Key Points
Interaction of the integrin β3 cytoplasmic tail with kindlin-2 selectively promotes outside-in signaling through αVβ3.
Disruption of the kindlin-2/αVβ3 interaction impairs outside-in signaling and endothelial cell functions, both in vitro and in vivo.
Abstract
The bidirectional signaling and hemostatic functions of platelet αIIbβ3 are regulated by kindlin-3 through interactions with the β3 cytoplasmic tail. Little is known about kindlin regulation of the related “vitronectin receptor,” αVβ3. These relationships were investigated in endothelial cells, which express αVβ3 and kindlin-2 endogenously. “β3ΔRGT” knock-in mice lack the 3 C-terminal β3 tail residues, whereas in “β3/β1(EGK)” mice, RGT is replaced by the corresponding residues of β1. The wild-type β3 tail pulled down kindlin-2 and c-Src in vitro, whereas β3ΔRGT bound neither protein and β3/β1(EGK) bound kindlin-2, but not c-Src. β3ΔRGT endothelial cells, but not β3/β1(EGK) endothelial cells, exhibited migration and spreading defects on vitronectin and reduced sprouting in 3-dimensional fibrin. Short hairpin RNA silencing of kindlin-2, but not c-Src, blocked sprouting by β3 wild-type endothelial cells. Moreover, defective sprouting by β3ΔRGT endothelial cells could be rescued by conditional, forced interaction of αVβ3ΔRGT with kindlin-2. Stimulation of β3ΔRGT endothelial cells led to normal extracellular ligand binding to αVβ3, pin-pointing their defect to one of outside-in αVβ3 signaling. β3ΔRGT mice, but not β3/β1(EGK) mice, exhibited defects in both developmental and tumor angiogenesis, responses that require endothelial cell function. Thus, the β3/kindlin-2 interaction promotes outside-in αVβ3 signaling selectively, with biological consequences in vivo.
Introduction
Integrins are transmembrane receptors composed of α and β subunits that mediate bidirectional signaling between the extracellular matrix and the cell interior. Among the best studied integrins is αIIbβ3, which is required for hemostasis and platelet involvement in thrombosis.1,2 The related β3 integrin, αVβ3, is expressed at low levels in platelets and better expressed in several other hematopoietic cells and in cells of the vasculature, particularly proliferative endothelial cells (ECs).3 Studies of αVβ3 function in vitro and in genetically modified mice appear somewhat contradictory. Antibodies or small molecules that block the binding of vitronectin and other matrix ligands to αVβ3 can inhibit αVβ3-dependent cell migration and angiogenesis.4 However, β3 knockout mice show increased tumor growth and angiogenesis, in part from elevated expression of vascular endothelial growth factor receptor-2 (VEGFR-2) in β3−/− ECs.5 Furthermore, acute but not long-term depletion of β3 in ECs inhibits tumor growth and angiogenesis in mice.6 Global or tissue-specific ablation of β3 may lead to altered expression of nontargeted genes, VEGFR-2 being one example. Generation of knock-in mutations in β3 may avoid this problem, as exemplified by normal VEGFR-2 expression in “β3(DiY>F)” knock-in mice, in which 2 β3 cytoplasmic tail tyrosines (747 and 759) are mutated to phenylalanine, resulting in reduced tumor angiogenesis.7 This result was attributed to effects on bidirectional integrin signaling and to disruption of normal interactions between αVβ3 and VEGFR-2. Thus, further studies of β3 knock-in mice might lead to a deeper understanding of αVβ3 function.
Integrin signaling encompasses “inside-out” regulation of adhesive ligand binding to integrins and ligand-dependent “outside-in” regulation of cellular responses.8 In both contexts, signaling can be influenced by interactions of integrin cytoplasmic tails with specific enzymes and adapters.8,9 Among proteins capable of interacting with the β3 cytoplasmic tail are the Src family protein tyrosine kinases (SFKs) and the adapters talin and kindlin. One function of SFKs in platelets and ECs is the phosphorylation of β3 Y747 and Y759, resulting in reduced interaction of β3 with talin10 and kindlins,11,12 respectively. Direct interaction between c-Src and the extreme C terminus of the β3 cytoplasmic tail appears to be required for normal αIIbβ3-dependent platelet functions in vivo13 and for the αVβ3-dependent functions of stem cells14 and tumor cells.15 Consequently, binding of c-Src to the β3 tail in ECs might regulate αVβ3 signaling. Indeed, “β3(DiY>F)” knock-in mice with phenylalanine substitutions at the 2 putative SFK phosphorylation sites exhibit reduced angiogenesis.10,16
The kindlin family of adapters (kindlin-1, kindlin-2, and kindlin-3) plays key roles in multiple cell types to promote bidirectional signaling involving β1, β2, and β3 integrins.11,17-21 For example, kindlin-3 cooperates with talin to effect β2 activation in leukocytes and β3 activation in platelets.22,23 Moreover, interaction between kindlin-3 and β2 integrins seems to be required for T-cell homing in vivo.24 Kindlin-2 is the predominant isoform in ECs, and kindlin-2+/− mice exhibit reduced tumor vessel density and growth,25 suggesting a role for kindlin-2 in EC functions. However, because kindlins may also function in integrin-independent processes,26 the extent to which any function of kindlin-2 in ECs is due to its direct interaction with integrin β cytoplasmic tails remains to be determined.
Here we sought to clarify the role of αVβ3 signaling in ECs and by so doing shed more light on the molecular underpinnings of αVβ3 function. We have taken advantage of 2 β3 knock-in mouse strains with deletion or mutations of the 3 most C-terminal β3 residues (R760GT762).13 Studies of αIIbβ3 in murine platelets and in heterologous expression systems have demonstrated that the C-terminal 3 amino acids of the β3 cytoplasmic tail (Arg-Gly-Thr, RGT) are required for β3 interactions with c-Src and certain other SFKs.13,27,28 In addition, residues at or near the C terminus of integrin β tails support tail interaction with kindlins.11,18,19,29 For example, the last 3 amino acids (Glu-Gly-Lys, EGK) of β1 are required for kindlin-2 interaction18 ; however, they do not enable c-Src binding to β1.27 Therefore, we hypothesized that deletion of RGT would prevent normal kindlin-2 binding to β3, whereas substitution of RGT with EGK would enable kindlin-2 binding to β3. By studying ECs as a biologically relevant experimental platform, we have established the differential roles of the β3/kindlin-2 and the β3/c-Src interactions in αVβ3 functions. Specifically, the interaction of β3 with kindlin-2, but not with c-Src, is required for outside-in signaling through αVβ3, with implications for EC function in vitro and in vivo.
