Key Points
Mx1+ stromal cells and/or their descendants provide functional niches for HSPCs and regulate their localization.
Targeting Ext1 or HSPG can mobilize more potent reconstituting cells and enable engraftment without cytotoxic conditioning.
Abstract
The glycosyltransferase gene, Ext1, is essential for heparan sulfate production. Induced deletion of Ext1 selectively in Mx1-expressing bone marrow (BM) stromal cells, a known population of skeletal stem/progenitor cells, in adult mice resulted in marked changes in hematopoietic stem and progenitor cell (HSPC) localization. HSPC egressed from BM to spleen after Ext1 deletion. This was associated with altered signaling in the stromal cells and with reduced vascular cell adhesion molecule 1 production by them. Further, pharmacologic inhibition of heparan sulfate mobilized qualitatively more potent and quantitatively more HSPC from the BM than granulocyte colony-stimulating factor alone, including in a setting of granulocyte colony-stimulating factor resistance. The reduced presence of endogenous HSPC after Ext1 deletion was associated with engraftment of transfused HSPC without any toxic conditioning of the host. Therefore, inhibiting heparan sulfate production may provide a means for avoiding the toxicities of radiation or chemotherapy in HSPC transplantation for nonmalignant conditions.
Introduction
Heparan sulfate proteoglycans (HSPGs) are thought to serve as extracellular binding partners for secreted signaling molecules. They participate in establishing and maintaining morphogen gradients that play central roles in establishing the position and identity of cells to create the architecture of developing tissues.1-4 Gradients are also recognized determinants of events in adult organisms, although these have largely been explored on the level of particular cytokines.5,6 Electrostatic interactions of cytokines with HSPGs restrict diffusion and permit gradients to persist, perhaps revealing why HSPG are uniformly present in all metazoa.7-9
In hematopoiesis, HSPGs have been implicated in a variety of processes. In vitro studies performed in the 1980s and 1990s described the interaction of HSPGs with key hematopoietic cytokines and theorized a potential role in bone marrow (BM) compartmentalization.10-12 These studies provided the first evidence that the effect exerted by cytokines such as granulocyte macrophage colony-stimulating factor (GM-CSF) and interleukin 3 depended on the integrity of the HSPGs to which they are bound; enzymatic or chemical degradation of HSPGs impaired the effects of the cytokines in vitro. More recently, in vivo administration of naturally occurring and synthetic HSPG mimetics has been shown to induce rapid mobilization of hematopoietic stem cells (HSCs) and progenitor cells13-15 from the BM to the peripheral blood (PB), likely by modulating CXC chemokine ligand 12 (CXCL12) levels.14 In contrast, overexpression of the HSPG-cleaving enzyme heparanase in mice results in an accumulation of HSPCs in the BM as a result of an increase in CXCL12 turnover and reduced activity of proteolytic enzymes in the BM.16 Moreover, Khurana and colleagues recently demonstrated that glypican 3, a HSPG family member, inhibits the extracellular dipeptidylpeptidase CD26,17 implicated in HSPC homing and mobilization.18,19
Our laboratory recently described a population of BM skeletal stem/progenitors characterized by the interferon-inducible expression of the Myxovirus resistance 1 (Mx1) gene.20 These cells participate in bone homeostasis and partially overlap with the Nestin1+ stromal population, shown to be a component of the HSPC niche.21 We hypothesized that cytokines and morphogens maintained by interaction with locally secreted matrix proteins are essential in maintaining the HSC/progenitor cell (HSPC) niche. To test this, we conditionally deleted the Ext1 gene, a glycosyltransferase essential for the synthesis of heparan sulfate,9,22 in Mx1+ stromal cells. Our data demonstrate that Ext1/HSPG expressed in Mx1+ stromal cells and their descendants control HSPC localization and retention in the BM, in part by modulating vascular cell adhesion molecule 1 (Vcam1). Competitive pharmacologic inhibition of endogenous heparan sulfate enhanced the mobilization efficacy of G-CSF, including in the setting of mobilization resistance in a diabetes model. Further, the mobilized HSPCs had improved kinetics of reconstitution in primary and secondary transplants in irradiated hosts. Finally, engraftment of transplanted HSPCs occurred efficiently without cytotoxic conditioning shortly after mice were rendered Ext-1-deficient. These findings demonstrate the critical role of heparan sulfate in the BM HSC niche and suggest that targeting heparan sulfate or the enzyme, Ext1, may provide novel methods for achieving outcomes in either mobilization or engraftment that are of importance for clinical HSPC transplantation.
Materials and methods
Mice
EXT1flox/flox mice were previously described.9 C57BL/6J, B6.SJL-Ptprca Pep3b/BoyJ (B6.SJL), Mx1-Cre (B6.Cg-Tg[Mx1-cre]1Cgn/J), Rosa26-loxP-stop-loxP-EYFP (Rosa-YFP, B6.129X1Gt[ROSA]26Sortm1[EYFP]Cos/J), and Col2.3-GFP (B6.Cg-Tg[Col1a1*2.3-GFP]1Rowe/J) mice were purchased from Jackson Laboratory. Six- to 12-week-old male mice were used. Polyinosinic-polycytidylic acid (pIpC) was obtained from Amersham (GE-Healthcare Life Sciences) and administered by intraperitoneal injection at a dose of 25 mg/kg total body weight (TBW) in phosphate-buffered saline every other day for 4 days. The Harvard University Institutional Animal Care and Use Committee and the Subcommittee on Research Animal Care of the Massachusetts General Hospital approved all animal work.
