In this issue of Blood, Martelli et al1 and El Hajj et al2 independently report that nucleophosmin-1 (NPM1)-mutant leukemia is particularly vulnerable to a novel strategy combining all-trans retinoic acid (ATRA) with arsenic trioxide (ATO). The era of targeted therapy has seen some of its greatest successes in the hematologic arena (eg, breakpoint cluster region [BCR]/Abelson [ABL] kinase inhibitors in chronic myeloblastic leukemia and ATRA in acute promyelocytic leukemia [APL]). Moreover, addition of ATO, an agent that induces oxidative stress and interferes with protein translation, to ATRA sharply increases APL cell killing to the extent that cures in this disease are no longer unrealistic.3 A theoretical (and practical) basis for translating ATRA/ATO-based strategies to non-APL acute myelocytic leukemia (AML) is currently lacking.
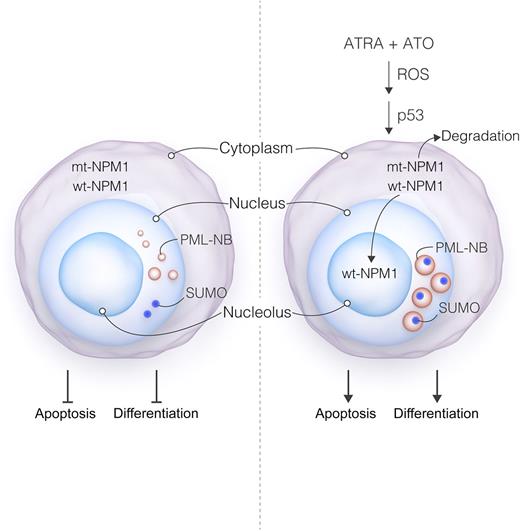
Proposed model of ATRA/ATO actions in NPM1-mutant cells. Mutant NPM1 leads to delocalization of both mutant (mt) and wild-type (wt) NPM1 (ie, from their normal nucleolar distribution to the cytoplasm). NPM1 mutation also results in disorganization of PML nuclear bodies (PML-NB), manifested by small size, heterogeneous appearance, nucleoplasmic localization, and dissociation from SUMO-1. These events, through mechanisms that have yet to be determined, disrupt both cell differentiation and apoptosis, contributing to leukemogenesis. Exposure of mutant NPM1-expressing cells to ATRA + ATO triggers pronounced oxidative stress and p53 activation, culminating in mutant NPM1 degradation. The loss of mutant NPM1 leads in turn to re-localization of wt NPM1 to the nucleolus, accompanied by reversal of PML-NB disorganization (eg, reflected by larger size, more homogeneous appearance, and restoration of the SUMO-1 association). These events culminate in leukemic cell differentiation and/or cell death. Professional illustration by Luk Cox, Somersault18:24.
Proposed model of ATRA/ATO actions in NPM1-mutant cells. Mutant NPM1 leads to delocalization of both mutant (mt) and wild-type (wt) NPM1 (ie, from their normal nucleolar distribution to the cytoplasm). NPM1 mutation also results in disorganization of PML nuclear bodies (PML-NB), manifested by small size, heterogeneous appearance, nucleoplasmic localization, and dissociation from SUMO-1. These events, through mechanisms that have yet to be determined, disrupt both cell differentiation and apoptosis, contributing to leukemogenesis. Exposure of mutant NPM1-expressing cells to ATRA + ATO triggers pronounced oxidative stress and p53 activation, culminating in mutant NPM1 degradation. The loss of mutant NPM1 leads in turn to re-localization of wt NPM1 to the nucleolus, accompanied by reversal of PML-NB disorganization (eg, reflected by larger size, more homogeneous appearance, and restoration of the SUMO-1 association). These events culminate in leukemic cell differentiation and/or cell death. Professional illustration by Luk Cox, Somersault18:24.