Methods
Reagents, DNA constructs, cell lines, and mice are described in detail in the supplemental Methods on the Blood Web site. All animal procedures were performed using protocols approved by the University of California San Diego Institutional Animal Care and Use Committee.
Western blotting and pull-down assays
Western blotting and pull-down assays using recombinant integrin tails were performed as described elsewhere.30-33
Cell migration
Transwells with 8-μm pores were coated on both sides with 10 ng/mL vitronectin overnight at 4°C. Then 105 immortalized lung ECs (imLECs) in 200 μL of endothelial basal medium were added to the upper chamber and 700 μL of medium (with or without 10% fetal bovine serum) were added to the lower chamber. After 16 hours at 37°C and 5% CO2, cells on the upper surface of the chamber were removed with a cotton swab and migrated cells on the lower surface were fixed with methanol at −20°C for 15 minutes and stained with 0.1% crystal violet. Migration was quantified by eluting crystal violet in 10% acetic acid and measuring absorbance at 590 nm.
Detection of αVβ3 activation
A ligand-mimetic antibody, WOW-1 Fab, was used to detect activated αVβ3 in ECs.32 For suspended cells, 4-6 × 105 imLECs in 50 µL modified Tyrode’s buffer were incubated for 30 minutes at 22°C with WOW-1 Fab ± 200 nM phorbol 12,13-dibutyrate (PDBu), a protein kinase C activator. After addition of 50 μg/mL Alexa Fluor 647-labeled F(ab′)2 fragment of goat anti-mouse immunoglobulin G (H + L chains), WOW-1 Fab binding was determined by flow cytometry. Nonspecific binding was determined in the presence of 5 mM EDTA, and specific binding was taken as total minus nonspecific binding.32 For adherent cells, imLECs were plated on coverslips coated with 25 μg/mL fibrinogen and incubated ± 200 nM PDBu for 1 hour at 37°C. Cells were fixed and permeabilized before staining with WOW-1 Fab and rhodamine-phalloidin for F-actin.33 Confocal images were acquired using an Olympus FV1000 confocal microscope.
Three-dimensional fibrin gel bead assay
Retinal angiogenesis
Mouse eyes were obtained at postnatal day 3 and onwards by enucleation. After fixation with 2% paraformaldehyde in phosphate-buffered saline (PBS) for 1 hour at 4°C, lens, sclera, cornea, and vitreous were excised and the retina dissected.36,37 Tissues were fixed, permeabilized, and stained overnight at 4°C with rat anti-mouse CD31 antibody MEC13.3 (1:100). Retinas were washed in PBS, stained with Alexa Fluor 647–conjugated goat anti-rat secondary antibody and mounted by making 4 incisions in anti-fade mounting medium. Images of retinal vessels were acquired using LSM 5 software on a Zeiss 510 confocal microscope with a ×10 oil-objective lens (Nikon) and analyzed with Adobe Photoshop CS4 (Adobe Systems) and ImageJ (NIH). To assess proliferation, retinal endothelium was labeled with fluorescein isothiocyanate–isolectin B4 and stained with rat anti-mouse Ki67. Endothelial tip cells and filopodia per 25 μm of vessel length were quantified in sprouting vascular fronts after photographing at ×40 and ×100, respectively, using an Olympus FV1000 confocal microscope and ImageJ.
Pathological angiogenesis
β3+/+ (wild-type; WT), β3+/−, β3ΔRGT, and β3/β1(EGK) mice were inoculated subcutaneously with 2 × 105 B16F1 murine melanoma cells in 300 μL PBS. Twenty-one days later, tumors were excised and weighed. Frozen sections were fixed with acetone/4% paraformaldehyde, permeabilized with 0.1% Triton X-100 in PBS, blocked with 10% goat serum, and stained with anti-mouse CD31, appropriate secondary antibody, and with Hoechst 33342 for nuclei. Images were acquired using a PlanFluor ×10 objective and an Eclipse TE2000-U microscopy system (Nikon). The area of CD31-positive blood vessels was quantified by calculating mean positive pixels from ≥9 representative images of each tumor using MATLAB software.7
Bone marrow transplantation
Bone marrow was harvested from euthanized donor mice by flushing femurs with complete medium (Dulbecco’s modified Eagle medium, 10% fetal bovine serum) and dissociated by passage through a 22G needle. After red blood cell lysis, 2 × 106 bone marrow cells were injected intravenously into irradiated (1000 rads) recipient mice. B16 tumors were grown in recipient mice >4 weeks after transplant.
Results
Investigation of the β3/kindlin-2 interaction
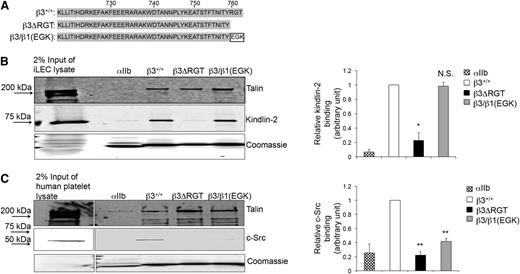
Because specific regions of the β3 tail may support its interaction with 1 or more intracellular proteins,38-40 we sought to establish a means to evaluate the selective interaction of kindlin-2 with β3 in the context of integrin αVβ3. Therefore, affinity chromatography27,28,31 was used to identify mutant β3 tails that would enable selective study of the β3/kindlin-2 interaction (Figure 1A). Unlike the WT β3 tail that bound kindlin-2 and c-Src, a β3ΔRGT tail lacking the C-terminal RGT sequence did not bind c-Src and bound little kindlin-2 (Figure 1B-C). In contrast, the β3/β1(EGK) tail, where RGT was replaced by the corresponding residues in β1 (Glu-Gly-Lys),13 retained the ability to bind kindlin-2, but bound little if any c-Src (Figure 1B-C). All these β3 tails bound talin, which interacts with more membrane-proximal β3 tail residues.8,39,40 Thus, we reasoned that a comparison of αVβ3 function in the context of the β3ΔRGT tail vs the β3/β1(EGK) tail would allow a determination of the selective role of the β3/kindlin-2 interaction in cells.