Flow cytometry analysis
Immunophenotypic characterization of the hematopoietic and stromal compartments was performed as previously described.23 For details, see supplemental Data, available on the Blood Web site. Vcam1 and Cxcl12 protein levels were evaluated with an anti-Vcam1-APC and an anti-Cxcl12-APC antibody, respectively, and with the corresponding isotype controls (R&D Systems). All data collection was performed on an LSRII or FACS Aria II (Beckon Dickinson), and data analysis was performed with FlowJo (Treestar).
Transplantation assays
For noncompetitive BM transplantation, to create the chimeras described in Figure 1C, 1 million whole-BM cells from B6.SJL (CD45.1) mice were transplanted into lethally irradiated (9.5 Gy from a cesium source 4 to 24 hours before transplantation) Ext1-loxP/loxP-Mx1cre (CD45.2) recipients 6 to 8 weeks before pIpC administration. Neutrophil and platelet recovery assay was performed as previously described.24 Briefly, 3 million mobilized PB mononuclear cells from C57BL/6J (CD45.2) mice were transplanted into lethally irradiated B6.SJL (CD45.1) mice and followed for at least 36 days. For transplantation without cytotoxic conditioning, 1, 4, or 8 million whole-BM cells from B6.SJL mice were transplanted into Ext1-loxP/loxP-Mx1cre+ or Ext1-loxP/loxP-Mx1cre− recipients 3 weeks after pIpC administration. For competitive transplantation of mobilized PB, 150 μL PB (CD45.2) was mixed with 2 × 105 congenic BM support cells and injected into lethally irradiated CD45.1 recipients. For competitive transplantation of splenocytes, 1 million whole-spleen cells from Ext1 control or mutant chimeric mice (CD45.1) were mixed with 106 congenic whole-spleen support cells (CD45.2) and injected into lethally irradiated CD45.2 recipients. Cells were infused via lateral tail vein injection. Engraftment was monitored at 4-week intervals by fluorescence-activated cell sorter analysis.
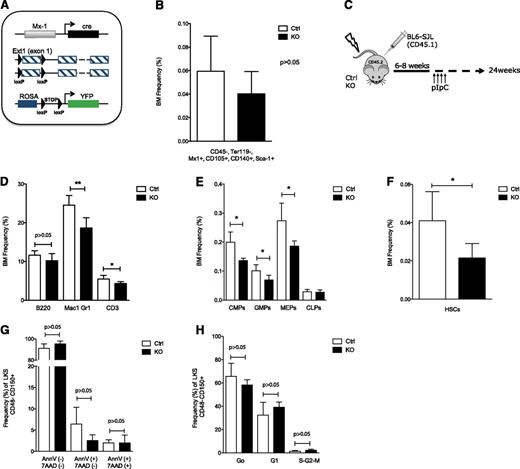
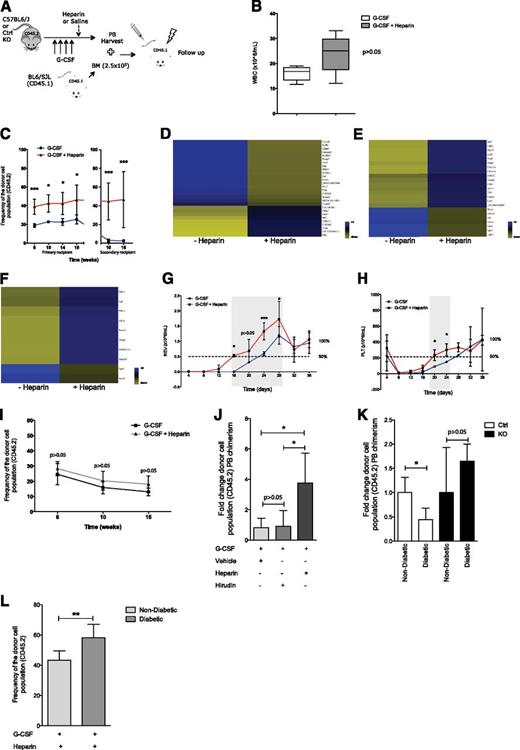
Stromal cell heparan sulfate controls HSPC localization in the BM. (A) Schematic overview of the breeding strategy to facilitate prospective isolation of CD45− Ter119− Mx1+ cells. Control and mutant mice were bred into a Rosa-YFP strain. (B) BM analysis showing contribution to CD45− Ter119− Mx1+ CD105+ CD140+ Sca1+ stromal stem cells in control and mutant mice. (C) Schematic overview of experimental design. (D-H and Figure 2A-H) Data collected 24 weeks after pIpC induction. (D-F) BM analysis showing (D) contribution to B cells (B220), myeloid cells (Mac1 and Gr1), and T cells (CD3); (E) committed myeloid (CMP, GMP, and MEP) and common lymphoid progenitors; and (F) to LKS CD48−CD150+ HSCs. (G) Apoptosis in the BM KLS CD48−CD150+ cell population. (H) Cell cycle analysis of BM KLS CD48−CD150+ HSCs; cells in G0, G1, and S-G2-M phases of the cell cycle are shown. CMP: Lin−cKit+Sca1−CD34+CD16/32−; GMPs: Lin−cKit+Sca1−CD34+CD16/32+; MEPs: Lin−cKit+Sca1−CD34−CD16/32−; common lymphoid progenitors: Lin−cKitlowSca1lowCD127+. Data are representative of at least 3 independent experiments; n = 5 to 8 mice per genotype per experiment. Data are represented as mean ± SD. * P < .05; ** P < .01. Ctrl, control; KO, knockout.
