Key Points
Adoptive transfer of TH-1 cells is a safe and effective treatment of refractory AdV infection after stem cell transplantation.
AdV-related mortality was 9.5% in patients with a response to ACT (overall survival 71%) compared with 100% mortality in nonresponders.
Abstract
Hematopoietic stem cell transplantation (HSCT) has improved over the last few decades. However, viral infections are often refractory to pharmacologic treatment and require alternative treatment strategies such as immunotherapy. Adenovirus (AdV) is th predominant disease-causing pathogen in pediatric HSCT. In a clinical trial, we analyzed safety and efficacy of ex vivo adoptive T-cell transfer (ACT) with hexon-specific T cells, predominantly of the T-helper cell 1 (Th1) phenotype, in 30 patients with AdV disease or viremia. ACT was feasible with no acute toxicities or significant onset of graft-versus-host disease. ACT led to in vivo antiviral immunity for up to 6 months with viral control, resulting in complete clearance of viremia in 86% of patients with antigen-specific T-cell responses. After ACT and a follow-up of 6 months, overall survival was markedly increased in responders (mean, 122 days; 15 survivors) compared with nonresponders who all died shortly after ACT (mean, 24 days; no survivors). AdV-related mortality was 100% in nonresponders compared with 9.5% in responders (≥1 log reduction of DNA copies per milliliter after ACT). In summary, ex vivo ACT of AdV-specific Th1 cells was well tolerated and led to successful and sustained restoration of T-cell immunity correlated with virologic response and protection from virus-related mortality. This cellular immunotherapy is a short-term available and broadly applicable treatment. The study is registered at European Union Clinical Trials Register as 2005-001092-35.
Introduction
Hematopoietic stem cell transplantation (HSCT) has become an accepted treatment strategy for patients with various malignant and nonmalignant disorders. Major medical progress has been made in recent years in reducing treatment-related morbidity and mortality.1,2 However, after HSCT, patients develop a transient state of combined immunodeficiency with increased susceptibility to serious viral infections.3,4 Persistent viruses are well-known pathogens associated with severe morbidity and mortality despite prospective monitoring and early intervention with pharmacologic treatment. Herpesviruses such as cytomegalovirus or Epstein-Barr virus are predominant pathogens in adult HSCT recipients whereas adenovirus (AdV) is the most common virus in children after HSCT, with associated mortality rates up to 83%.5-8 Treatment with antiviral drugs, including ribavirin and cidofovir, has substantial side effects and is often insufficient for eradicating adenoviral infection.9,10 Since T cells are the most potent effectors in the human immune system, control of T-cell responses will improve morbidity and mortality of various infectious and malignant diseases.11 Furthermore, a sustained protection against infectious agents will reduce toxic therapies and treatment costs.8
Because T-cell function is crucial for the clearance of adenoviral infection, adoptive T-cell transfer (ACT) has been developed as a therapeutic option for restoring virus-specific T-cell immunity.12 It has been shown to be a safe and successful approach for treating viral complications after HSCT and establishing sustained T-cell responses.13-20 However, treatment was limited to a few specialized centers, and generation of the cellular products was time and labor intensive.3 In recent years, it has been shown in single cases that antigen-specific T cells could be generated by short-term ex vivo isolation under good manufacturing practice conditions. Infusion of small numbers of specific CD4+ and CD8+ T cells could lead to in vivo expansion of the infused T cells and could result in durable reductions of the viral load.13,21,22
In a clinical trial, we analyzed the safety and efficacy of ACT with hexon-specific T cells predominantly of the T-helper cell 1 (Th1) phenotype. Thirty patients suffering from chemotherapy refractory AdV disease or viremia after HSCT were treated according to the study protocol for further analysis of in vivo expansion of AdV-specific T cells. AdV-specific T cells were isolated within 30 hours by using interferon gamma (IFN-γ) capture technique and were directly infused into the patients to restore protective T-cell immunity for treating infection and preventing AdV-related complications.
Patients and methods
Ex vivo generation of AdV-specific T cells
Generation of AdV-specific T cells was performed for all patients in a central GMP laboratory at the University Children’s Hospital Tübingen, as described previously.23 After cell processing, generated AdV-specific T cells were transferred to the treating centers and infused on the same day of the cell isolation procedure or they were cryopreserved after isolation and infused at a later date (supplemental Methods available online at the Blood Web site). AdV-specific T cells were isolated from whole blood or unstimulated apheresis of the HSCT donor after obtaining written informed consent for ACT. Peripheral blood mononuclear cells were isolated by Ficoll-Paque (Biochrome, Berlin, Germany) density gradient centrifugation of heparinized blood from healthy donors, diluted to 1 × 107 cells per milliliter with culture medium (RPMI 1640 [Biochrome, Berlin, Germany] + 10% human AB serum), and stimulated with 6 µg per milliliter hexon protein (Virion-Serion GmbH, Würzburg, Germany). In total, 0.1 to 1 × 109 peripheral blood mononuclear cells were stimulated for 16 hours in a 37°C humidified incubator. Enrichment of IFN-γ–secreting cells was performed by using the Cytokine Secretion System and the CliniMACS device for immunomagnetic separation (both Miltenyi Biotec, Bergisch Gladbach, Germany). A minimum of 10% purity for IFN-γ+ cells was defined for the release of the product.
Patient characteristics and follow-up after ACT
Thirty patients fulfilled inclusion and exclusion criteria: AdV infection refractory to treatment with antiviral drugs over 14 days and absence of grade 3 to 4 graft-versus-host disease (GVHD). Patients were treated with hexon-specific T cells from their stem cell donor. Treatment with AdV-specific T cells was performed in a clinical trial for further analysis of immune responses after ACT. All patients previously underwent allogeneic HSCT. Clinical data for the treated patients are summarized in the Tables 1 and 2. Patients were eligible for ACT as preemptive treatment when diagnosis of refractory infection was confirmed. Infection was defined as 2 consecutive positive results for AdV. Presence of the virus together with appropriate symptoms in the absence of any other recognizable cause was defined as AdV disease. A “refractory infection” was defined as a persistent number of AdV copies detected by quantitative polymerase chain reaction (qPCR) despite antiviral therapy for at least 2 weeks. Changes in copy numbers were defined as ≥1 log change. Patients received preemptive treatment with cidofovir and additional antiviral prophylaxis according to institutional guidelines. Antiviral chemotherapy with cidofovir was continued during and after ACT except in 7 patients because of impaired kidney function (Table 1).
