In this issue of Blood, Jitschin et al demonstrate a microenvironmental glycolytic shift in chronic lymphocytic leukemia (CLL) cells mediated by Notch-c-Myc signaling. Interfering in the Notch-c-Myc pathway and reprogramming glycolytic metabolism could contribute to overcoming drug resistance in CLL.1
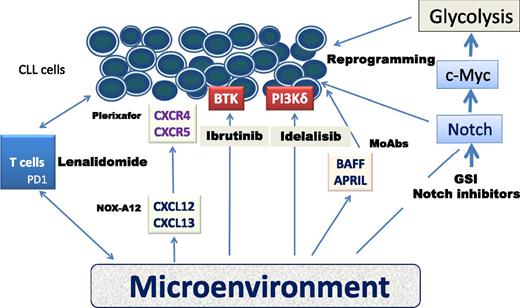
Schematic representation of the microenvironment and agents with the potential of targeting specific CLL pathways. APRIL, a proliferation-inducing ligand; BAFF, B cell–activating factor of the TNF family; BTK, Bruton tyrosine kinase; PD1, programmed death 1 receptor; PI3Kδ, phosphoinositide 3-kinase δ.
Schematic representation of the microenvironment and agents with the potential of targeting specific CLL pathways. APRIL, a proliferation-inducing ligand; BAFF, B cell–activating factor of the TNF family; BTK, Bruton tyrosine kinase; PD1, programmed death 1 receptor; PI3Kδ, phosphoinositide 3-kinase δ.
CLL is the prototypical tumor in which the microenvironment plays a key role in the physiopathology of the disease. CLL cells cultivated in vitro die quickly unless they are grown in a microenvironment-mimicking milieu. The CLL microenvironment (an admixture of stromal cells, monocyte-derived nursing cells, T cells, and macrophages) promotes cell growth, traffic of cells between tissues and blood, and cells homing in protected niches. Importantly, the microenvironment also protects CLL cells from both spontaneous- and cytotoxic-mediated apoptosis. Unraveling the mechanisms accounting for these effects is therefore crucial to understanding CLL physiopathology and identifying potential treatment targets.
The microenvironment activates and protects CLL cells through several mechanisms. CLL surface molecules (eg, B-cell receptor, CD38, CXC chemokine receptor 4 [CXCR4]), chemokines (eg, CXC chemokine ligands 12, 13 [CXCL12, CXCL13]), adhesion molecules (eg, fibroblast growth factor, platelet-derived growth factor, very late antigen-4, stromal-derived factor), and tumor necrosis factor (TNF) receptor members (eg, CD40, B-cell maturation antigen, B cell–activating factor receptor, transmembrane activator and calcium modulator and cyclopholin ligand interactor) engage in crosstalk with their respective tissue ligands. This results in survival and expansion of the CLL clone, and protects CLL cells from apoptosis.2
On the other hand, a main feature of cancer cells is their ability to avidly take up glucose and convert it to lactate, even in the presence of sufficient oxygen (“Warburg effect”). This altered glycolytic dependency leads to a less efficient generation of adenosine triphosphate compared with the oxidative phosphorylation process which occurs in normal cells.3 It has been shown that CLL cells exert increased oxidative phosphorylation in mitochondria,4 but whether stromal cells may induce metabolic changes in CLL cells is largely unknown. In this context, it is worth mentioning that oncogenes and tumor suppressor genes are strongly linked to metabolic pathways through transcriptional or posttranscriptional regulation of metabolic enzymes.3
To shed light on these issues, Jitschin et al studied the glycolytic metabolism in 49 peripheral blood samples coculturing CLL cells with the HS-5 human bone marrow stromal cell line, primary bone marrow–derived mesenchymal stromal cells, or lymph node–derived fibroblasts. They found that CLL cells showed increased glycolysis accompanied by higher lactic acid production, glucose uptake, and glucose transportation, as well as expression of key enzymes (eg, hexokinase-2, lactate dehydrogenase A, enolase-1) involved in this process. Furthermore, they demonstrated that Notch-c-Myc signaling participates in these events. In line with recent evidence linking Notch signaling with stromal cell–mediated effects,5 cocultivated CLL cells upregulated the expression of the Notch-1 receptor and the downstream target gene Hes-1, reflecting a canonical Notch activation. Interestingly, a correlation between Notch-1 mutated CLL cells and an increased glycolytic metabolism was also found. To close the loop, CLL cells cocultured in a microenvironment-mimicking milieu displayed a significant upregulation of the c-Myc gene and its protein expression. In fact, the inhibition of the Notch signaling pathway by a γ-secretase inhibitor (GSI) resulted not only in decreased stromal-mediated upregulation of c-Myc and its target gene cyclinb1, but also in the abolishment of the glycolytic shift. As a result, a decrease in glycolytic enzymes and extracellular acidification rate were observed. Furthermore, this inhibition conveyed a synergistic effect on fludarabine-induced cell death on CLL cells under stromal contact.
In short, besides well-known physiopathological connections between the microenvironment and CLL cells, it appears that the microenvironment has an important role in shifting glycolysis in CLL cells, and that this effect is at least mediated in part by the Notch-c-Myc axis.
From the clinical perspective, CLL treatment is rapidly moving from cytotoxic agents, which in most cases are given in combination with an anti-CD20 monoclonal antibody (MoAb), to noncytotoxic compounds targeting specific CLL pathways revolving around the microenvironment (see figure). Some of these agents (eg, ibrutinib, idelalisib) have entered into clinical practice, have shown remarkable effectiveness, and are changing CLL treatment algorithms.6 Now, to the plethora of microenvironment-governed chemokines that operate in a complex and the intertwined network of different pathways, an old and well-known actor in the physiopathology of cancer, the so-called Warburg effect, does apply for a prominent role in CLL biology and its therapy. In fact, reprogramming glycolytic metabolism is again being considered a target for cancer treatment. There is no reason why CLL should be an exception to this appealing approach. In the exciting era of precision medicine for CLL, the study reported by Jitschin et al paves the road for further studies on CLL metabolism and its potential implications in the treatment of this still incurable disease.
Conflict-of-interest disclosure: The author declares no competing financial interests.