Stromal cell heparan sulfate controls HSPC localization in the BM. (A) Schematic overview of the breeding strategy to facilitate prospective isolation of CD45− Ter119− Mx1+ cells. Control and mutant mice were bred into a Rosa-YFP strain. (B) BM analysis showing contribution to CD45− Ter119− Mx1+ CD105+ CD140+ Sca1+ stromal stem cells in control and mutant mice. (C) Schematic overview of experimental design. (D-H and Figure 2A-H) Data collected 24 weeks after pIpC induction. (D-F) BM analysis showing (D) contribution to B cells (B220), myeloid cells (Mac1 and Gr1), and T cells (CD3); (E) committed myeloid (CMP, GMP, and MEP) and common lymphoid progenitors; and (F) to LKS CD48−CD150+ HSCs. (G) Apoptosis in the BM KLS CD48−CD150+ cell population. (H) Cell cycle analysis of BM KLS CD48−CD150+ HSCs; cells in G0, G1, and S-G2-M phases of the cell cycle are shown. CMP: Lin−cKit+Sca1−CD34+CD16/32−; GMPs: Lin−cKit+Sca1−CD34+CD16/32+; MEPs: Lin−cKit+Sca1−CD34−CD16/32−; common lymphoid progenitors: Lin−cKitlowSca1lowCD127+. Data are representative of at least 3 independent experiments; n = 5 to 8 mice per genotype per experiment. Data are represented as mean ± SD. * P < .05; ** P < .01. Ctrl, control; KO, knockout.
Intravital microscopy
In vivo imaging of HSPCs in the calvaria BM cavity and data analysis were performed as previously described.25 Briefly, fluorescence-activated cell sorter-sorted HSCs were stained in PBS for 15 minutes at 37°C with DiD (1,1′dioctadecil-3,3,3′-tetramethylindodicarbocyanine perchlorate; Invitrogen), using a 1:200 dilution and injected into lethally irradiated Ext1 control and mutant Col2.3-GFP+ recipients. Mice were imaged 24 hours later. Distance between HSCs, GFP+ osteoblastic cells, and bone were measured using Image J software.
HSC mobilization and blood collection
Recombinant human G-CSF (Neupogen, Filgrastim) was administered at 125 μg/kg of TBW every 12 hours for 8 consecutive injections. Heparin sodium (APP Pharmaceuticals) was injected intraperitoneally at a single dose of 100 U. Hirudin was used at 40 mg/kg of TBW in a single dose. Vcam1 neutralizing antibody and the corresponding isotype control (Rat IgG2a, κ) were injected intravenously at 2 mg/kg of TBW every day for 3 doses, and PB samples were obtained through retroorbital bleeding the day after the last injection. AMD3100 was administered subcutaneously at a single dose of 5 mg/kg TBW. PB samples were obtained through retroorbital bleeding 3 hours after the last injection of G-CSF and 1 hour after heparin, AMD3100, or hirudin injection.
Diabetic mouse model
Diabetes was induced in 4- to 6-week-old C57BL/6J or Ext1-loxP/loxP-Mx1cre male mice, as previously described.26 Only animals with glucose values higher than 300 mg/dL were used for experiments.
Statistical analysis
Unpaired, 2-tailed Student t-test or one-way analysis of variance, followed by the appropriate post hoc test, was used. Data have been plotted as average ± standard deviation (SD) for samples following a normal (Gaussian) distribution. Alternatively, Mann-Whitney U test was used and data have been plotted as median ± interquartile range. Statistical significance is indicated as follows: *P < .05, **P < .01, and ***P < .001.
Results
Heparan sulfate controls HSPC localization
Mx1 is expressed in the hematopoietic system and in osteolineage stromal cells after interferon induction by Poly(I):Poly(C) (pIpC).20,27 Biallelic deletion of floxed alleles by cre recombinase driven by the Mx1 promoter is highly efficient in the hematopoietic system23 but is less characterized in the stromal compartment. To evaluate the efficiency of Ext1 deletion in the stromal compartment upon pIpC administration, control and mutant Ext1 mice were crossed with the ROSA26-loxP-stop-loxP-EYFP (Rosa-YFP) reporter mice to generate Ext1-loxP/loxP-Mx1cre+;Rosa-YFP+ (mutant-YFP) and Ext1+/+;Mx1cre+;Rosa-YFP+ (control-YFP) animals (Figure 1A). Mutant and control YFP+, CD45−, Ter119-skeletal progenitors were flow-sorted 21 days after pIpC induction, and efficient deletion of Ext1 was verified in ex vivo-expanded cells by western blotting (supplemental Figure 1A-B). Furthermore, significant abrogation of heparan sulfate production upon Ext1 deletion was detected in ex vivo-expanded as well as freshly isolated Mx1+ stromal cells (supplemental Figure 1C-D). Heparan sulfate has been previously implicated in the maintenance of mesenchymal stem cells.28 Importantly, deletion of Ext1 in our model did not affect the abundance of immunophenotypically defined mesenchymal/stromal stem cells in the BM (Figure 1B). To restrict Ext1 deletion to Mx1+ skeletal progenitors, we transplanted total BM cells from CD45.1-expressing congenic animals (B6.SJL) into lethally irradiated mutant (Ext1-loxP/loxP-Mx1cre+) or control (Ext1-loxP/loxP-Mx1cre−) mice (both in C57BL/6J background) (Figure 1C) to create control and mutant chimeras. Six to 8 weeks after transplantation, the hematopoietic system was replaced by more than 95% CD45.1 cells (supplemental Figure 1E) and chimeric animals displayed equivalent hematopoietic parameters such as white blood cells (WBC), red blood cells, platelet counts, and WBC lineage distribution in the PB (supplemental Figure 1F-I). Ext1 was then deleted in nonhematopoietic cells by pIpC induction and hematopoiesis was monitored for 24 weeks. At 24 weeks post pIpC, both control and mutant chimeric mice displayed comparable TBW and BM cellularity (supplemental Figure 1J-K). BM immunophenotypic analysis revealed a significant decrease in the proportion of Mac1+Gr1+ and CD3+ cells in mutant chimeras without a change in the B220+ population (Figure 1D). Similarly, we found a significant decrease in the proportion of mature myeloid progenitors (common myeloid progenitors or CMPs, megakaryocyte erythroid progenitors or MEPs, and GM progenitors or GMPs) (Figure 1E), a comparable frequency of common lymphoid progenitors, as well as a twofold decrease in the proportion of LKS (Lin−cKit+Sca1+) CD48−CD150+ HSCs in the BM of mutant chimeric mice (Figure 1F). These changes, however, could be attributed neither to an increase in HSC death nor to a proliferation defect (Figure 1G-H).