Patient characteristics (n = 30)
| . | No. of patients/No. of evaluable patients . | Mean . | Range . |
|---|---|---|---|
| Age, y | |||
| <10 | 13 | 14 | 0.5-45 |
| 10-20 | 11 | ||
| 21-40 | 4 | ||
| >40 | 2 | ||
| Body weight, kg | 39 | 5-83 | |
| Karnofsky index, % | 65 | 20-100 | |
| Diagnosis | |||
| Acute lymphoblastic leukemia | 14 | ||
| Acute myeloid leukemia | 4 | ||
| Chronic myeloid leukemia | 1 | ||
| Myelodysplastic syndrome | 1 | ||
| Solid tumor | 1 | ||
| Immunodeficiency | 7 | ||
| Congenital bone marrow failure | 1 | ||
| Non-Hodgkin lymphoma | 1 | ||
| Graft | |||
| Matched unrelated donor | 7 | ||
| Matched sibling donor | 3 | ||
| Mismatched unrelated donor (<9/10 matched) | 3 | ||
| Haploidentical HSCT from parents or siblings | 17 | ||
| T-cell depletion | |||
| In vivo depletion (ATG) | 7 | ||
| Graft manipulation (plus serotherapy) | 17 | ||
| Alemtuzumab | 4 | ||
| Antiviral drugs for treatment of AdV | |||
| Cidofovir | |||
| Before ACT | 28/29 | ||
| After ACT | 20/27 | ||
| Ribavirin | 19/26 | ||
| Time of first positive AdV PCR in days after HSCT | |||
| In stool (n = 30) | 29 | –80-275 | |
| In blood (n = 28) | 36 | –71-305 | |
| Days to ACT after HSCT (± SD)] | 56 ± 61 | ||
| <50 | 25 | ||
| 50-100 | 7 | ||
| >100 | 6 |
| . | No. of patients/No. of evaluable patients . | Mean . | Range . |
|---|---|---|---|
| Age, y | |||
| <10 | 13 | 14 | 0.5-45 |
| 10-20 | 11 | ||
| 21-40 | 4 | ||
| >40 | 2 | ||
| Body weight, kg | 39 | 5-83 | |
| Karnofsky index, % | 65 | 20-100 | |
| Diagnosis | |||
| Acute lymphoblastic leukemia | 14 | ||
| Acute myeloid leukemia | 4 | ||
| Chronic myeloid leukemia | 1 | ||
| Myelodysplastic syndrome | 1 | ||
| Solid tumor | 1 | ||
| Immunodeficiency | 7 | ||
| Congenital bone marrow failure | 1 | ||
| Non-Hodgkin lymphoma | 1 | ||
| Graft | |||
| Matched unrelated donor | 7 | ||
| Matched sibling donor | 3 | ||
| Mismatched unrelated donor (<9/10 matched) | 3 | ||
| Haploidentical HSCT from parents or siblings | 17 | ||
| T-cell depletion | |||
| In vivo depletion (ATG) | 7 | ||
| Graft manipulation (plus serotherapy) | 17 | ||
| Alemtuzumab | 4 | ||
| Antiviral drugs for treatment of AdV | |||
| Cidofovir | |||
| Before ACT | 28/29 | ||
| After ACT | 20/27 | ||
| Ribavirin | 19/26 | ||
| Time of first positive AdV PCR in days after HSCT | |||
| In stool (n = 30) | 29 | –80-275 | |
| In blood (n = 28) | 36 | –71-305 | |
| Days to ACT after HSCT (± SD)] | 56 ± 61 | ||
| <50 | 25 | ||
| 50-100 | 7 | ||
| >100 | 6 |
AdV disease and GVHD status before and after ACT
| . | No. of patients/No. ofevaluable patients . |
|---|---|
| Site of local infection | |
| Stool | 29 |
| Urine | 6 |
| Nasopharyngeal fluid | 9 |
| Bronchoalveolar lavage | 1 |
| Adenoviral disease | 24 |
| Symptoms of AdV disease | |
| Diarrhea | 19 |
| Nephritis | 4 |
| Hepatitis | 6 |
| Cystitis | 4 |
| Pneumonia | 5 |
| Encephalitis | 1 |
| Course of AdV infection after ACT | |
| Viral clearance in patients with in vivo T-cell expansion | 12/14 |
| Responders*† | 21/30 |
| With final clearance of AdV in blood‡ | 14/21 |
| Without final clearance of viremia | 3/21 |
| With negative viremia at transfer§ | 4/21 |
| Nonresponders|| | 8/30 |
| GVHD prophylaxis at ACT | |
| Cyclosporine | 11 |
| Mycophenolate mofetil | 13 |
| Steroids | 16 |
| None | 5 |
| GVHD status prior to ACT | |
| No GVHD | 23 |
| Grade 1-2 | 2 |
| Grade 3-4 | 4 |
| Chronic GVHD | 1 |
| Early onset of GVHD after ACT† | |
| No onset of GVHD | 27 |
| Grade 1 | 2 |
| Grade 2-4 | 0 |
| . | No. of patients/No. ofevaluable patients . |
|---|---|
| Site of local infection | |
| Stool | 29 |
| Urine | 6 |
| Nasopharyngeal fluid | 9 |
| Bronchoalveolar lavage | 1 |
| Adenoviral disease | 24 |
| Symptoms of AdV disease | |
| Diarrhea | 19 |
| Nephritis | 4 |
| Hepatitis | 6 |
| Cystitis | 4 |
| Pneumonia | 5 |
| Encephalitis | 1 |
| Course of AdV infection after ACT | |
| Viral clearance in patients with in vivo T-cell expansion | 12/14 |
| Responders*† | 21/30 |
| With final clearance of AdV in blood‡ | 14/21 |
| Without final clearance of viremia | 3/21 |
| With negative viremia at transfer§ | 4/21 |
| Nonresponders|| | 8/30 |
| GVHD prophylaxis at ACT | |
| Cyclosporine | 11 |
| Mycophenolate mofetil | 13 |
| Steroids | 16 |
| None | 5 |
| GVHD status prior to ACT | |
| No GVHD | 23 |
| Grade 1-2 | 2 |
| Grade 3-4 | 4 |
| Chronic GVHD | 1 |
| Early onset of GVHD after ACT† | |
| No onset of GVHD | 27 |
| Grade 1 | 2 |
| Grade 2-4 | 0 |
Defined as patients with significant reduction (>1 log) of viremia after ACT (straight or transient).
Course of viremia and onset of GVHD is not evaluable for patient 1 because of death 2 days after ACT.
Patient 10 had AdV-negative blood at ACT but developed viremia thereafter with consequent re-clearance of the viral load and is therefore classified as responder with final clearance of viremia.
Patients with viremia-negative blood at ACT who showed clearance of AdV at other sites of infection (stool, urine, or nasopharyngeal fluid).