Effects of β3 cytoplasmic tail mutations on β3/protein interactions. (A) Amino acid sequences of the β3+/+, β3ΔRGT, and β3/β1(EGK) cytoplasmic tails used in these studies. Pull-down of c-Src, kindlin-2, or talin from (B) immortalized mouse lung endothelial cell lysate (as a convenient source of talin and kindlin-2) or (C) human platelet lysate (as a convenient source of talin and c-Src) by recombinant β3+/+ or mutant β3 cytoplasmic domain model proteins. The αIIb cytoplasmic domain was used as a negative control. Bound talin, kindlin-2, and c-Src were detected by immunoblotting. Loading of pull-down beads with recombinant cytoplasmic domains was monitored by staining with Coomassie brilliant blue. (Left panel) Representative images of pull-downs; (right panel) quantification of binding of kindlin-2 (B) or c-Src (C). Binding of kindlin-2 or c-Src to αIIb or mutant β3 tails was expressed relative to their binding to the β3+/+ tail, which was arbitrarily taken as 1.0. Data represent means ± standard error of the mean (SEM) of 3 independent experiments. **P < .01; *P < .05; NS, not significant (paired Student t test).
Effects of β3 cytoplasmic tail mutations on β3/protein interactions. (A) Amino acid sequences of the β3+/+, β3ΔRGT, and β3/β1(EGK) cytoplasmic tails used in these studies. Pull-down of c-Src, kindlin-2, or talin from (B) immortalized mouse lung endothelial cell lysate (as a convenient source of talin and kindlin-2) or (C) human platelet lysate (as a convenient source of talin and c-Src) by recombinant β3+/+ or mutant β3 cytoplasmic domain model proteins. The αIIb cytoplasmic domain was used as a negative control. Bound talin, kindlin-2, and c-Src were detected by immunoblotting. Loading of pull-down beads with recombinant cytoplasmic domains was monitored by staining with Coomassie brilliant blue. (Left panel) Representative images of pull-downs; (right panel) quantification of binding of kindlin-2 (B) or c-Src (C). Binding of kindlin-2 or c-Src to αIIb or mutant β3 tails was expressed relative to their binding to the β3+/+ tail, which was arbitrarily taken as 1.0. Data represent means ± standard error of the mean (SEM) of 3 independent experiments. **P < .01; *P < .05; NS, not significant (paired Student t test).
Disruption of the β3/kindlin-2 interaction impairs αVβ3-dependent cellular functions
Primary murine lung ECs (LECs) from β3 WT, and β3ΔRGT homozygous and β3/β1(EGK) homozygous mice were isolated.13 Surface expression of β3 in β3ΔRGT LECs was ∼50% of that in β3+/+ LECs and similar to β3 expression in β3+/− LECs. β3/β1(EGK) LECs expressed ∼80% the level of β3 in β3+/+ LECs (supplemental Figure 1A). Unlike the elevated expression of VEGFR-2 in ECs from β3−/− mice,5 VEGFR-2 expression was normal in β3ΔRGT ECs. Moreover, VEGF-induced tyrosine phosphorylation of VEGFR-2 and serine-threonine phosphorylation of its downstream target, ERK1/2, were normal in β3ΔRGT ECs (supplemental Figure 1B).
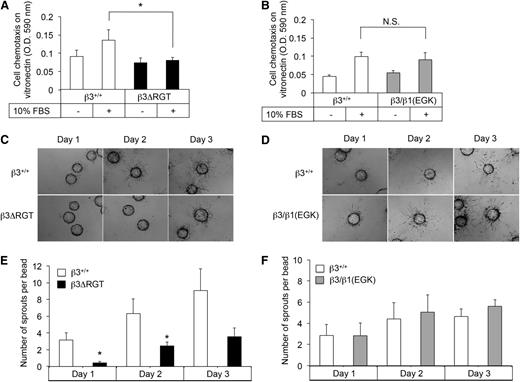
To obtain unlimited quantities of ECs for further study, LECs were immortalized41 and single colonies were selected. imLECs grew well in culture at the permissive temperature of 33°C and displayed an EC morphology at 37°C (supplemental Figure 2A). Surface expression of β3 and αV was similar in β3ΔRGT and β3+/+ imLECs (supplemental Figure 2B). Because αVβ3 promotes EC migration,42 we tested whether the deletion or substitution of β3 RGT affected migration in a chemotactic Transwell assay. Compared with β3+/+ imLECs, β3ΔRGT imLECs showed reduced migration across vitronectin in the presence of a serum gradient (P < .05) (Figure 2A). In contrast, migration of β3/β1(EGK) imLECs was similar to that of β3+/+ imLECs (Figure 2B). β3ΔRGT imLECs also showed reduced migration across another matrix ligand for αVβ3 (fibrinogen), but not across collagen, a ligand for certain β1 integrins (not shown). Thus, the β3 tail C terminus promotes αVβ3-dependent EC migration in a manner that likely requires β3 tail interaction with kindlin-2, the only kindlin isoform we could detect in these cells by western blotting.
Immortalized β3ΔRGT ECs exhibit reduced migration and angiogenic sprouting. (A-B) Cell migration across vitronectin-coated Transwells. (C-D) Representative images of fibrin gel bead assays to assess EC sprouting from day 1 to day 3; bead diameter, 60-87 μm. (E-F) Quantification of EC sprouting in the 3-dimensional fibrin gel bead assay. Data represent means ± SEM of 4 experiments. *P < .05; NS, not significant (paired Student t test); OD, optical density.
Immortalized β3ΔRGT ECs exhibit reduced migration and angiogenic sprouting. (A-B) Cell migration across vitronectin-coated Transwells. (C-D) Representative images of fibrin gel bead assays to assess EC sprouting from day 1 to day 3; bead diameter, 60-87 μm. (E-F) Quantification of EC sprouting in the 3-dimensional fibrin gel bead assay. Data represent means ± SEM of 4 experiments. *P < .05; NS, not significant (paired Student t test); OD, optical density.