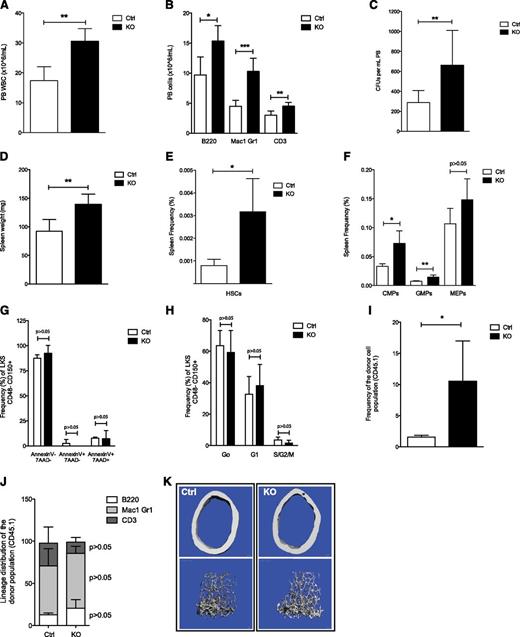
Given the lack of changes in apoptosis and cell cycle of HSCs in mutant chimeric mice, we assessed for mislocalization of HSPCs by examining the PB and spleen. During the 24-week time course, mutant chimeras developed a marked leukocytosis (Figure 2A), with a significant increase in the numbers of lymphoid and myeloid cells in PB (Figure 2B) and a moderate thrombocytopenia with normal red blood cell counts (supplemental Figure 1L-M). In addition, PB colony-forming assays (CFU-Cs) displayed a significant increase in circulating progenitors in mutant chimeric mice (Figure 2C). Mutant chimeras had significantly larger spleens at 24 weeks after pIpC injection (Figure 2D). Immunophenotypic characterization of the spleen revealed a substantial increase in the proportion of HSCs (Figure 2E), CMPs, and GMPs, but not MEPs, in these mice (Figure 2F). The accumulation of HSCs in the spleen cannot be attributed to a resistance to cell death or to an increase in their proliferation (Figure 2G-H). Furthermore, competitive transplantation of spleen cells from mutant chimeric mice with equal numbers of spleen cells from C57BL/6J mice revealed a significant, long-term, competitive advantage compared with spleen cells from control chimeras (Figure 2I). Contribution to blood cell lineages by HSCs from control and mutant chimeras in this transplantation experiment was equivalent (Figure 2J). Mislocalization of HSPCs has been associated with neutrophil turnover and neutrophil-induced BM microenvironment changes.29 Distribution of circulating “aged” neutrophils (supplemental Figure 1N) and BM Cxcl12 levels (supplemental Figure 1O) was equivalent in control and mutant chimeric Ext1 mice, suggesting the neutrophilia observed in our model does not account for the HSPC mislocalization. Given that osteolineage cells have been shown to affect hematopoiesis in a number of murine models,30 we evaluated whether the observed HSPC mislocalization in Ext1 mutant mice was a consequence of impaired osteolineage cell function. Bone histomorphometric, micro-computed tomography, and histologic analysis showed no significant differences, suggesting Ext1 deletion in Mx1+ skeletal progenitors is not essential for skeletal homeostasis (Figure 2K; supplemental Figure 1P; supplemental Tables 1 and 2), at least over the intervals examined here. Taken together, these results indicate that production of heparan sulfate by a population of Mx1+ skeletal progenitors regulates HSPC retention in the BM cavity.