Defined as patients with persistent or increasing viral loads or with negative viremia at ACT and without virus clearance at other sites of infection.
The study protocol (EudraCT-No. 2005-001092-35) was in accordance with the Declaration of Helsinki and was approved by the institutional ethical review board and by the national authority (Paul-Ehrlich-Institute). Patients and/or their legal representatives signed informed consent and were treated according to a common management plan. Institutional standard criteria for donor lymphocyte infusion were applied for toxicity evaluation. Monitoring included heart rate, blood pressure, oxygen saturation, and physical examinations during and for 2 hours after ACT. Toxicity criteria were acute allergic reaction or any change in vital signs during and after ACT, and impairment of blood count and liver and kidney function, according to institutional guidelines, as well as signs of GVHD for up to 8 weeks after ACT. T-cell responses were evaluated for all patients in a central laboratory with a uniform detection threshold (supplemental Methods). Qualitative PCR and qPCR of the AdV load was performed in the treating centers. Response was defined as reduction of viral load (≥1 log DNA copies per milliliter). Follow-up of the patients enrolled onto the study was performed until at least 6 months after immunotherapy. The mean observation period was 482 days (range, 2 to 2764 days) after ACT (Table 3).
Outcome of patients after ACT
| . | Outcome of patients/patients evaluable until 6 mo after ACT* . | Days after ACT . | Outcome of patients/patients evaluable until last observation after ACT† . | Days after ACT . | ||
|---|---|---|---|---|---|---|
| Mean . | Range . | Mean . | Range . | |||
| Survivors after ACTठ| 15/30 | 7/30 | ||||
| Responders | 15/21 | 7/21 | ||||
| Nonresponders | 0/8 | 0/8 | ||||
| Death after ACT§ | 15 | 61 | 2-184 | 23 | 137 | 2-428 |
| Responders|| | 6 | 122 | 33-184 | 14 | 211 | 33-428 |
| With final clearance of viremia | 4 | 151 | 134-184 | 9 | 209 | 134-391 |
| Without final clearance of viremia | 2 | 62 | 33-91 | 62 | 33-91 | |
| Nonresponders | 8 | 24 | 9-56 | 24 | 9-56 | |
| Cause of death | ||||||
| AdV-associatedठ| 11/15 | 11/22 | ||||
| Nonresponders | 8/8 | 8/8 | ||||
| Responders with final clearance of viremia | 0/4 | 0/11 | ||||
| Responders without final clearance of viremia | 2/2 | 2/2 | ||||
| Other cause | 4/15 | 11/22 | ||||
| Nonresponders | 0/8 | 0/8 | ||||
| Responders | 4/6 | 11/13 | ||||
| . | Outcome of patients/patients evaluable until 6 mo after ACT* . | Days after ACT . | Outcome of patients/patients evaluable until last observation after ACT† . | Days after ACT . | ||
|---|---|---|---|---|---|---|
| Mean . | Range . | Mean . | Range . | |||
| Survivors after ACTठ| 15/30 | 7/30 | ||||
| Responders | 15/21 | 7/21 | ||||
| Nonresponders | 0/8 | 0/8 | ||||
| Death after ACT§ | 15 | 61 | 2-184 | 23 | 137 | 2-428 |
| Responders|| | 6 | 122 | 33-184 | 14 | 211 | 33-428 |
| With final clearance of viremia | 4 | 151 | 134-184 | 9 | 209 | 134-391 |
| Without final clearance of viremia | 2 | 62 | 33-91 | 62 | 33-91 | |
| Nonresponders | 8 | 24 | 9-56 | 24 | 9-56 | |
| Cause of death | ||||||
| AdV-associatedठ| 11/15 | 11/22 | ||||
| Nonresponders | 8/8 | 8/8 | ||||
| Responders with final clearance of viremia | 0/4 | 0/11 | ||||
| Responders without final clearance of viremia | 2/2 | 2/2 | ||||
| Other cause | 4/15 | 11/22 | ||||
| Nonresponders | 0/8 | 0/8 | ||||
| Responders | 4/6 | 11/13 | ||||
End point of the study.
Data are presented as number of patients. “Days after ACT” represents days to last observation >6 mo after ACT: P2/2689; P3/1805; P7/292; P8/231; P9/391; P11/277; P12/204; P13/1771; P14/1840; P15/218; P19/187; P30/242; P31/2764; P32/428; P35/189.
AdV association with death is unclear for patient 15 (death on day 218 after ACT).
Patient 1 is not classified as a responder or nonresponder because of death 2 days after ACT, but death was AdV-associated.
Patients with negative viremia in blood at ACT who showed clearance of AdV at other sites of infection are included.
Four patients were treated with repetitive ACTs (patients 5, 24, 31, and 35). The interval between repetitive ACTs was 21 to 41 days after previous T-cell infusion. Indications for a subsequent T-cell infusion were nonresponse and availability of hexon-specific T cells. T-cell dose was limited to 5000 cells per kilogram in HLA-mismatched donors and 25 000 cells per kilogram in HLA-matched donors. In patient 5, the first dose was divided into several infusions (1307 T cells per kilogram per infusion on days 0, 4, and 11). This was done because of safety concerns related to the unstable clinical condition of the patient.
Results
Feasibility and safety of treatment with AdV-specific T cells
Antigen-specific T cells could be generated in 100% of isolations. Thirty patients received the T-cell transfer. Purity of the polyclonal T-cell graft was 72% ± 17% (mean ± standard deviation [SD]; note that the plus or minus symbol hereinafter indicates SD; supplemental Methods, supplemental Figure 1, and supplemental Table 1). T cells were reconstituted in a volume of 5 to 10 mL and immediately infused intravenously into the patients over 5 to 10 minutes or cryopreserved for later use. To achieve a sustained immunologic response, a second T-cell administration after 4 to 41 days was performed in 4 patients and a third T-cell administration was performed within 7 days in 1 patient. Mean T-cell dose at ACT was 4.1 × 103 CD3+ cells per kilogram of body weight (range, 0.3 to 24 × 103 cells per kilogram of body weight; supplemental Table 1). T-cell infusion was well tolerated by all patients with no acute side effects. Patient 14 developed liver failure on day 14 after ACT with proof of AdV-related hepatopathy in the liver biopsy but without morphologic evidence of lymphocyte infiltration or signs of GVHD. Therefore, liver failure was attributed to AdV disease and not to immunopathology related to ACT.