When ECs are cultured on microcarrier beads in a 3-dimensional fibrin matrix, they extend sprouts analogous to those formed by ECs during angiogenesis.34,43 In this system, β3+/+ imLECs started sprouting from beads within 1 day, and sprouts continued to elongate for up to 72 hours (Figure 2C-D). Sprout formation in fibrin could be blocked by cilengitide, an inhibitor of αVβ3 and αVβ5,44 suggesting that this process is dependent on αVβ3 under these experimental conditions. Significantly fewer sprouts were observed at 24 and 48 hours from beads coated with β3ΔRGT imLECs (P < .05) (Figure 2C,E), whereas β3/β1(EGK) imLECs formed sprouts normally (Figure 2D,F). Similar results were obtained with primary β3ΔRGT ECs (supplemental Figure 3). Thus, the C terminus of the β3 cytoplasmic tail promotes EC sprouting in a manner that likely requires tail interaction with kindlin-2.
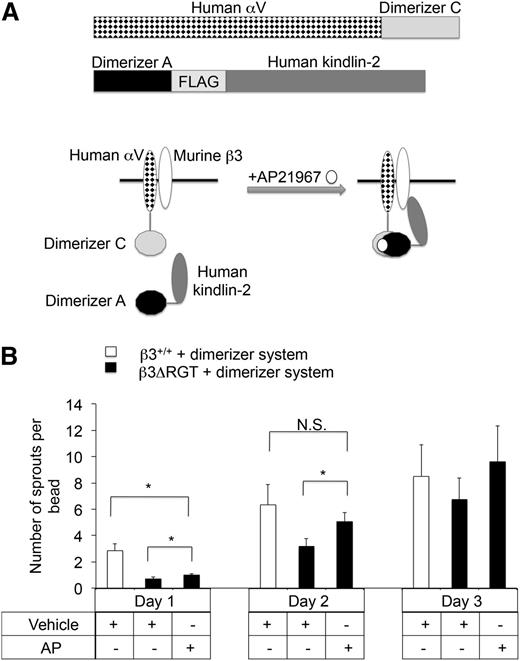
To provide direct evidence that a proximal interaction between kindlin-2 and β3 promotes EC sprout formation, a heterodimerizer system was used to enforce this interaction in imLECs expressing β3ΔRGT. Following short hairpin RNA (shRNA) knock-down of endogenous αV, β3ΔRGT or β3+/+ imLECs were engineered to stably coexpress human αV and kindlin-2 fusion proteins designed to heterodimerize upon addition of a small molecule, AP2196745,46 (Figure 3A). Expression of the fusion proteins was similar in β3ΔRGT and β3+/+ imLECs (supplemental Figure 4). Incubation of the β3ΔRGT cells with AP21967 caused a significant increase in sprouting (P < .05) that by day 2 was similar to the level observed in control β3+/+ imLECs that had not been exposed to AP21967 (Figure 3B). Thus conditional, enforced interaction between kindlin-2 and αVβ3ΔRGT rescues angiogenic sprouting by ECs.
Enforced interaction of αVβ3ΔRGT with kindlin-2 rescues angiogenic sprouting by ECs. (A) Schematic of the inducible heterodimerizer system used here. (Top panel) The 2 fusion proteins employed: human αV-dimerizer C and dimerizer A-FLAG-human kindlin-2. β3+/+ or β3ΔRGT immortalized lung endothelial cells were transduced with lentiviruses encoding both of these fusion proteins and an shRNA specific for mouse αV. In theory, after addition of a cell-permeable heterodimerizer (AP21967), human αV-dimerizer C should associate with dimerizer A-human kindlin-2, thereby enforcing interaction between αVβ3 and kindlin-2. (B) Cytodex beads coated with either β3+/+ or β3ΔRGT endothelial cells expressing the dimerizer system described in panel A were incubated overnight at 37°C with 250 nM AP21967 (AP) or vehicle control. Then sprout formation by the cells was evaluated using the 3-dimensional fibrin gel bead assay. Data represent means ± SEM of 4 independent experiments; *P < .05; NS, not significant (paired Student t test).
Enforced interaction of αVβ3ΔRGT with kindlin-2 rescues angiogenic sprouting by ECs. (A) Schematic of the inducible heterodimerizer system used here. (Top panel) The 2 fusion proteins employed: human αV-dimerizer C and dimerizer A-FLAG-human kindlin-2. β3+/+ or β3ΔRGT immortalized lung endothelial cells were transduced with lentiviruses encoding both of these fusion proteins and an shRNA specific for mouse αV. In theory, after addition of a cell-permeable heterodimerizer (AP21967), human αV-dimerizer C should associate with dimerizer A-human kindlin-2, thereby enforcing interaction between αVβ3 and kindlin-2. (B) Cytodex beads coated with either β3+/+ or β3ΔRGT endothelial cells expressing the dimerizer system described in panel A were incubated overnight at 37°C with 250 nM AP21967 (AP) or vehicle control. Then sprout formation by the cells was evaluated using the 3-dimensional fibrin gel bead assay. Data represent means ± SEM of 4 independent experiments; *P < .05; NS, not significant (paired Student t test).
Kindlin-2, but not c-Src, promotes sprout formation by ECs
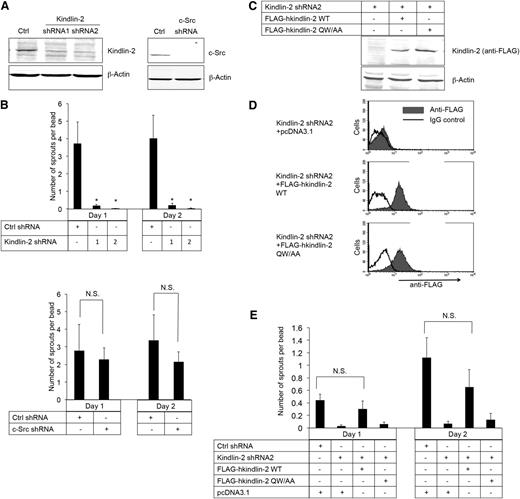
To establish directly whether kindlin-2 and/or c-Src is required for EC sprouting, shRNAs were used to knock down each of these proteins in β3+/+ imLECs. Knock-down was achieved with each of 2 kindlin-2 shRNAs and 1 c-Src shRNA, but not with a control, scrambled shRNA (Figure 4A). Knock-down of kindlin-2 blocked angiogenic sprout formation by β3+/+ imLECs (Figure 4B, upper panel), an effect that was rescued by coexpression of shRNA-resistant human kindlin-2 (Figure 4C-E). In contrast, knock-down of c-Src in β3+/+ imLECs had no effect on sprout formation (Figure 4B, lower panel), nor did incubation of cells with SFK inhibitors, PP1 or PP2 (not shown). Moreover, when β3+/+ imLECs depleted of kindlin-2 were used to express a human kindlin-2 mutant (Gln614 → Ala; Trp615 → Ala) with reduced binding to β3,11,47 angiogenic sprout formation failed to be rescued (Figure 4C-E). These results establish that the binding of both kindlin-2 and c-Src to β3 requires an intact β3 tail C terminus, but only the former interaction promotes EC sprout formation.
