 treated for 48 hours. (E) Western blot analysis for p53, P-p53, p21, or actin in THP-1 and OCI-AML3 cells treated for 48 hours as described.
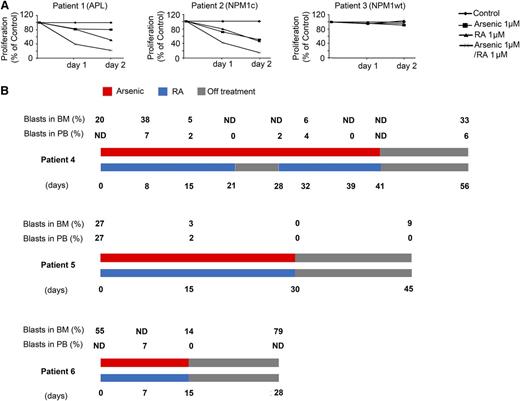
Primary leukemic cells derived from BM of 3 AML patients (patients 1-3), presenting initially with 52%, 70%, and 74% of BM blasts, respectively, were treated in vitro with RA and/or arsenic, as indicated above. Patient 1 had APL with PML/RARA rearrangement; patient 2 harbored an NPM1 mutation without FLT3-ITD; and patient 3 had AML-M6 with WT NPM1. Cells from patients 1 and 2 were much more sensitive to RA and arsenic treatment than those derived from patient 3 (Figure 2A). Again, a strong synergy between RA and arsenic was observed, exclusively in patients 1 and 2 at any time point and dose (Figure 2A and data not shown). Collectively, RA and arsenic exert selective apoptosis on NPM1 mutant AMLs ex vivo.
RA and arsenic induce growth inhibition in NPM1 mutated AML cells ex vivo and in vivo. (A) Primary AML cells from 3 different AML patients were treated with arsenic (1 μM), RA (1 μM) or a combination of both for 48 hours. Cell growth (percent of control) was assayed in duplicate wells. (B) Percent of PB and BM blasts and treatment schedule in 3 NPM1–mutated AML patients treated with RA and arsenic as indicated. ND, not done.
RA and arsenic induce growth inhibition in NPM1 mutated AML cells ex vivo and in vivo. (A) Primary AML cells from 3 different AML patients were treated with arsenic (1 μM), RA (1 μM) or a combination of both for 48 hours. Cell growth (percent of control) was assayed in duplicate wells. (B) Percent of PB and BM blasts and treatment schedule in 3 NPM1–mutated AML patients treated with RA and arsenic as indicated. ND, not done.
RA/Arsenic reduce marrow blasts in NPM1 mutant AML patients
Compassionate use of RA and arsenic was initiated in 5 previously untreated or relapsed elderly AML patients (patients 4-8) with normal karyotype and mutated NPM1 that were judged unfit for chemotherapy. As expected from APL patients, this treatment was very well tolerated.24 BM blasts significantly decreased in 3 patients (patients 4-6) on day 15 posttreatment (Figure 2B) as detailed below. In patient 4, analysis of BM aspirates revealed that blasts increased initially from day 1 to day 15 of RA treatment (day 8 of RA/arsenic) (15% to 38%, respectively), and subsequently normalized at day 23 of RA treatment (day 16 of RA/arsenic) (5%) in a normocellular marrow. Furthermore, peripheral blood (PB) blasts gradually decreased to disappear at day 21 of RA/arsenic. Throughout the treatment period, the patient became transfusion independent. In patient 5, BM and PB blasts decreased from 27% at day 1 to 2% to 3% at day 15 of RA/arsenic treatment. In patient 6, BM blasts decreased from day 1 to day 15 of RA/arsenic (from 55% to 14%) with a clearance of PB blasts at day 15. In these 3 patients, blast counts reincreased with discontinuation of treatment. Two additional patients7,8 were treated; patient 7 died of invasive Aspergillosis at day 21 from RA/arsenic treatment with no evidence of response and patient 8 rapidly died of bilateral interstitial pneumonia at day 10 from RA/arsenic treatment (data not shown). Thus, RA and arsenic exerted a transient in vivo antileukemic effect in this subset of AML patients.