In the study protocol, GVHD was attributed to ACT when onset or aggravation occurred within less than 8 weeks after ACT, although it cannot be excluded that the AdV-specific T cells contributed to a later GVHD occurrence. No relevant induction of GVHD was observed. Only two patients developed mild GVHD grade 1 within 2 weeks after ACT. Four patients developed GVHD grade 2 to 3 at more than 7 weeks after ACT. GVHD responded to steroid treatment (supplemental Data). We could not find a difference in GVHD rate between responders and nonresponders.
Reconstitution of AdV-specific T-cell immunity through transfusion of hexon-specific T cells
Prior to ACT, AdV-specific T cells could not be detected in any of the evaluable patients. AdV-specific T cells were monitored for a period of up to 6 months after ACT in 23 of 30 patients. In 7 patients, analysis of specific T cells was not possible, which limits the interpretation of the specific T-cell responses.
In 14 (61%) of 23 evaluable patients, ACT led to successful in vivo expansion of AdV-specific T cells (Figure 1, supplemental Data, supplemental Figure 2, and supplemental Table 2). In vivo expansion was detectable after 24 ± 17 days (first detection of IFN-γ+ T cells; n = 14) and up to 6 months. Last positive follow-up of IFN-γ+ T cells was 72 ± 47 days (n = 14) after immunotherapy. In vivo expansion of T lymphocytes was found within both the CD4+ and CD8+ T-cell compartments in 13 (93%) of the 14 patients with detectable IFN-γ+ T cells. In patients with detectable virus-specific T-cell responses, viremia was cleared in 86% of patients (12 of 14) within the observation period (Figure 2). Cryopreserved T cells could also lead to virologic response after ACT (patients 10, 13, 24, 30, and 35) and to in vivo expansion of AdV-specific T cells (patients 13 and 30, in vivo expansion; patients 5 and 24, no in vivo expansion; patients 10 and 35, not evaluable).
In vivo T-cell response after adoptive transfer of hexon-specific T cells after allogeneic HSCT. Antigen-specific T cells were analyzed by flow cytometry after stimulation of blood samples with hexon antigen, followed by intracellular cytokine staining. (A) Fourteen of 23 evaluable patients developed successful in vivo T-cell expansion after ACT. The threshold of positive antigen-specific T-cell responses was defined as 0.01% IFN-γ+ T cells of viable T cells. In vivo expansion could be detected until a mean of 72 ± 47 days (range, 11 to 177 days) after ACT and was found within both the CD4+ and CD8+ T-cell compartments in 13 (93%) of the 14 patients with detectable IFN-γ+ T cells. Exclusive expansion in the CD4+ T-cell compartment was found in patient 7 (and in patient 30 after first ACT). Detailed information on in vivo T-cell expansion of the patients is shown in supplemental Figure 2. (B) Time course of in vivo T-cell response and virologic response of patient (Pat.) 31 is shown. In vivo expansion of AdV-specific T cells after adoptive transfer was associated with clearance of the viral load. Patient 31 shows a straight and fast virologic response with clearance of viremia <6 weeks after second ACT. (C) Immune reconstitution status prior to and after ACT. Number of leukocytes (white blood cell count [WBC]) and CD3+ T cells are shown prior to and 1 week and 1 month after ACT. WBC counts did not change after ACT, but CD3 counts significantly increased after ACT, confirming that the response was not related to an overall reconstitution of WBCs. ns, not significant.
In vivo T-cell response after adoptive transfer of hexon-specific T cells after allogeneic HSCT. Antigen-specific T cells were analyzed by flow cytometry after stimulation of blood samples with hexon antigen, followed by intracellular cytokine staining. (A) Fourteen of 23 evaluable patients developed successful in vivo T-cell expansion after ACT. The threshold of positive antigen-specific T-cell responses was defined as 0.01% IFN-γ+ T cells of viable T cells. In vivo expansion could be detected until a mean of 72 ± 47 days (range, 11 to 177 days) after ACT and was found within both the CD4+ and CD8+ T-cell compartments in 13 (93%) of the 14 patients with detectable IFN-γ+ T cells. Exclusive expansion in the CD4+ T-cell compartment was found in patient 7 (and in patient 30 after first ACT). Detailed information on in vivo T-cell expansion of the patients is shown in supplemental Figure 2. (B) Time course of in vivo T-cell response and virologic response of patient (Pat.) 31 is shown. In vivo expansion of AdV-specific T cells after adoptive transfer was associated with clearance of the viral load. Patient 31 shows a straight and fast virologic response with clearance of viremia <6 weeks after second ACT. (C) Immune reconstitution status prior to and after ACT. Number of leukocytes (white blood cell count [WBC]) and CD3+ T cells are shown prior to and 1 week and 1 month after ACT. WBC counts did not change after ACT, but CD3 counts significantly increased after ACT, confirming that the response was not related to an overall reconstitution of WBCs. ns, not significant.
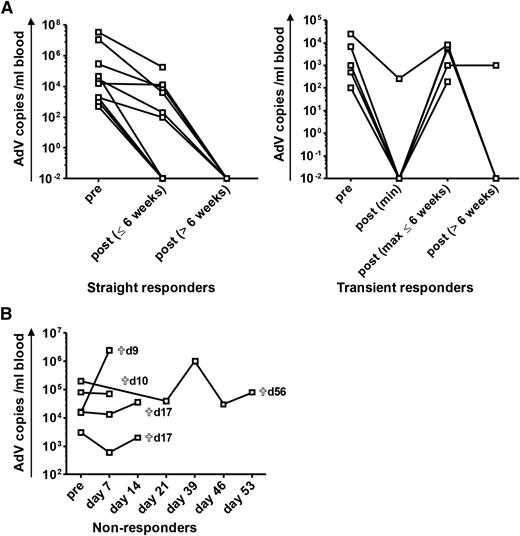
Virologic response after adoptive transfer of AdV-specific T cells for treatment of refractory AdV infection after allogeneic HSCT. ACT of hexon-specific T cells was performed in patients after allogeneic HSCT. The virologic response to ACT is shown in terms of viral copies in peripheral blood. Pre ACT refers to data analyzed before or at ACT. Patients are subdivided into 3 different groups according to the change of viral load after ACT: straight responders (n = 10) are defined as patients with a significant reduction of viremia (≥1 log) or clearance of the viral load after ACT, transient responders (n = 5) show a temporary decrease of viremia (≥1 log) or clearance of AdV with subsequent re-increase of the viral load thereafter, and nonresponders (n = 5) are patients with persistent or increasing levels of the viral load after ACT (detailed information on individual patients is provided in supplemental Data, supplemental Figure 2, and supplemental Table 2). qPCR results of <1000 copies per milliliter are represented as 1000 copies per milliliter in the figure. (A) The course of viremia after ACT for straight and transient responders. For transient responders, the minimum of AdV copies after ACT, the maximum of re-increasing AdV copies within the time period of 6 weeks after ACT, and the course of viremia in the time period >6 weeks after ACT are shown. Clearance of viremia after 6 weeks took place at a mean of 93 ± 37 days (range, 55 to 176 days). (B) Course of viremia in nonresponders. Time of death (days after ACT) of the different patients is indicated.