β3 interaction with kindlin-2, but not with c-Src, promotes angiogenic sprouting by ECs. (A) Western blots of kindlin-2 and c-Src in β3+/+ immortalized lung endothelial cells transduced with lentivirus encoding kindlin-2 shRNA (shRNA1 and shRNA2), a c-Src shRNA, or a control (Ctrl) shRNA. β-actin was monitored as a loading control. (B) Quantification of sprouts by the cells transduced with lentiviruses encoding the kindlin-2 (upper panel), c-Src (lower panel), or Ctrl shRNAs. (C-E) Cells were transduced with kindlin-2 shRNA2 and then transiently transfected with shRNA-resistant complementary DNAs encoding either FLAG-human kindlin-2 (FLAG-hkindlin-2 WT), FLAG-hkindlin-2 QW/AA mutant, or nothing (pcDNA3.1 empty vector). (C) Western blots of human kindlin-2 detected with an anti-FLAG antibody. (D) Flow cytometry histograms depicting intracellular expression of shRNA-resistant kindlin-2 using anti-FLAG antibody. (E) Kindlin-2 rescue of endothelial cell sprout formation in the 3-dimensional fibrin gel bead assay. Note that transfection by electroporation decreased cell vitality so that the number of the sprouts was lower compared with parental immortalized lung endothelial cells. Quantification of EC sprouting is depicted as means ± SEM of 5 experiments. *P < .05; NS, not significant (paired Student t test).
β3 interaction with kindlin-2, but not with c-Src, promotes angiogenic sprouting by ECs. (A) Western blots of kindlin-2 and c-Src in β3+/+ immortalized lung endothelial cells transduced with lentivirus encoding kindlin-2 shRNA (shRNA1 and shRNA2), a c-Src shRNA, or a control (Ctrl) shRNA. β-actin was monitored as a loading control. (B) Quantification of sprouts by the cells transduced with lentiviruses encoding the kindlin-2 (upper panel), c-Src (lower panel), or Ctrl shRNAs. (C-E) Cells were transduced with kindlin-2 shRNA2 and then transiently transfected with shRNA-resistant complementary DNAs encoding either FLAG-human kindlin-2 (FLAG-hkindlin-2 WT), FLAG-hkindlin-2 QW/AA mutant, or nothing (pcDNA3.1 empty vector). (C) Western blots of human kindlin-2 detected with an anti-FLAG antibody. (D) Flow cytometry histograms depicting intracellular expression of shRNA-resistant kindlin-2 using anti-FLAG antibody. (E) Kindlin-2 rescue of endothelial cell sprout formation in the 3-dimensional fibrin gel bead assay. Note that transfection by electroporation decreased cell vitality so that the number of the sprouts was lower compared with parental immortalized lung endothelial cells. Quantification of EC sprouting is depicted as means ± SEM of 5 experiments. *P < .05; NS, not significant (paired Student t test).
The β3/kindlin-2 interaction appears dispensable for inside-out activation of αVβ3
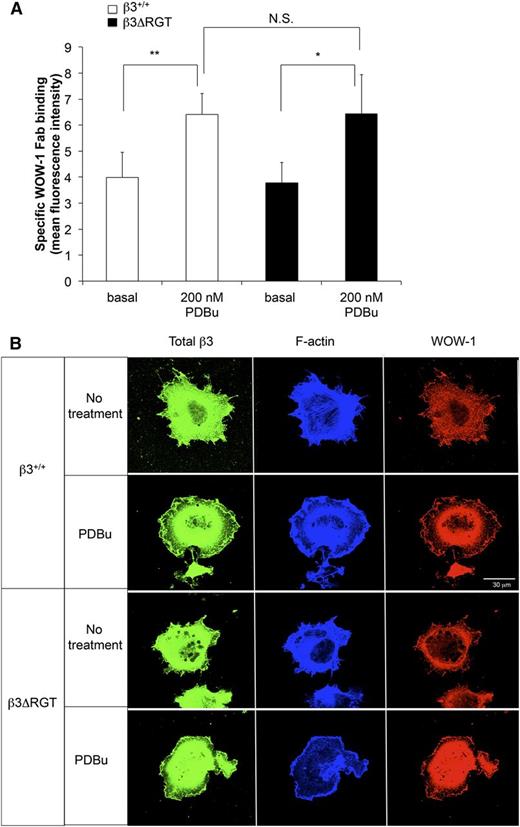
αVβ3 is responsive to inside-out signals that lead to αVβ3 activation and extracellular ligand binding.48 We wondered if the C terminus of β3 was required for this process in ECs. imLECs were stimulated briefly with the protein kinase C activator, PDBu, and activation of αVβ3 was monitored with WOW-1 Fab.32,33 Incubation of β3ΔRGT imLECs with PDBu led to an approximately 2-fold increase in specific WOW-1 Fab binding, a response similar to that observed with β3+/+ imLECs (Figure 5A). When imLECs were plated on fibrinogen-coated coverslips and examined by confocal microscopy, WOW-1 Fab binding was observed at protrusive edges of cells expressing either β3ΔRGT or β3+/+ (Figure 5B). Thus, the C-terminal 3 amino acids of β3, and by extrapolation the binding of kindlin-2 to β3, may not be required to initiate activation of αVβ3 in ECs, suggesting rather that they promote outside-in αVβ3 signaling.