Inhibition of stromal cell heparan sulfate synthesis promotes HSPC egress from the BM and accumulation in extramedullary sites. (A-C) PB analysis showing (A) the WBC count; (B) the contribution to B cells (B220), myeloid cells (Mac1 and Gr1), and T cells (CD3); and (C) the number of circulating progenitor cells measured by CFU assay in methylcellulose in chimeric mice. (D-F) Spleen analysis showing (D) total weight, (E) the contribution to KLS CD48−CD150+ HSCs, and (F) the contribution to CMP, GMP, and MEP cells. (G-H) Spleen-resident KLS CD48− CD150+ HSCs analysis showing (G) cell cycle analysis; cells in G0, G1, and S-G2-M phases of the cell cycle are shown, as well as (H) apoptosis in control and mutant chimeric mice. (I) Relative PB reconstitution and (J) contribution to B cells (B220), myeloid cells (Mac1 and Gr1), and T cells (CD3), 16 weeks after transplantation, of recipient C57BL/6J (CD45.2) mice transfused with spleen cells from control or mutant chimeric mice (CD45.1) competed with equal numbers of wild-type (WT) CD45.2 spleen cells. (K) Representative 3-dimensional micro-computed tomography images of femoral cortical bone (top) and cancellous bone (bottom) of control and mutant chimeric mice. Data are representative of at Ctrl, control; KO, knockout.
Inhibition of stromal cell heparan sulfate synthesis promotes HSPC egress from the BM and accumulation in extramedullary sites. (A-C) PB analysis showing (A) the WBC count; (B) the contribution to B cells (B220), myeloid cells (Mac1 and Gr1), and T cells (CD3); and (C) the number of circulating progenitor cells measured by CFU assay in methylcellulose in chimeric mice. (D-F) Spleen analysis showing (D) total weight, (E) the contribution to KLS CD48−CD150+ HSCs, and (F) the contribution to CMP, GMP, and MEP cells. (G-H) Spleen-resident KLS CD48− CD150+ HSCs analysis showing (G) cell cycle analysis; cells in G0, G1, and S-G2-M phases of the cell cycle are shown, as well as (H) apoptosis in control and mutant chimeric mice. (I) Relative PB reconstitution and (J) contribution to B cells (B220), myeloid cells (Mac1 and Gr1), and T cells (CD3), 16 weeks after transplantation, of recipient C57BL/6J (CD45.2) mice transfused with spleen cells from control or mutant chimeric mice (CD45.1) competed with equal numbers of wild-type (WT) CD45.2 spleen cells. (K) Representative 3-dimensional micro-computed tomography images of femoral cortical bone (top) and cancellous bone (bottom) of control and mutant chimeric mice. Data are representative of at Ctrl, control; KO, knockout.
Heparan sulfate modulates HSCs microanatomical positioning
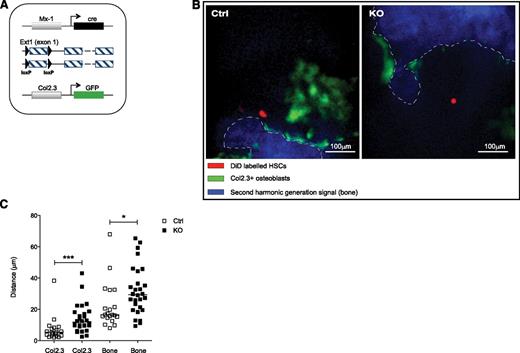
Microanatomic positioning of HSPCs in the BM influences their heterotypic interactions, altering activity and response to stimuli. We evaluated whether HSPC positioning in the BM was affected upon Ext1 deletion in Mx1+ skeletal progenitor cells. Control and mutant mice were bred into a reporter strain expressing GFP under the Col2.3 promoter (Col2.3-GFP+), specifically labeling mature osteoblastic cells (Figure 3A). Ext1 control and mutant Col2.3-GFP+ mice were lethally irradiated and transplanted with 104 WT LKS CD48−, CD150+ DiD-labeled HSCs. Labeled HSCs were visualized in the calvarial BM 24 hours after injection by means of intravital high-resolution 2-photon and confocal microscopy, and their relative distance to osteoblastic cells (Col2.3-GFP+) and the endosteal surface was quantified. The anatomic positioning of DiD+ cells in the mutant animals was markedly changed with cells at a greater distance from GFP+ osteolineage cells (Figure 3B-C).
Heparan sulfate modulates HSCs microanatomical positioning. (A-C) Relative positioning of HSCs in the BM cavity. (A) Control and mutant mice were bred into a strain expressing GFP under the Col2.3 promoter labeling osteoblastic cells. (B) Representative pictures of the BM cavity of control and mutant mice injected with equal numbers of DiD-labeled HSCs (red); blue, second-harmonic generation signal (bone); green, Col2.3GFP (osteoblasts). Scale bars, 100 μm. (C) Distances of LKS CD48−CD150+ HSCs 24 hours after transplantation, from Col2.3GFP cells (left) and endosteal surface (right) in micrometers. Data represent 2 independent experiments; n = 2 mice per genotype per experiment. Data are represented as mean ± SD. *P < .05; **P < .01; ***P < .001.
Heparan sulfate modulates HSCs microanatomical positioning. (A-C) Relative positioning of HSCs in the BM cavity. (A) Control and mutant mice were bred into a strain expressing GFP under the Col2.3 promoter labeling osteoblastic cells. (B) Representative pictures of the BM cavity of control and mutant mice injected with equal numbers of DiD-labeled HSCs (red); blue, second-harmonic generation signal (bone); green, Col2.3GFP (osteoblasts). Scale bars, 100 μm. (C) Distances of LKS CD48−CD150+ HSCs 24 hours after transplantation, from Col2.3GFP cells (left) and endosteal surface (right) in micrometers. Data represent 2 independent experiments; n = 2 mice per genotype per experiment. Data are represented as mean ± SD. *P < .05; **P < .01; ***P < .001.