The central question addressed in the 2 studies is whether the ATRA/ATO strategy might be appropriate for non-APL leukemias, and particularly for leukemia characterized by another mutant oncoprotein, NPM1. NPM1 is a gene encoding a nucleolar shuttling protein that is frequently mutated in AML (30%) and which has been implicated in leukemogenesis. Although it carries a favorable prognosis, this feature is overcome by the presence of FMS-like tyrosine kinase-3 internal tandem duplication mutations. Moreover, despite initial responses to induction chemotherapy, relapses often occur in NPM1-mutant AML, and cures remain elusive, justifying the search for novel approaches. Although the mechanism of action (MOA) of NPM1 is not known with certainty, its deregulation leads to delocalization of mutant NPM1 from the nucleolus associated with disorganization of promyelocytic leukemia (PML) nuclear bodies. Interestingly, in APL cells, correction of PML disorganization accompanied by PML-retinoic acid receptor-α (PML-RAR-α) degradation and differentiation induction occurs with ATO treatment.4 Although pharmacologic inhibitors of NPM1 function (eg, deguelin) have been described,5 their clinical relevance remains to be established. Notably, previous studies have shown that genetic or pharmacologic NPM1 inhibition sensitizes mutant NPM1 leukemia cells to ATRA.6
Anecdotal evidence from the clinic indicating that NPM1 mutations may render AML patients susceptible to ATRA raised the possibility that a mutant oncoprotein-targeting strategy involving ATRA (possibly in combination with ATO) could be a viable alternative to specific pharmacologic NPM1 inhibitors. However, the mechanism(s) by which such a strategy might operate in this setting are unknown. The results of the 2 studies provide a theoretical foundation for applying this approach to mutant NPM1. Specifically, the authors report that exposure of NPM1-mutant AML cells to ATRA and ATO induces selective proteasomal degradation of mutant NPM1 protein accompanied by nucleolar redistribution of wild-type NPM1, reversal of the characteristic disorganization of PML bodies, and pronounced apoptosis and/or differentiation. Significantly, these events did not occur in non–NPM1-mutant leukemias, were dependent upon oxidative stress induction and p53 activation and, importantly, were also observed in primary NPM1-mutant AML blasts (see figure). Of note, El Hajj et al2 treated 5 elderly patients ineligible for chemotherapy with the ATRA/ATO regimen on a compassionate basis, and reductions in blasts and/or other hematologic improvements were observed in some of these patients. Collectively, these findings argue that, in addition to its established role in APL, the ATRA/ATO strategy may represent a viable option in NPM1-mutant AML and raise the possibility that analogous MOAs may be operative.
The findings presented in these studies could have implications well beyond NPM1-mutant AML and could be applicable to other leukemias (and possibly other hematologic malignancies) characterized by diverse mutant oncoproteins. They also highlight fundamental differences between approaches that directly inhibit specific mutant oncoproteins (driver mutations) vs those targeting so-called “orthogonal” processes required to circumvent neoplastic cell oncogenic stress.7 For example, a major success in leukemia therapy involved the introduction of inhibitors that specifically disrupt the function of the primary leukemogenic BCR/ABL kinase. More recently, specific IDH1/2 kinase inhibitors (eg, AG-221) have shown highly promising results in IDH2-mutant AML.8 Unfortunately, for many mutant oncoproteins, including transcription factors or proteins whose functions are not clearly defined, development of specific inhibitors can be problematic. However, such proteins may be vulnerable to agents that nonspecifically disrupt stress-related pathways required for oncoprotein maintenance. In this context, mutant oncoproteins are highly dependent upon chaperone proteins (eg, HSP90) to maintain stability, prompting the development of HSP90 antagonists in this setting.9 For various reasons, including host toxicity, a clear role for HSP90 antagonists has not yet been established in AML or other malignancies. Conversely, ATO induces oxidative stress in neoplastic cells10 and also interferes with the translation of mutant oncoproteins.11 It appears that, as in the case of PML-RARα APL, the mutant NPM1 protein may be particularly vulnerable to this approach and that its resulting degradation and redistribution may circumvent the need for specific NPM1 inhibitors. The possibility also exists that other mutant oncoproteins implicated in leukemogenesis, and for which specific inhibitors are not yet available, could represent additional targets for this strategy.
There are, however, several questions and caveats that could clearly determine the potential of this approach. For example, it remains to be established whether the ATRA/ATO strategy targets mutant NPM1-expressing leukemia-initiating cells (stem cells) which, at least theoretically, could be responsible for disease relapse. In addition, although both cultured and primary NPM1-mutant AML cells were very susceptible to this regimen, it is less certain whether the degree of cell killing can approximate that observed in the case of APL cells. Finally, although the ATRA/ATO regimen showed some activity in patients with mutant NPM1 AML, it is generally recognized that survival benefits in this (or other) disease(s) absent objective complete responses are unlikely. In this regard, the finding that ATRA/ATO enhanced the activity of an active anti-leukemic agent (eg, daunorubicin) in mutant NPM1 AML is noteworthy and raises the possibility of a more effective future approach. Alternatively, combining the ATRA/ATO strategy with other targeted therapies, including those directed against NPM1 itself, may provide opportunities for cure in this disease, as observed in some patients with APL. Finally, extrapolation of this strategy to other AML subtypes that display different oncogenic mutant proteins represents a promising possibility and one that clearly deserves further investigation.
Conflict-of-interest disclosure: The author declares no competing financial interests.