Deletion of RGT from the β3 cytoplasmic tail does not impair activation of endothelial cell αVβ3. (A) Immortalized lung endothelial cells were incubated for 30 minutes at room temperature with WOW-1 Fab in the presence or absence of 200 nM PDBu (to activate protein kinase C) or with 5 mM EDTA (to inhibit specific WOW-1 Fab binding). WOW-1 Fab binding was measured by flow cytometry as described in “Methods.” Data represent specific WOW-1 Fab binding, defined as binding inhibited by EDTA, and represent means ± SEM of 3 independent experiments. **P < .01; *P < .05; NS, not significant (paired Student t test). (B) Localization of activated αVβ3 at lamellipodial edges. Cells were plated onto coverslips coated with fibrinogen and allowed to spread for 1 hour in the presence or absence of 200 nM PDBu as indicated. Cells were fixed, permeabilized, and stained with WOW-1 Fab, with antibody to total β3, and with phalloidin for F-actin as described in “Methods.” Bar represent 30 μm.
Deletion of RGT from the β3 cytoplasmic tail does not impair activation of endothelial cell αVβ3. (A) Immortalized lung endothelial cells were incubated for 30 minutes at room temperature with WOW-1 Fab in the presence or absence of 200 nM PDBu (to activate protein kinase C) or with 5 mM EDTA (to inhibit specific WOW-1 Fab binding). WOW-1 Fab binding was measured by flow cytometry as described in “Methods.” Data represent specific WOW-1 Fab binding, defined as binding inhibited by EDTA, and represent means ± SEM of 3 independent experiments. **P < .01; *P < .05; NS, not significant (paired Student t test). (B) Localization of activated αVβ3 at lamellipodial edges. Cells were plated onto coverslips coated with fibrinogen and allowed to spread for 1 hour in the presence or absence of 200 nM PDBu as indicated. Cells were fixed, permeabilized, and stained with WOW-1 Fab, with antibody to total β3, and with phalloidin for F-actin as described in “Methods.” Bar represent 30 μm.
Role of the integrin β3/kindlin-2 interaction in vivo
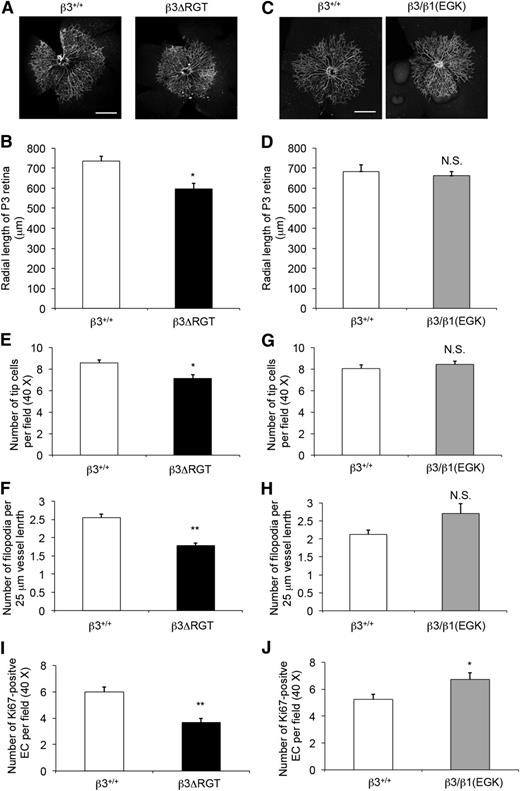
One example of EC function in vivo is angiogenesis. Retinal angiogenesis occurs postnatally in the mouse.49 When compared with β3+/+ pups, β3ΔRGT homozygous pups exhibited a delay in radial expansion of their retinal vascular beds at postnatal day 3 (P < .05) (Figure 6A-B), whereas radial expansion was normal by day 7. In contrast, retinal angiogenesis in β3/β1(EGK) homozygous (Figure 6C-D) and β3+/− pups was similar to that of β3+/+ pups. Thus, the C-terminal 3 residues of β3, and by extrapolation the interaction of β3 with kindlin-2, are required for the normal pace of vascular development in the retina. This interpretation is consistent with the reported role of kindlin-2 in developmental angiogenesis in zebrafish and mice.25
Postnatal retinal angiogenesis is delayed in β3ΔRGT mice, but not in β3/β1(EGK) mice. Retinas on postnatal day 3 (P3) from β3+/+ and the indicated β3 mutant littermates were stained with anti-CD31 (to mark blood vessels) (A-D) or with fluorescein isothiocyanate–isolectin B4 (for vessels) and anti-Ki67 (for cell proliferation) (E-J) (supplemental Figure 5), and cells were imaged as described in “Methods.” (A,C) Representative images of whole mount retinas with ×10 oil-dipping objective. Bar represents 500 μm. (B,D) Quantification of retinal vessels based on radial length from optic nerve to the periphery. (E,G) Quantification of endothelial tip cells at the retinal vascular front. (F,H) Quantification of endothelial cell filopodial extensions per 25-μm vessel length. (I,J) Quantification of Ki67-positive endothelial cells at the retinal vascular front. (B,D) Data from 12-14 retinas; (E,F,I), data from 8 retinas and 4 mice; (G,H,J) data from 4 retinas and 2 mice. Data are means ± SEM. *P < .05; **P < .01; NS, not significant (unpaired Student t test).
Postnatal retinal angiogenesis is delayed in β3ΔRGT mice, but not in β3/β1(EGK) mice. Retinas on postnatal day 3 (P3) from β3+/+ and the indicated β3 mutant littermates were stained with anti-CD31 (to mark blood vessels) (A-D) or with fluorescein isothiocyanate–isolectin B4 (for vessels) and anti-Ki67 (for cell proliferation) (E-J) (supplemental Figure 5), and cells were imaged as described in “Methods.” (A,C) Representative images of whole mount retinas with ×10 oil-dipping objective. Bar represents 500 μm. (B,D) Quantification of retinal vessels based on radial length from optic nerve to the periphery. (E,G) Quantification of endothelial tip cells at the retinal vascular front. (F,H) Quantification of endothelial cell filopodial extensions per 25-μm vessel length. (I,J) Quantification of Ki67-positive endothelial cells at the retinal vascular front. (B,D) Data from 12-14 retinas; (E,F,I), data from 8 retinas and 4 mice; (G,H,J) data from 4 retinas and 2 mice. Data are means ± SEM. *P < .05; **P < .01; NS, not significant (unpaired Student t test).