Virologic response after adoptive transfer of AdV-specific T cells for treatment of refractory AdV infection after allogeneic HSCT. ACT of hexon-specific T cells was performed in patients after allogeneic HSCT. The virologic response to ACT is shown in terms of viral copies in peripheral blood. Pre ACT refers to data analyzed before or at ACT. Patients are subdivided into 3 different groups according to the change of viral load after ACT: straight responders (n = 10) are defined as patients with a significant reduction of viremia (≥1 log) or clearance of the viral load after ACT, transient responders (n = 5) show a temporary decrease of viremia (≥1 log) or clearance of AdV with subsequent re-increase of the viral load thereafter, and nonresponders (n = 5) are patients with persistent or increasing levels of the viral load after ACT (detailed information on individual patients is provided in supplemental Data, supplemental Figure 2, and supplemental Table 2). qPCR results of <1000 copies per milliliter are represented as 1000 copies per milliliter in the figure. (A) The course of viremia after ACT for straight and transient responders. For transient responders, the minimum of AdV copies after ACT, the maximum of re-increasing AdV copies within the time period of 6 weeks after ACT, and the course of viremia in the time period >6 weeks after ACT are shown. Clearance of viremia after 6 weeks took place at a mean of 93 ± 37 days (range, 55 to 176 days). (B) Course of viremia in nonresponders. Time of death (days after ACT) of the different patients is indicated.
Analysis of the CD4:CD8 ratio of the T-cell graft shows that adoptive transfer with very few CD8+ T cells (<100 CD8+IFNγ+ T cells per kilogram in the T-cell grafts for patients 6, 7, 12, 16, 25, 31, and 35) led to fast immune recovery associated with favorable outcomes. Thus, small amounts of AdV-specific CD8+ T cells are sufficient for the fast and successful development of in vivo T-cell responses (see supplemental Data for individual patient outcomes).
Seven of the evaluable patients were treated with the T-cell–depleting agents antithymocyte globulin (ATG) or alemtuzumab. Only 2 (29%) of 7 showed AdV-specific T-cell responses after ACT. Five patients (71%) had no in vivo expansion of T cells after ACT. Three of these 5 patients without specific T-cell responses received the T-cell product within <50 days after HSCT and both patients with detectable AdV-specific T cells were also treated in this early posttransplant setting. Therefore, treatment with ATG/alemtuzumab seems to reduce the efficacy of ACT. Since specific T-cell responses can develop despite the use of T-cell–depleting antibodies and early infusion posttransplantation, use of ATG/alemtuzumab should not be an absolute contraindication for T-cell therapy. Detection of residual in vivo activity of these antibodies will clarify this issue in future trials.
Virologic response after ACT
Among patients responding to ACT (n = 21), complete clearance of viremia was observed in 14 patients (67%); 3 patients responded to ACT (>1 log change in copy numbers) without final clearance of viremia (14%), and 4 patients had AdV-negative blood at time of ACT but cleared AdV at other sites of infection (19%). Eight patients showed no response to ACT (no reduction >1 log change in copy numbers or clearance of viremia), and 1 patient with viral copies of more than a billion died of infectious morbidity on day 2 after ACT (Table 2, supplemental Figure 3, and supplemental Table 2).
Virologic responses revealed different kinetics: viral clearance took place in a fast response (defined as <6 weeks after ACT) or in a slower response (defined as >6 weeks after ACT). Response was either straight with continuous reduction of viremia or transient with temporary reduction/clearance and subsequent re-increase of viremia within 6 weeks after ACT (Figure 2 and supplemental Table 2). AdV-specific T-cell responses could be detected in 3 of the 5 transient responders (patients 11, 14, and 19). Straight clearance of viremia was observed in 12 patients. Fast clearance within 6 weeks was observed in 5 patients at a mean of 25 ± 12 days. In 10 patients, clearance of viremia occurred at a later time point (93 ± 37 days). This slow clearance of viremia could take place in a straight (n = 7) or in a transient (n = 3) way (Figure 2, supplemental Figure 2, and supplemental Table 2). Time period until first clearance of viremia was dependent on the patients’ level of viral load at ACT and was faster in patients with viral loads of <10 000 copies per milliliter (Figure 3D). Repetitive ACT was successfully performed for patients 31 and 35 who both cleared viremia after second ACT (supplemental Data).
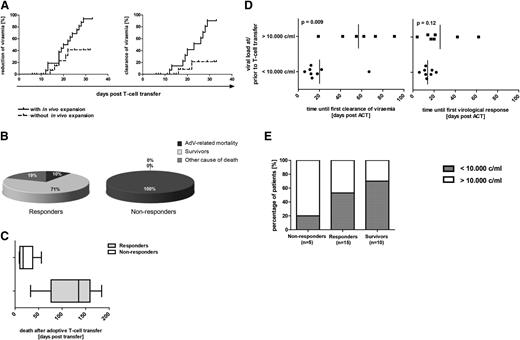
Cumulative probability of response to ACT. (A) Cumulative probability of reduction or clearance of viremia after adoptive transfer of AdV-specific T cells with and without in vivo expansion of specific T cells. The two plots demonstrate the influence of in vivo expansion of AdV-specific T cells (defined as detected ≥0.01% IFN-γ+ T cells of viable T cells in blood samples of patients after ACT) for the cumulative probability of reduction (left) and clearance (right) of viremia until day 35 after adoptive T-cell therapy (n = 17). Patients 1, 4, 6, 7, 8, 10, 13, 23, 27, 32, 33, 34, and 35 are not represented in the figure because T-cell response could not be analyzed within the time period, patients already had negative viremia at time of ACT, or no quantitative analysis of the viral load was available. (B) AdV-related mortality in responders and nonresponders. Shown are the percentage of responders (defined as reduction >1 log change in copy numbers of viremia; n = 21) and nonresponders (defined as no reduction >1 log change in copy numbers or clearance of viremia; n = 8) who died as a result of AdV infection, as a result of other cause of death, or who survived within the observation period of 6 months after ACT. Numbers in the figure are rounded up so that 2 of 21 represents 10% instead of 9.52%. (C) Time to AdV-related mortality. Time of death within the follow-up of 6 months after ACT is demonstrated for the deceased responders (n = 6) and nonresponders (n = 8). (D) Correlation of the level of viremia with the velocity of viral clearance and virologic response after ACT. Time period until first viral clearance after ACT in correlation to the level of viremia at or prior to ACT is shown in the left graph. In evaluable patients with viral loads <10 000 copies per milliliter at time of ACT, first clearance of viremia could be achieved at a mean of 20 days (range, 6 to 67 days) after ACT. First viral clearance of evaluable patients with AdV loads >10 000 copies per milliliter was reached significantly later, at a mean of 57 days after ACT (range, 19 to 91 days). (Right) Time until first virologic response (defined as reduction of the viral load in copy numbers ≥1 log) in correlation to the level of viremia at or before ACT. In patients with viral loads <10 000 copies per milliliter at time of ACT, first virologic response could be achieved at a mean of 14 days (range, 6 to 22 days) after ACT. Significant reduction of the viral load in patients with AdV loads >10 000 copies per milliliter at ACT occurred for the first time at a mean of 26 days (range, 4 to 62 days) after ACT. For patients 24 and 25, viral load is indicated in copies per microgram of DNA and for patient 31, in genome equivalents per 20 000 cells. For patients 30, 31, and 35, time until first viral clearance or virologic response after first ACT is shown. (E) Correlation of the viral load at ACT with virologic response to ACT and with outcome of patients. The percentage of patients with viral loads <10 000 and >10 000 copies per milliliter at ACT within the groups of evaluable responders (n = 15), survivors (n = 10), and nonresponders (n = 5) in the time period of 6 months after ACT are shown. Patients 7, 8, 10, 13, 32, and 33 are not represented because viremia was negative at time of ACT; for patients 15, 22, and 34, only qualitative polymerase chain reaction results were available, and patient 1 is not included in the figure because the patient died shortly after ACT and could therefore not be classified as responder or nonresponder. For patients 24 and 25, viral load is indicated as 1000 copies per microgram of DNA, and for patient 31, in genome equivalents per 20 000 cells.