Degradation of mutant NPM1 drives RA and/or arsenic-induced growth inhibition
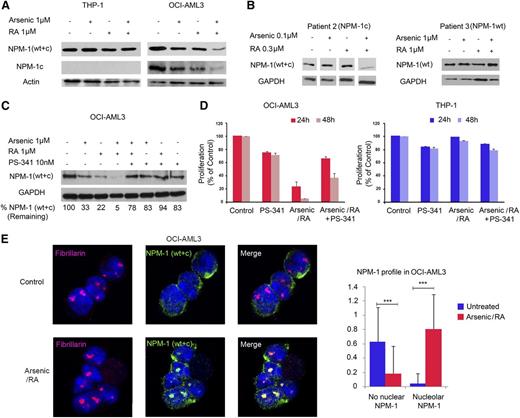
Because of the similarities between RA/arsenic effects on NPM1 mutant AML cells and APL cells (growth arrest, apoptosis and differentiation in vitro, in vivo anti-leukemic activity), we investigated the ability of these drugs to induce degradation of mutant NPM1 oncoproteins. In contrast, RA and, to a lesser extent, arsenic decreased NPM1 protein levels in OCI-AML3 cells, as assessed with an antibody detecting both WT and mutant proteins (Figure 3A). Using an antibody selective for NPM1 mutant protein, the amplitude of NPM1 downregulation was even higher. Similar findings were observed in primary patient cells (Figure 3B). In the IMS-M2 cells, degradation of the NPM1 mutant protein was observed with arsenic, not RA (data not shown). No effect of RA or arsenic on NPM1 expression was observed in THP-1, ML-2, HEL, or MOLM-13 cells (Figure 3A; supplemental Figure 1B). Critically, both NPM1 downregulation and growth arrest were reversed with the addition of the proteasome inhibitor PS-341 (Figures 3C-D and data not shown) (CI = 4.58 at 24 hours). These results suggest that RA/arsenic-induced growth arrest of AML cells with mutant NPM1 is caused by proteasomal degradation of the mutated oncoprotein.
RA and arsenic induce proteasomal degradation of mutant NPM1 and restore NPM1 nucleolar localization. (A) Western blot analysis for NPM1 recognizing both WT and mutated NPM1 (WT + c), mutated NPM1 (NPM1c), actin in THP-1, and OCI-AML3 cells treated with arsenic (1 μM), RA (1 μM), or a combination of both for 48 hours as indicated. A representative of 3 independent experiments is shown. (B) Western blot analysis for NPM1 (WT + c) and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) in primary leukemic cells derived from AML patients treated with arsenic (0.1 or 1 μM), RA (0.3 or 1 μM), or a combination of both for 48 hours as indicated. (C) Western blot analysis for NPM1 (WT + c) and GAPDH in OCI-AML3 cells treated with arsenic (1 μM), RA (1 μM), PS-341 (10 nM), either alone, or in combination for 48 hours as indicated. Percentages indicate the amount of remaining NPM1 (WT + c) after normalization to GAPDH. (D) Cell count with trypan blue staining (percent of control) of OCI-AML3 and THP-1 cells treated with arsenic (1 μM), RA (1 μM), PS-341 (10 nM), either alone, or in combination for up to 48 hours. Cell growth (percent of control) was assayed in triplicate wells. The results depict one representative experiment among 3 independent ones. (E) Confocal microscopy analysis of nucleolar NPM1 localization in OCI-AML3 or THP-1 cells after treatment with RA/arsenic for 48 hours. NPM1 was stained with an antibody recognizing NPM1 (WT + c) (green), nucleoli were stained with anti-Fibrillarin (red), and nuclei were stained with 4,6 diamidino-2-phenylindole (blue). Images represent Z sections. Graphs show quantification of nucleolar NPM1 as averages of one Z section/cell from 30 different cells of 3 independent experiments. Significant P values are indicated by asterisks.