Heparan sulfate modulates Vcam1-dependent adhesion
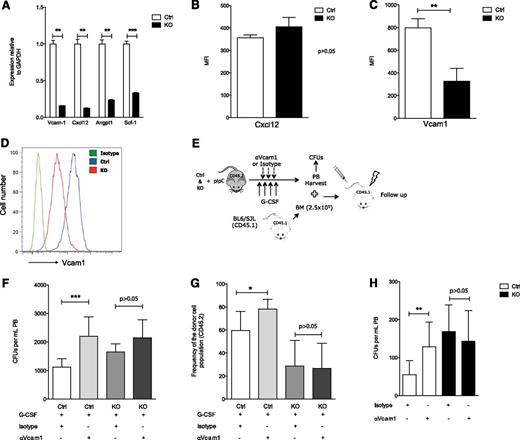
To investigate whether heparan sulfate affected the regulation of molecules known to modulate HSPC localization, control and mutant YFP+ (Figure 1A), CD45−, and Ter119− skeletal progenitors were flow-sorted 21 days after pIpC induction. Assessment of mRNA levels revealed that the expression of Cxcl12, Vcam1, Scf, and angiopoietin-130 was significantly down-regulated in mutant mice (Figure 4A). Conditional deletion of Cxcl12 and Scf in osteolineage cells does not affect HSPC biology,31-33 and angiopoietin1 controls HSC quiescence,34 a state that was not changed in HSCs upon Ext1 deletion (Figure 1H). In addition, Cxcl12 levels in the BM, as well as Cxcl12 protein produced by Mx1+ stromal cells, were comparable in control and mutant mice (supplemental Figure 1O; Figure 4B). Hence, we then evaluated whether the HSPC defects observed upon Ext1 deletion in Mx1+ stromal cells were the result of changes in Vcam1 expression. Vcam1 is the ligand for the alpha4-beta1 integrin, Vla4.35 The Vla4–Vcam1 axis plays a role in HSPC adhesion to their niches, and pharmacologic inhibition of this interaction results in HSPC mobilization.36,37 Vcam1 protein levels were significantly reduced in Mx1+ stromal cells in mutant mice (Figure 4C-D). To determine whether the observed reduction in Vcam1 expression functionally contributed to the mislocalization of HSPCs seen in Ext1-mutant chimeric mice, we evaluated how the addition of a Vcam1-neutralizing antibody influenced G-CSF-induced HSPC mobilization in control and mutant animals (Figure 4E). Mobilized PB from control animals receiving G-CSF, together with Vcam1-neutralizing antibody, displayed a significant increase in circulating CFU-Cs (Figure 4F, left),36 as well as increased donor chimerism in lethally irradiated congenic recipients after transplantation (Figure 4G, left; supplemental Figure 2) compared with animals receiving G-CSF alone. However, PB from mutant Ext1 mice mobilized with the combination of G-CSF and Vcam1-neutralizing antibody displayed an equivalent number of CFU-Cs (Figure 4F, right) and an equivalent capacity to reconstitute hematopoiesis in lethally irradiated congenic recipients (Figure 4G, right; supplemental Figure 2) as G-CSF alone. Comparable results were obtained in response to Vcam1 neutralization without G-CSF administration (Figure 4H). In summary, deletion of Ext1 and its HSPG products in Mx1+ skeletal progenitors reduces production of Vcam1 and other adhesion mediators, rendering the region less functional as a site of stem cell retention.
Heparan sulfate modulates Vcam1-dependent adhesion. (A-D) Expression of niche-related molecules in Mx1+ cells. (A) Real-time polymerase chain reaction for Vcam1, Cxcl12, Angpt1, and Scf1. (B) Cxcl12 protein level quantification in CD45− Ter119− Mx1+ stromal cells from control and mutant Ext1 mice. (C) Vcam1 protein level quantification and (D) representative histogram of Vcam1 protein levels in CD45− Ter119− Mx1+ stromal cells from control and mutant Ext1 mice. (E-H) Functional evaluation of Vcam1 in Mx1+ cells. (E) Overview of the experimental design. (F) Number of circulating progenitors measured by CFU-C assay in PB of control and mutant mice injected with G-CSF and Vcam1 neutralizing antibody. (G) Total PB reconstitution, 16 weeks after transplantation, in recipient mice transfused with 150 μL PB from control or mutant mice mobilized with G-CSF and Vcam1 neutralizing antibody or isotype control. CD45.1 BM cells were transplanted for radioprotection in equal numbers in control and mutant mice. (H) Quantification of PB circulating progenitor cells measured by CFU assay from Ext1 control or mutant mice receiving Vcam1 neutralizing antibody or isotype control. Data are representative of at least 2 independent experiments; n = 5 to 8 mice per genotype per experiment. In mobilization experiments, PB was collected from at least 5 mice per experimental group. Data are represented as mean ± SD. *P < .05; **P < .01; ***P < .001.