The migration of a specific group of ECs called tip cells helps to provide guidance during vascular development.50 We assessed EC growth patterning at the vascular front of the developing retina, with particular attention to tip cell proliferation, tip cell numbers, and filopodia formation (supplemental Figure 5A-B). β3ΔRGT retinas showed reduced tip cell numbers (16.5% reduction; P < .05) (Figure 6E) and filopodia density (29.8% reduction; P < .01) (Figure 6F), compared with β3+/+ littermates. In addition, proliferation at the vascular front, taken as the number of Ki67-positive ECs, was reduced by 38.2% in β3ΔRGT retinas (P < .01) (Figure 6I). Tip cell numbers and filopodia formation were normal in β3/β1(EGK) retinas, (Figure 6G-H) and EC proliferation was even somewhat increased compared with β3+/+ littermates (P < .05) (Figure 6J). Although αVβ3 is expressed in vascular cells other than ECs, these results when taken together with the EC studies in vitro point to a role for the β3/kindlin-2 interaction in developmental angiogenesis.
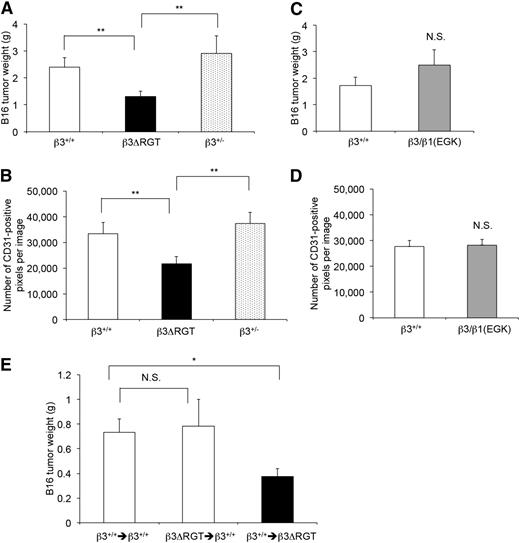
To investigate αVβ3 function in tumor angiogenesis, β3 mutant mice were injected subcutaneously with 2 × 105 B16F1 murine melanoma cells and tumors were evaluated 21 days later.5,51 β3ΔRGT homozygous mice showed reduced tumor weights (1.24 ± 0.19 g, n = 20) compared with β3+/+ (2.23 ± 0.34 g, n = 19; P < .01) or β3+/− littermates (3.26 ± 0.59 g, n = 21; P < .01) (Figure 7A). Tumor blood vessel density, assessed by staining tumors with an antibody to CD31, was also reduced in tumors from β3ΔRGT mice compared with those from β3+/+ or β3+/− mice (Figure 7B; supplemental Figure 5C). In contrast, tumor weights and tumor vessel density in β3/β1(EGK) homozygous mice were similar to those in β3+/+ littermates (Figure 7C-D; supplemental Figure 5C). Thus, the extreme C terminus of β3 and its interaction with kindlin-2 promote αVβ3-dependent developmental and pathological angiogenesis.
Tumor growth and tumor vessel density are reduced in β3ΔRGT mice, but not in β3/β1(EGK) mice. β3+/+, β3+/−, and β3 mutant mice were inoculated subcutaneously with 300 μL of 2 × 105 B16F1 melanoma cells. (A,C) Quantification of tumor weights 21 days after inoculation. (B,D) Quantification of CD31-positive vessels per field in tumor sections as described in “Methods” (also see supplemental Figure 5C). (E) β3+/+ or β3ΔRGT mice were transplanted with the indicated bone marrow cells. There were 9-10 transplant recipients/group. Four to 6 weeks later, mice were inoculated with tumor cells and tumor weights were quantified 21 days after inoculation. Data represent means ± SEM. *P < .05; **P < .01; NS, not significant (unpaired Student t test).
Tumor growth and tumor vessel density are reduced in β3ΔRGT mice, but not in β3/β1(EGK) mice. β3+/+, β3+/−, and β3 mutant mice were inoculated subcutaneously with 300 μL of 2 × 105 B16F1 melanoma cells. (A,C) Quantification of tumor weights 21 days after inoculation. (B,D) Quantification of CD31-positive vessels per field in tumor sections as described in “Methods” (also see supplemental Figure 5C). (E) β3+/+ or β3ΔRGT mice were transplanted with the indicated bone marrow cells. There were 9-10 transplant recipients/group. Four to 6 weeks later, mice were inoculated with tumor cells and tumor weights were quantified 21 days after inoculation. Data represent means ± SEM. *P < .05; **P < .01; NS, not significant (unpaired Student t test).
Signaling from bone marrow–derived cells can contribute to pathological angiogenesis in mouse model systems.52-54 To determine the role of bone marrow–derived cells, tumor growth was examined in mice transplanted with bone marrow from β3+/+ or β3ΔRGT homozygous mice. When compared with B16 tumor growth in β3+/+ mice transplanted with β3+/+ bone marrow, tumor growth in β3ΔRGT mice transplanted with β3+/+ bone marrow was reduced (P < .05) (Figure 7E). In contrast, tumor growth in β3+/+ mice transplanted with β3ΔRGT bone marrow was similar to that observed in β3+/+ mice transplanted with β3+/+ bone marrow (P = .84) (Figure 7E). These results suggest that the defects in angiogenesis observed in β3ΔRGT mice are not dictated primarily by mutant β3 in bone marrow–derived cells. Rather, they are consistent with a role for mutant β3 in ECs.
Discussion
The mechanisms whereby bidirectional integrin signaling by αVβ3 regulate cellular functions through interacting proteins, such as talin, kindlins, and SFKs remain incompletely understood. Although αVβ3 is recognized to regulate angiogenesis in murine models, conclusions about its precise role have seemed contradictory.7,11,17-21 Interpretation of αVβ3 function in gene-targeted mice may be limited in some cases because embryonic lethality,19,55 developmental compensation,5 gene function redundancy,56 or a requirement for the precise experimental control of timing and length of genetic manipulation.6 Furthermore, although direct interactions of the β3 cytoplasmic tail with intracellular proteins are important for integrin signaling,8,38-40 interaction with αVβ3 is unlikely the only manner in which these proteins regulate cellular functions. Here we sought to clarify the role of the β3 tail interaction with kindlin-2 in αVβ3 signaling.