RA and arsenic induce proteasomal degradation of mutant NPM1 and restore NPM1 nucleolar localization. (A) Western blot analysis for NPM1 recognizing both WT and mutated NPM1 (WT + c), mutated NPM1 (NPM1c), actin in THP-1, and OCI-AML3 cells treated with arsenic (1 μM), RA (1 μM), or a combination of both for 48 hours as indicated. A representative of 3 independent experiments is shown. (B) Western blot analysis for NPM1 (WT + c) and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) in primary leukemic cells derived from AML patients treated with arsenic (0.1 or 1 μM), RA (0.3 or 1 μM), or a combination of both for 48 hours as indicated. (C) Western blot analysis for NPM1 (WT + c) and GAPDH in OCI-AML3 cells treated with arsenic (1 μM), RA (1 μM), PS-341 (10 nM), either alone, or in combination for 48 hours as indicated. Percentages indicate the amount of remaining NPM1 (WT + c) after normalization to GAPDH. (D) Cell count with trypan blue staining (percent of control) of OCI-AML3 and THP-1 cells treated with arsenic (1 μM), RA (1 μM), PS-341 (10 nM), either alone, or in combination for up to 48 hours. Cell growth (percent of control) was assayed in triplicate wells. The results depict one representative experiment among 3 independent ones. (E) Confocal microscopy analysis of nucleolar NPM1 localization in OCI-AML3 or THP-1 cells after treatment with RA/arsenic for 48 hours. NPM1 was stained with an antibody recognizing NPM1 (WT + c) (green), nucleoli were stained with anti-Fibrillarin (red), and nuclei were stained with 4,6 diamidino-2-phenylindole (blue). Images represent Z sections. Graphs show quantification of nucleolar NPM1 as averages of one Z section/cell from 30 different cells of 3 independent experiments. Significant P values are indicated by asterisks.
RA and arsenic restore the normal localization of both NPM1 and PML
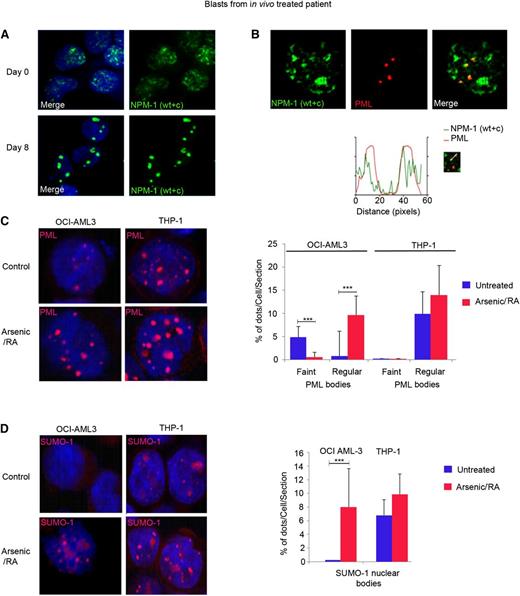
In NPM1 mutant AMLs, NPM1 becomes delocalized to the cytoplasm (Figure 3E; supplemental Figure 3).25 Importantly, treatment with combined RA/arsenic, but not single agents alone, resulted in complete degradation of mutant NPM1 and accordingly restored the nucleolar localization of the remaining WT NPM1 protein in OCI-AML3 cells (Figure 3E; supplemental Figure 3). In vivo RA/arsenic treatment resulted in complete NPM1 nucleolar relocalization in the blasts of one patient (patient 7, Figure 4A), although mutant NPM1 was not fully degraded (data not shown). Thus, therapy corrects the defects in nucleolar organization (and presumably function) imposed by NPM1 mutation.