Cumulative probability of response to ACT. (A) Cumulative probability of reduction or clearance of viremia after adoptive transfer of AdV-specific T cells with and without in vivo expansion of specific T cells. The two plots demonstrate the influence of in vivo expansion of AdV-specific T cells (defined as detected ≥0.01% IFN-γ+ T cells of viable T cells in blood samples of patients after ACT) for the cumulative probability of reduction (left) and clearance (right) of viremia until day 35 after adoptive T-cell therapy (n = 17). Patients 1, 4, 6, 7, 8, 10, 13, 23, 27, 32, 33, 34, and 35 are not represented in the figure because T-cell response could not be analyzed within the time period, patients already had negative viremia at time of ACT, or no quantitative analysis of the viral load was available. (B) AdV-related mortality in responders and nonresponders. Shown are the percentage of responders (defined as reduction >1 log change in copy numbers of viremia; n = 21) and nonresponders (defined as no reduction >1 log change in copy numbers or clearance of viremia; n = 8) who died as a result of AdV infection, as a result of other cause of death, or who survived within the observation period of 6 months after ACT. Numbers in the figure are rounded up so that 2 of 21 represents 10% instead of 9.52%. (C) Time to AdV-related mortality. Time of death within the follow-up of 6 months after ACT is demonstrated for the deceased responders (n = 6) and nonresponders (n = 8). (D) Correlation of the level of viremia with the velocity of viral clearance and virologic response after ACT. Time period until first viral clearance after ACT in correlation to the level of viremia at or prior to ACT is shown in the left graph. In evaluable patients with viral loads <10 000 copies per milliliter at time of ACT, first clearance of viremia could be achieved at a mean of 20 days (range, 6 to 67 days) after ACT. First viral clearance of evaluable patients with AdV loads >10 000 copies per milliliter was reached significantly later, at a mean of 57 days after ACT (range, 19 to 91 days). (Right) Time until first virologic response (defined as reduction of the viral load in copy numbers ≥1 log) in correlation to the level of viremia at or before ACT. In patients with viral loads <10 000 copies per milliliter at time of ACT, first virologic response could be achieved at a mean of 14 days (range, 6 to 22 days) after ACT. Significant reduction of the viral load in patients with AdV loads >10 000 copies per milliliter at ACT occurred for the first time at a mean of 26 days (range, 4 to 62 days) after ACT. For patients 24 and 25, viral load is indicated in copies per microgram of DNA and for patient 31, in genome equivalents per 20 000 cells. For patients 30, 31, and 35, time until first viral clearance or virologic response after first ACT is shown. (E) Correlation of the viral load at ACT with virologic response to ACT and with outcome of patients. The percentage of patients with viral loads <10 000 and >10 000 copies per milliliter at ACT within the groups of evaluable responders (n = 15), survivors (n = 10), and nonresponders (n = 5) in the time period of 6 months after ACT are shown. Patients 7, 8, 10, 13, 32, and 33 are not represented because viremia was negative at time of ACT; for patients 15, 22, and 34, only qualitative polymerase chain reaction results were available, and patient 1 is not included in the figure because the patient died shortly after ACT and could therefore not be classified as responder or nonresponder. For patients 24 and 25, viral load is indicated as 1000 copies per microgram of DNA, and for patient 31, in genome equivalents per 20 000 cells.
Because of renal insufficiency, 7 patients did not receive cidofovir after ACT. Among them were 2 nonresponders (patients 6 and 22), 4 responders (patients 8, 12, 16, and 26), and 1 patient who was not classified as either a responder or nonresponder because of death on day 2 after ACT. Therefore, we could not find a difference in response to ACT among patients who were treated with or without cidofovir after ACT. Patients could reach clearance of viremia after ACT without additional antiviral treatment with cidofovir.
Outcome and follow-up after ACT
Prior to treatment with antigen-specific T cells, patients suffered from AdV infection refractory to antiviral pharmacologic therapy. AdV infection was first detected in stool at a mean of 29 days after HSCT and at a mean of 7 days before the onset of viremia (Table 1). ACT with subsequent in vivo expansion of AdV-specific T cells was strongly associated with clearance or significant reduction of viremia within the observation period (Figures 1B and 3A). Follow-up was performed at least until 6 months and up to 2764 days after ACT (Table 3).