In pull-down assays, the WT β3 cytoplasmic tail interacted with kindlin-2 and c-Src, but the β3ΔRGT tail did not bind c-Src and bound little kindlin-2, and the β3/β1(EGK) tail interacted with kindlin-2 but not c-Src (Figure 1). Availability of β3ΔRGT and β3/β1(EGK) knock-in mice thus made it possible to evaluate the differential roles of β3/kindlin-2 and β3/c-Src interactions in vivo. The following major conclusions can be drawn: (1) Deletion of the β3 C-terminal 3 amino acids (R760GT762) disrupts binding of kindlin-2 and c-Src to β3, resulting in defects in EC migration and angiogenic sprouting in vitro and in developmental and tumor angiogenesis in vivo. Furthermore, bone marrow transplantation studies suggest that β3ΔRGT ECs and not β3ΔRGT bone marrow–derived cells are responsible for the angiogenesis defects. (2) Inside-out activation of αVβ3 in β3ΔRGT ECs appears to be normal. (3) Substitution of β3 RGT with the corresponding C-terminal residues of β1 (EGK) restores the binding capacity of β3 for kindlin-2, but not for c-Src, and it restores EC spreading, migration, and sprouting as well as angiogenesis. (4) Knock-down of kindlin-2 in ECs disrupts migration and sprouting, whereas knock-down or inhibition of c-Src does not. (5) Enforced proximity of kindlin-2 to β3ΔRGT in ECs promotes αVβ3-dependent sprout formation. Although these studies do not formally exclude a role for other β3-expressing vascular cells in the angiogenesis phenotypes observed in β3ΔRGT mice, they extend previous work on integrins, SFKs, and kindlin-2 in ECs,25,56,57 and they provide a mechanistic context for kindlin-2 in αVβ3 outside-in signaling.
Kindlin-2 enhances talin-dependent activation of platelet integrin αIIbβ3 in a CHO cell expression system.11,19 We did not detect an impairment in the binding of activation-dependent antibody, WOW-1 Fab, to αVβ3ΔRGT ECs (Figure 5). This suggests that a direct interaction between kindlin-2 and β3 is not absolutely required for inside-out αVβ3 signaling. This conclusion may seem at odds with studies of platelets deficient in kindlin-3, which do not bind fibrinogen normally in response to agonists.22,23,58 However, agonist-induced fibrinogen binding to αIIbβ3 in β3ΔRGT murine platelets is only minimally impaired,13 and similar observations were made in knock-in platelets expressing a β3 integrin binding-defective kindlin-3 mutant.59 WOW-1 Fab binding to β3(DiY>F) ECs stimulated by VEGF is reportedly impaired.7 However, unlike αVβ3ΔRGT, interactions of β3(DiY>F) with both talin and kindlin-2 are likely to be impaired. Given normal WOW-1 Fab binding to αVβ3ΔRGT ECs, their reduced spreading on vitronectin (supplemental Figure 2C) suggests a predominant role for the β3/kindlin-2 interaction in outside-in αVβ3 signaling.
Although SFKs have been shown to play a role in VEGF-induced angiogenesis,34,57 we found that mice expressing β3/β1(EGK), which cannot bind c-Src, exhibit normal retinal and tumor angiogenesis (Figures 6 and 7). Moreover, c-Src knock-down (Figure 4) or SFK inhibition failed to prevent EC sprouting in vitro. This is consistent with previous studies showing that c-Src knockout mice exhibit tumor growth and angiogenesis equivalent to c-Src replete controls,53 and that c-Src or Yes knock-down fails to affect VEGF-induced EC tube formation.60 We suggest that β3/β1(EGK) ECs retain angiogenesis-related αVβ3-dependent functions because the β3/β1(EGK) cytoplasmic tail can still interact with kindlin-2.
Unlike the WT or β3/β1(EGK) cytoplasmic tails, the β3ΔRGT cytoplasmic tail cannot bind kindlin-2 (Figure 1). Our preliminary studies show that the same is true for kindlin-3, which unlike kindlin-2, is highly expressed in platelets. This may help to explain previous findings that β3ΔRGT mice, but not β3/β1(EGK) mice, show impaired platelet thrombus formation in a carotid artery injury model.13 It is consistent with the observation that knock-in mice expressing a kindlin-3 mutant with impaired binding to β3 exhibit reduced thrombus formation.59 Expression of kindlin-3 has been reported in some types of human ECs61 ; however, we could not detect it by western blotting in the murine ECs used here.
RGD-based small-molecule antagonists or antibodies to αVβ3 and other integrins are being evaluated in clinical trials for inflammatory and neoplastic disorders.62,63 Our results suggest that were selective disruption of the αVβ3/kindlin-2 interaction possible, it might offer an alternative pharmacological approach by which to interfere with pathological processes dependent on this interaction. Inhibition of integrin cytoplasmic tail–protein interactions in cells is feasible. Su et al showed that a synthetic cell–permeable RGT peptide corresponding to the last 3 amino acids of β3 inhibits platelet function ex vivo.64 The present studies suggest that this peptide might block both β3/kindlin-2 and β3/c-Src interactions. For translational purposes, selective blockade of the β3 tail interaction with kindlin-2 in ECs or with kindlin-3 in platelets would be required. Initial structural analyses of the β3 tail in complex with the c-Src SH3 domain have been conducted.65 Analogous studies of the β3 tail in complex with kindlins could shed more light on the feasibility of such a therapeutic approach.
The online version of this article contains a data supplement.
There is an Inside Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Jian Kang, John Mitsios, Lea Scheppke, and Feng Ye for technical advice or support; Alexandra Newton for PDBu; David Cheresh for advice on the retinal angiogenesis experiments; and the University of California San Diego Neuroscience Microscopy Shared Facility (P30 NS047101) for technical assistance.
This work was supported by National Institutes of Health, National Heart, Lung, and Blood Institute (HL56595, HL57900, and HL78784) and a postdoctoral fellowship award from the American Heart Association (Z.L.).
Authorship
Contribution: Z.L. designed experiments, performed experiments, and wrote the paper; H.K. designed and performed experiments; M.P. and J.M.C. performed experiments; A.J.A. contributed vital reagents; M.H.G. designed experiments; S.J.S. designed experiments and wrote the paper; and all authors read and approved of the final version of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Zhongji Liao, Department of Medicine, University of California-San Diego, Leichtag Biomedical Research Building- Room 132, 9500 Gilman Dr, Mail Code 0726, La Jolla, CA 92093-0726; e-mail: zhliao@ucsd.edu.