Combination of RA and arsenic restores PML and SUMO-1 nuclear body formation. (A-B) Primary leukemic blasts from one NPM1 mutated AML patient on days 0 and 8 after in vivo treatment with RA/arsenic were analyzed by confocal miscroscopy. NPM1 was stained with anti-NPM1 (WT + c) antibody (green); PML was stained with anti-PML antibody (red). (C-D) OCI-AML3 cells (left panels) and THP-1 cells (right panels) treated with RA/arsenic for up to 48 hours and analyzed by confocal miscroscopy. (C) Treatment of OCI-AML3 cells with RA/arsenic leads to PML nuclear body reorganization. (D) Treatment of OCI-AML3 cells with RA/arsenic leads to SUMO-1 nuclear body formation. The results (C-D) depict one representative of 3 independent experiments. Graphs show quantification of PML and SUMO-1 NBs, as averages of one Z-section/cell from 30 different cells. Significant P values are indicated by asterisks.
Combination of RA and arsenic restores PML and SUMO-1 nuclear body formation. (A-B) Primary leukemic blasts from one NPM1 mutated AML patient on days 0 and 8 after in vivo treatment with RA/arsenic were analyzed by confocal miscroscopy. NPM1 was stained with anti-NPM1 (WT + c) antibody (green); PML was stained with anti-PML antibody (red). (C-D) OCI-AML3 cells (left panels) and THP-1 cells (right panels) treated with RA/arsenic for up to 48 hours and analyzed by confocal miscroscopy. (C) Treatment of OCI-AML3 cells with RA/arsenic leads to PML nuclear body reorganization. (D) Treatment of OCI-AML3 cells with RA/arsenic leads to SUMO-1 nuclear body formation. The results (C-D) depict one representative of 3 independent experiments. Graphs show quantification of PML and SUMO-1 NBs, as averages of one Z-section/cell from 30 different cells. Significant P values are indicated by asterisks.
PML NBs constitute platforms for posttranslational modifications, notably sumoylation, that have been repeatedly implicated in transformation.26-28 Yet, alterations in PML NBs were not previously described in NPM1 mutant AMLs. Unexpectedly, in OCI-AML3 cells, PML NBs were significantly smaller than in THP-1 cells (Figure 4B). Accordingly, we could not detect nuclear bodies using SUMO-1 antibodies (Figure 4C). In primary NPM1 mutated AML cells, we again observed abnormal and heterogenous PML NBs, together with a significant overlap between nuclear, but extranucleolar, NPM1, and PML (Figure 4A). Treatment with RA/arsenic restored PML NB organization in both OCI-AML3 and THP-1 cells, however this effect was more pronounced in NPM1 mutated cells and was accompanied by enhanced SUMO-1 NB formation (Figure 4B-C). These results are highly suggestive for mutant NPM1-mediated disorganization of PML/SUMO-1 NBs. As in APL, NB restoration could contribute to the therapeutic efficacy of the RA/arsenic combination.17,29
Discussion
In APL, both ourselves and others, have demonstrated that PML/RARA degradation by arsenic or RA restores PML nuclear bodies and activates a p53 senescence checkpoint that is required for APL eradication.15,30,31 The results obtained here with NPM1 mutant AML unexpectedly bear some similarities with APL. First, mutant NPM1 is degraded with RA or arsenic exposure, and p53 signaling is activated. Second, mutant NPM1 disorganizes PML bodies and RA/arsenic restores nuclear organization (nucleolar NPM1 with normal PML/SUMO bodies) ex vivo and in vivo. Finally, BM blasts are reduced in some treated patients. Mechanistically, these AMLs are addicted to continuous expression of the mutant protein32 so that degradation of mutant NPM1 most likely triggers cell cycle arrest, apoptosis, and differentiation. Reorganization of PML bodies could also contribute to P53 activation and therapy response.17,33
Cell cycle arrest and apoptosis induced by RA most likely reflect P53 activation with degradation of mutant NPM1. RA very efficiently degrades mutant NPM1 in OCI-AML3 cells, but not IMS-M2 cells. It remains obscure how RA selectively targets mutant NPM1 protein and this should be the focus of future studies. A variety of mutations were described in NPM1. Some of these may only confer sensitivity to RA-initiated and/or arsenic-initiated degradation. PML NBs are SUMO-dependent degradation factories activated by interferons and oxidative stress.27 Arsenic enhances formation of PML NBs and promotes the degradation of some PML-associated proteins. That pretreatment with interferon-α significantly accelerated arsenic-induced degradation of mutant NPM1 (data not shown), which could suggest a role of PML and/or SUMOs in the arsenic-triggered degradation process. In that respect, altered PML NBs biogenesis in NPM1 mutant AMLs could reflect a physical interaction between PML and mutant NPM1, a protein which is massively sumoylated.34 Other similarities between PML and NPM1 exist, such as link to p53 control, interferon signaling, or oxidative stress.35