Heparan sulfate modulates Vcam1-dependent adhesion. (A-D) Expression of niche-related molecules in Mx1+ cells. (A) Real-time polymerase chain reaction for Vcam1, Cxcl12, Angpt1, and Scf1. (B) Cxcl12 protein level quantification in CD45− Ter119− Mx1+ stromal cells from control and mutant Ext1 mice. (C) Vcam1 protein level quantification and (D) representative histogram of Vcam1 protein levels in CD45− Ter119− Mx1+ stromal cells from control and mutant Ext1 mice. (E-H) Functional evaluation of Vcam1 in Mx1+ cells. (E) Overview of the experimental design. (F) Number of circulating progenitors measured by CFU-C assay in PB of control and mutant mice injected with G-CSF and Vcam1 neutralizing antibody. (G) Total PB reconstitution, 16 weeks after transplantation, in recipient mice transfused with 150 μL PB from control or mutant mice mobilized with G-CSF and Vcam1 neutralizing antibody or isotype control. CD45.1 BM cells were transplanted for radioprotection in equal numbers in control and mutant mice. (H) Quantification of PB circulating progenitor cells measured by CFU assay from Ext1 control or mutant mice receiving Vcam1 neutralizing antibody or isotype control. Data are representative of at least 2 independent experiments; n = 5 to 8 mice per genotype per experiment. In mobilization experiments, PB was collected from at least 5 mice per experimental group. Data are represented as mean ± SD. *P < .05; **P < .01; ***P < .001.
Pharmacological competitive inhibition of heparan sulfate proteoglycans in vivo induces HSPC mobilization
Heparan sulfate mimetics have been shown to induce rapid HSPC mobilization in mice, putatively through competitive inhibition of endogenous HSPGs.13-15 Heparin, a highly sulfated glycosaminoglycan, has been shown to lack the ability to induce mobilization on its own.13 Surprisingly, we observed that heparin administration (100 U/mouse 1 hour before PB harvest) induces a modest, yet significant, increase in the number of circulating HSPCs, as measured by CFU assays, without a significant change in WBC counts (supplemental Figure 3A-B). Given our observations that the genetic deletion of heparan sulfate production alters HSPC localization and that heparin is an inexpensive, US Food and Drug Administration-approved drug, which competitively inhibits HSPG signaling, we investigated whether it could cooperate with the current G-CSF mobilization regimen (Figure 5A). Mice treated with heparin (100 U/mouse 1 hour before PB harvest) in combination with G-CSF displayed a modest (P > .05) increase in the WBC count in PB compared with mice treated with G-CSF alone (Figure 5B). However, the combination of heparin and G-CSF significantly increased the mobilization of long-term reconstituting cells, as measured by PB competitive transplantation into lethally irradiated congenic mice (Figure 5C, left). Furthermore, these cells efficiently self-renewed, as shown by their ability to engraft secondary recipients (Figure 5C, right). Importantly, administration of heparin and G-CSF mobilized a population of HSCs with a distinct transcriptional signature compared with G-CSF alone (Figure 5D-F; supplemental Table 3), suggesting either heparan sulfate competitive inhibition mobilizes an additional population of HSPCs or the mobilization process is sufficiently different to rapidly alter gene expression characteristics.
Pharmacological inhibition of heparan sulfate proteoglycans induces HSPC mobilization. (A) Schematic overview of experimental design. (B) Peripheral WBC counts in C57BL/6J (CD45.2) mice receiving G-CSF or G-CSF plus heparin. (C) Total donor (CD45.2) reconstitution of G-CSF or G-CSF plus heparin mobilized PB from C57BL/6J (CD45.2) transplanted into lethally irradiated CD45.1 congenic recipients. CD45.1 BM cells were transplanted for radioprotection in equal numbers in both groups. The break in the X-axis represents serial BM transplantation into lethally irradiated recipients. (D-F) Clustering of genes differentially expressed in fluorescence-activated cell sorter sorted HSPCs (Lin−, cKit+, Sca1+) from PB upon G-CSF (n = 1) or G-CSF plus heparin (n = 2) induced mobilization showing changes in (D) cell adhesion genes, (E) cell proliferation genes, and (F) growth regulation genes. Blue and yellow represent higher and lower expression, respectively. (G-H) Noncompetitive transplantation of G-CSF or G-CSF plus heparin mobilized PB into lethally irradiated recipients showing (G) neutrophil and (H) platelet recovery. Gray shadowed area represents the window of time in which the recovery time differed between groups. (I) Total donor (CD45.2) reconstitution of G-CSF or G-CSF plus heparin mobilized PB from Ext1 mutant mice transplanted into lethally irradiated CD45.1 congenic recipients. CD45.1 BM cells were transplanted for radioprotection in equal numbers in both groups. (J) Relative PB reconstitution, 16 weeks after transplantation, of recipient CD45.1 mice transfused with PB from C57BL/6J mice (CD45.2) receiving G-CSF and heparin or G-SCF and hirudin. Recipient mice were lethally irradiated and received CD45.1 cells for radioprotection. (K-L) Ext1/heparan sulfate genetic deletion and pharmacological inhibition rescue type 1 diabetes-induced mobilopathy. Relative PB reconstitution, 16 weeks after transplantation, of recipient mice transfused with (K) G-CSF mobilized PB from nondiabetic and diabetic control and mutant mice (4 groups in total) or (L) G-CSF plus heparin mobilized PB from nondiabetic and diabetic C57BL/6J mice. For the transplants, lethally irradiated CD45.1 animals were used as recipients. CD45.1 BM was used in equal numbers for radioprotection. Data are representative of at least 2 independent experiments; n = 5 to 8 mice per genotype per experiment. In mobilization experiments, PB was collected from at least 5 mice per experimental group. Data are represented as mean ± SD or median ± interquartile range. *P < .05; **P < .01; ***P < .001.