All nonresponders (n = 8) died shortly after ACT at a mean of 24 days (range, 9 to 56 days) after immunotherapy. In 100% of nonresponders, death was related to AdV infection compared with 9.5% AdV-related mortality in responders. In contrast, within the observation period of 6 months after ACT, 71% (15) of the responders survived. Survival of patients who died within this follow-up period was considerably longer among responders (mean number of days after ACT until death, 122) compared with nonresponders (Table 3 and Figure 3C). Causes of death among responders with final clearance of viremia were not related to AdV. Death in these patients was the result of a progression or relapse of underlying disease (patients 10, 16, 26, and 28). Death was related to AdV in only 2 patients within the group of responders (patients 24 and 25). Neither patient showed in vivo expansion of AdV-specific T cells, and there was no final clearance of viremia. Thus, all responders with final clearance of viremia after immunotherapy either survived or died as a result of some other cause of death (Table 3). In case of a detectable T-cell response after ACT (n = 14), AdV-related mortality was 14% (n = 2). In contrast, AdV-specific T-cell responses were not detected in 82% (9 of 11) of the patients with AdV-related mortality. In follow-up of more than 6 months, AdV-related mortality was 0%.
Patients with AdV-related mortality who did not respond to ACT had a high viral load of >10 000 copies per milliliter of blood (Figure 3E). However, in case of a T-cell response, clearance of viremia could occur even with very high levels of viremia: patient 28 achieved viral clearance from a level of 11 000 000 copies per milliliter on day 73 after ACT and patient 26 (40 700 AdV copies per milliliter at ACT) developed in vivo expansion of AdV-specific T cells on day 14 with subsequent viral clearance on day 20 after ACT.
Most patients were treated preemptively if they had an increasing viral load, and some patients already had severe virus-related disease. Clinical appearance of AdV disease was variable, and diarrhea was the most frequent symptom. In addition, 5 patients suffered from pneumonia, 6 from hepatitis, and 1 from encephalitis. Outcome of this patient cohort was worse compared with those who received preemptive treatment: 3 patients (25%) survived within a follow-up of 6 months after ACT, and 9 patients (75%) died at a mean of 50 days after ACT.
Before ACT, we aimed at a steroid dose of ≤1 mg/kg prednisolone. Sixteen patients received treatment with steroids at the time of ACT (patients 7, 11, 12, 13, 14, 15, 22, 23, 26, 27, 28, 31, 32, 33, 34, and 35). Most of the patients being treated with steroids developed in vivo expansion of AdV-specific T cells (12 of 13 evaluable patients) with subsequent virologic response (12 of 16 evaluable patients). Although steroid dose was still above 1 mg/kg in 5 patients, 3 of the 5 responded to ACT. In vivo expansion of AdV-specific T cells and virologic response could therefore take place under a low dose of steroids and, in single patients, under even higher doses of steroids.
In vivo T-cell response and pharmacologic immunosuppression
In our study, we showed that the presence and proliferation of AdV-specific T cells resulted from ACT itself and was not due only to withdrawal of immunosuppression: 14 of 23 evaluable patients developed AdV-specific T cells after ACT. Sixty-four percent of the patients (9) with detectable AdV-specific T cells were treated with single (n = 4) or multiple immunosuppressive drugs (5 patients were treated with 2 or more immunosuppressive drugs) at time of T-cell recovery. After withdrawal from or without immunosuppression, 9 patients did not develop AdV-specific T cells after ACT. AdV-specific T-cell responses developed under unchanged immunosuppressive treatment in the majority of patients, even under triple immunosuppressive treatment (patients 13 and 14) and after intensification of immunosuppression (patient 13). We can thus conclude that development of AdV-specific T cells in these patients was not the result of a change in immunosuppression. More likely it reflects the adoptively transferred T cells. T-cell responses were independent of the HLA disparities between donor and recipient. Responses could be observed in 56% of patients with HLA-matched donors (5 of 9 evaluable patients) and in 80% of patients with haploidentical or mismatched donors (16 of 20 patients).
Discussion
HSCT has become a routine treatment of a variety of inborn and acquired diseases. However, cure through HSCT encompasses a considerable treatment-related risk for the patient. Reduction of toxicities and side effects has improved dramatically over the last few decades. In pediatric patients after HSCT, viral disease still represents the single most important factor influencing treatment-related mortality, and AdV is the most common viral pathogen in these children.24-26 Because virus-specific T cells have been demonstrated to be essential for controlling virus infection,7,15,27,28 transfer of antigen-specific T cells for the reconstitution of a protective T-cell immunity has become a successful approach for the treatment of viral infections after HSCT.13,17,19
In this clinical trial, we analyzed the safety and efficacy of adoptive transfer of hexon-specific T cells generated by IFN-γ capture technique as a treatment strategy for chemotherapy refractory AdV infections after HSCT. The short GMP process of about 30 hours was successfully performed in 100% of the patients by using the adenoviral hexon antigen. Small numbers of mainly IFN-γ+–secreting Th1 cells were infused into patients without any relevant side effects or GVHD induction or aggravation. Therefore, ACT was safe with no acute toxicities in all treated patients. This clinical trial is not a placebo controlled trial and thus will not allow confirmative statistical analysis of efficacy and safety. Our descriptive data on efficacy show that ACT was followed by in vivo expansion of virus-specific T cells. We cannot prove that efficacy was solely due to ACT in every single case. However, the data provide a strong correlation between induction of specific T-cell immunity and a virologic response. GVHD rate after ACT was attributed arbitrarily to ACT when onset occurred within an 8-week period after transfer, and it cannot be excluded that the AdV-specific T cells contributed to acute GVHD occurrence at later time points. Formal distinction between ACT-induced and HSCT-induced GVHD will be possible only in future trials with a control arm.
It has been demonstrated that in vivo repopulation of distinct T-cell patients can be achieved even by a single antigen-specific T cell29 and that in vitro amplification of T cells is associated with reduced potential for in vivo expansion because of acquisition of terminal effector properties.30 Small numbers of AdV-specific T cells were thus infused without further ex vivo expansion steps. Efficacy of ACT was independent of the initial T-cell dose. Even a T-cell dose of 312 CD3+ T cells per kilogram showed effective in vivo expansion of T cells with successful clearance of viremia and survival of the patient within the observation period of 6 months after ACT.
The generated T-cell graft consisted mainly of CD4+ and a few CD8+ T cells which are both supposed to be required for sustained and effective immune responses: CD8+ T cells exert rapid antiviral activity and CD4+ T cells are essential for maintaining immune responses.31,32 Because the isolation procedure is based on IFN-γ, a predominance of Th cells with a Th1 cytokine profile can lead to an effective containment of the virus.
AdV-specific T-cell grafts were composed of a mixture of naïve, central memory, effector memory, and effector T-cell populations with a predominance of late effector stages. This indicates proliferation and effector potential of the transferred T cells because repopulating capacity is attributed to naïve and/or central memory T cells and effector functions to further differentiated T-cell stages.33
AdV-specific T-cell immunity could be detected up to 6 months after adoptive T-cell therapy, indicating a sustained and long-term protective T-cell response. In vivo expansion of AdV-specific T cells was effective and resulted in clearance of viremia in 86% of patients. Virologic response to adoptive T-cell therapy could thereby be straight or transient, and clearance of viremia could occur within 6 weeks of ACT (fast responders) or beyond 6 weeks after ACT (slow responders).