Because elderly NPM1–mutated AML (>80 years old and/or with severe comorbidities) are unlikely to be eligible for treatment with chemotherapy, some patients were treated on a compassionate basis with the RA/arsenic combination used in APL. Although we did not observe complete remissions, the leukemia clearly regressed in several patients. This combination is unlikely to be curative alone, but may be part of a broader therapeutic strategy. We could demonstrate the relocalization of NPM1 to the nucleolus in primary AML blasts, pointing to therapy-induced restoration of nuclear organization. Yet, this combination was insufficient to obtain complete degradation of mutant NPM1 in AML patients in vivo. In APL, clinical response mirrors the extent of PML/RARA degradation and only full oncoprotein catabolism yields remissions.17,19 Some in vivo/ex vivo differences may be responsible for this blunted in vivo degradation by the RA/arsenic combination. For example, the arsenic-induced oxidative stress required for full NPM1 degradation may not be reached in vivo. Preclinical optimization, for example using xenografted mouse models,36 could address this point. Although five AML cell lines with WT NPM1 were not affected by RA/arsenic therapy ex vivo, we cannot exclude that other AML genotypes may be sensitive to RA/arsenic and/or that this combination may not elicit degradation of other oncoproteins.
These unexpected findings provide an intriguing parallel to the RA/arsenic-mediated degradation of PML/RARA in APL. They warrant further biochemical studies to elucidate the basis for the selective catabolism of NPM1 mutants. Our observations could explain the survival benefit of adding RA to chemotherapy in this subset of patients and warrants clinical evaluation of new therapeutic combinations incorporating frontline RA/arsenic in elderly ones.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
The online version of this article contains a data supplement.
There is an Inside Blood Commentary on this article in this issue.
Acknowledgment
The authors thank Drs Rihab Nasr and Kim Rice for critical reading of the manuscript and Miss Rabab El-Eit for help in synergy studies.
This work was supported by the Beirut Laboratory, which is supported by the American University of Beirut Medical Practice Plan, the University Research Board, the Lebanese National Council for Scientific Research, and ERC (StemAPL grant); the Paris Laboratory, which is supported by INSERM, CNRS, Université Paris-Diderot, Institut Universitaire de France, Ligue Contre le Cancer, Institut National du Cancer, the French National Research Agency (ANR) “Investissements d’Avenir” program (ANR-11-PHUC-002, ANR-10-IHUB-0002), Association pour la Recherche contre le Cancer (Griffuel Award to HdT), Canceropôle Ile de France, and the European Research Council (STEMAPL advanced grant to HdT); and by a fellowship of the Fondation pour la Recherche Medicale (U.S.).
Authorship
Contribution: H.E.H., H.d.T., and A.B. designed the study and wrote the manuscript; Z.D., C.B., R.H., N.T., A.S., and U.S. performed experiments; E.R., L.A., O.L., M.M., H.D., and P.F. treated AML patients; N.D. and K.Z. participated in study design and approved the final version of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Hiba El Hajj, American University of Beirut, Medical Center, P.O. Box 113-6044, Beirut, Lebanon; e-mail: he21@aub.edu.lb; and Hugues de Thé, UMR 944/7212, Hôpital St. Louis 1, Ave Claude Vellefaux, 75475 Paris, Cedex 10, France; e-mail: hugues.dethe@inserm.fr; and Ali Bazarbachi, American University of Beirut, Medical Center, P.O. Box 113-6044, Beirut, Lebanon; e-mail: bazarbac@aub.edu.lb.
References
Author notes
H.d.T. and A.B. contributed equally to this study.