Causes of death in responders with final clearance of viremia were not related to AdV in any of the patients. AdV-related mortality was 14% in case of a detectable T-cell response after ACT, indicating a strong correlation between virus-specific immunity and virus control. Nonresponse to adoptive T-cell therapy was associated with AdV-related mortality in 100% of patients and with very limited survival time (mean time to death was 24 days after ACT). In contrast, outcomes of patients with virologic response to ACT was superior, with 71% of the patients surviving within an observation period of 6 months after ACT.
It has previously been shown that high levels of AdV DNA in blood correlate with fatal outcomes.8,34 In our study, high AdV loads at time of ACT (>10 000 copies per milliliter) were associated with late first clearance of viremia and with inferior outcome. However, the successful in vivo expansion of AdV-specific T cells with consequent virus clearance after ACT in patients with very high viral loads (of several million copies per milliliter) demonstrates the power of adoptive immunotherapy. Conversely, in patients in whom AdV has already led to organ damage, ACT did not prevent AdV-related mortality (eg, AdV-related lung failure), even in the absence of viremia (supplemental Table 3).
Regarding cost-benefit, in Germany, one isolation and infusion of virus-specific T cells represents 4.9 doses of cidofovir in terms of price. In a study by Yusuf et al,35 a median of 5 doses (range, 1 to 22 doses) of cidofovir was given. In our study, refractory patients usually needed more than 5 doses of cidofovir. In patients already suffering from AdV disease, hospitalization further increases the costs. In these cases, cost-benefit considerations make it even more important to support ACT.
In line with previous studies,17 we show that repetitive ACT seems to be a promising option in case of nonresponsiveness or lack of viral clearance after first transfer. But the number of patients with repetitive infusions of AdV-specific T cells is small (n = 4), and thus the benefit of repetitive ACTs needs to be evaluated in further clinical trials.
In conclusion, this clinical trial showed the safety and efficacy of a cellular immunotherapy approach in multiple centers, encouraging the implication of individualized immunotherapy approaches into clinical routine. Ex vivo ACT followed by in vivo expansion of hexon-specific T cells strongly correlates with clearance of viremia, which is associated with a remarkable reduction in virus-related mortality—from 100% in nonresponders to 9.5% in responders. Induction of Th1-driven T-cell responses is a fast, safe, and potent treatment strategy suited to wide clinical application. In the future, further relevant factors for successful in vivo T-cell expansion have to be determined, and efficacy will have to be confirmed in controlled clinical trials.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Christiane Braun and Michael Schumm for excellent GMP support and technical assistance. Miltenyi Biotec GmbH supplied CliniMACS materials at no cost.
This work was supported by Grant No. SFB685 from the German Research Foundation, grants from the German Centre for Infection Research and Tübingen University, and by Tübingen University Hospital for this investigator-driven clinical trial.
Authorship
Contribution: T.F., P.L., and R.H. developed the study concept; B.M.-K. treated patients 1, 2, and 3; S.M.-M. treated patients 4 and 5; W.B. treated patients 6, 7, 8, and 12; T.F., R.H., P.L., and K.O. treated patients 9, 10, 11, 13, and 14; H.E. treated patient 15; P.-G.S. treated patient 16; J.G. treated patients 19 and 30; P.B. treated patients 22, 23, 34, and 35; F.R.S. treated patients 24 and 25; M.H.A. treated patients 31, 32, and 33; C.R. treated patient 26; B.G. treated patient 27; B.K. treated patient 28; J.F., K.O., T.F., S.K., and L.H. performed data analysis and collection; J.F. and T.F. wrote the manuscript; and all authors reviewed the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Tobias Feuchtinger, Department of Pediatric Hematology/Oncology, University Children’s Hospital, Eberhard-Karls University, Hoppe-Seyler Straße 1, D-72076 Tübingen, Germany; e-mail: tobias.feuchtinger@med.uni-tuebingen.de.

![Figure 1. In vivo T-cell response after adoptive transfer of hexon-specific T cells after allogeneic HSCT. Antigen-specific T cells were analyzed by flow cytometry after stimulation of blood samples with hexon antigen, followed by intracellular cytokine staining. (A) Fourteen of 23 evaluable patients developed successful in vivo T-cell expansion after ACT. The threshold of positive antigen-specific T-cell responses was defined as 0.01% IFN-γ+ T cells of viable T cells. In vivo expansion could be detected until a mean of 72 ± 47 days (range, 11 to 177 days) after ACT and was found within both the CD4+ and CD8+ T-cell compartments in 13 (93%) of the 14 patients with detectable IFN-γ+ T cells. Exclusive expansion in the CD4+ T-cell compartment was found in patient 7 (and in patient 30 after first ACT). Detailed information on in vivo T-cell expansion of the patients is shown in supplemental Figure 2. (B) Time course of in vivo T-cell response and virologic response of patient (Pat.) 31 is shown. In vivo expansion of AdV-specific T cells after adoptive transfer was associated with clearance of the viral load. Patient 31 shows a straight and fast virologic response with clearance of viremia <6 weeks after second ACT. (C) Immune reconstitution status prior to and after ACT. Number of leukocytes (white blood cell count [WBC]) and CD3+ T cells are shown prior to and 1 week and 1 month after ACT. WBC counts did not change after ACT, but CD3 counts significantly increased after ACT, confirming that the response was not related to an overall reconstitution of WBCs. ns, not significant.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/125/12/10.1182_blood-2014-06-573725/4/m_1986f1.jpeg?Expires=1769094591&Signature=ve0axNn8-LgVBcG~NHLPptOO6eOiFWdA5t3pJof9utKyAdO9c6~dT1rQFaYczJMT~WYrytoYV92LAHcvBEfZ5e~qhj8hgtSvkykMQD4OMWlyH33ZpR6060A5a-LCIZ-VbTG1ObfaF~8T2k0LgcCPFr6BbCgImHEATUgWNko23tJtx-R5EV6YcYwkSahJLqrcHH1xXQHTh9LYfGSbgfJSHD0t0pekWyBFXvDn5aooKbygp~t9O-vAkHRbN09h3sjALhJXb8-KP~wEU9zI~5PJ4Af-yb6KUaG9QSljQJaVn-8BiPYgVLP5auBbSiTdu0BnflqKYGkPWUaiNgRra3f-GQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)