Pharmacological inhibition of heparan sulfate proteoglycans induces HSPC mobilization. (A) Schematic overview of experimental design. (B) Peripheral WBC counts in C57BL/6J (CD45.2) mice receiving G-CSF or G-CSF plus heparin. (C) Total donor (CD45.2) reconstitution of G-CSF or G-CSF plus heparin mobilized PB from C57BL/6J (CD45.2) transplanted into lethally irradiated CD45.1 congenic recipients. CD45.1 BM cells were transplanted for radioprotection in equal numbers in both groups. The break in the X-axis represents serial BM transplantation into lethally irradiated recipients. (D-F) Clustering of genes differentially expressed in fluorescence-activated cell sorter sorted HSPCs (Lin−, cKit+, Sca1+) from PB upon G-CSF (n = 1) or G-CSF plus heparin (n = 2) induced mobilization showing changes in (D) cell adhesion genes, (E) cell proliferation genes, and (F) growth regulation genes. Blue and yellow represent higher and lower expression, respectively. (G-H) Noncompetitive transplantation of G-CSF or G-CSF plus heparin mobilized PB into lethally irradiated recipients showing (G) neutrophil and (H) platelet recovery. Gray shadowed area represents the window of time in which the recovery time differed between groups. (I) Total donor (CD45.2) reconstitution of G-CSF or G-CSF plus heparin mobilized PB from Ext1 mutant mice transplanted into lethally irradiated CD45.1 congenic recipients. CD45.1 BM cells were transplanted for radioprotection in equal numbers in both groups. (J) Relative PB reconstitution, 16 weeks after transplantation, of recipient CD45.1 mice transfused with PB from C57BL/6J mice (CD45.2) receiving G-CSF and heparin or G-SCF and hirudin. Recipient mice were lethally irradiated and received CD45.1 cells for radioprotection. (K-L) Ext1/heparan sulfate genetic deletion and pharmacological inhibition rescue type 1 diabetes-induced mobilopathy. Relative PB reconstitution, 16 weeks after transplantation, of recipient mice transfused with (K) G-CSF mobilized PB from nondiabetic and diabetic control and mutant mice (4 groups in total) or (L) G-CSF plus heparin mobilized PB from nondiabetic and diabetic C57BL/6J mice. For the transplants, lethally irradiated CD45.1 animals were used as recipients. CD45.1 BM was used in equal numbers for radioprotection. Data are representative of at least 2 independent experiments; n = 5 to 8 mice per genotype per experiment. In mobilization experiments, PB was collected from at least 5 mice per experimental group. Data are represented as mean ± SD or median ± interquartile range. *P < .05; **P < .01; ***P < .001.
Recovery of neutrophil and platelet counts defines successful engraftment after BM transplantation in clinical settings, and time to recovery predicts survival.38 Importantly, noncompetitive transplantation of PB mobilized by means of coadministration of G-CSF and heparin resulted in a 4- to 6-day quicker recovery of neutrophils (Figure 5G) and platelets (Figure 5H) compared with G-CSF alone. The combination of heparin and G-CSF did not increase HSPC mobilization over G-CSF alone in the Ext1 mutant mice, suggesting heparin enhances G-CSF-induced mobilization in WT mice by modulating endogenous HSPGs (Figure 5I). In addition, hirudin, a nonheparin-based anticoagulant, did not affect G-CSF-induced HSPC mobilization (Figure 5J), indicating the observed effect was not a consequence of heparin anticoagulation properties. HSC transplantation remains the gold standard curative therapy for a number of hematological disorders. However, G-CSF mobilization resistance may compromise HSPC transplant for some individuals. We therefore evaluated whether genetic or pharmacologic competitive inhibition of HSPG may facilitate G-CSF-induced HSPC egress from the BM in a murine model of type 1 diabetes-induced mobilopathy.26 G-CSF failed to efficiently mobilize HSPCs in type 1 diabetic mice. However, the compromised response to G-CSF was fully corrected in type 1 diabetic mice lacking Ext1 expression in Mx1+ stromal cells (Figure 5K). Furthermore, combination of heparin with G-CSF resulted in normal mobilization of long-term reconstituting cells in diabetic animals, as measured by PB competitive transplantation into lethally irradiated congenic mice (Figure 5L). These data demonstrate that functional competitive inhibition of endogenous HSPGs rescues the mobilization abnormalities noted in animals with pharmacologically induced diabetes.
Pharmacological competitive inhibition of heparan sulfate modulates Vcam1-dependent adhesion
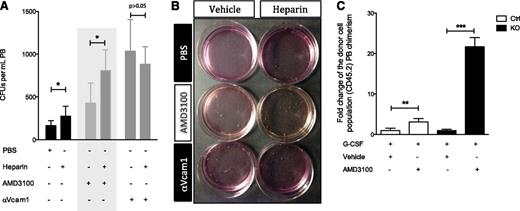
Next we evaluated whether heparin-induced mobilization was abrogated upon Vcam1 inhibition in the absence of G-CSF stimulation, as Vcam1 is, at least partially, responsible for the defect observed in the Ext1 mutant mice. Administration of heparin-enhanced HSPC mobilization compared with vehicle, as measured by CFU assays (Figure 6A-B). Conversely, coadministration of heparin and Vcam1-neutralizing antibodies failed to improve HSPC mobilization over Vcam1 neutralization alone, suggesting heparin induces mobilization by modulating Vcam1-dependent adhesion (Figure 6A-B). However, administration of heparin 1 hour before PB harvest does not modulate Vcam1 levels in Mx1+ stromal cells (supplemental Figure 3C), suggesting alternative mechanisms are implicated in the effect of heparin on Vcam1-dependent adhesion, such as allosteric interactions affecting the Vcam1–Vla4 interaction, as previously shown.39 Importantly, our results also show that coadministration of heparin enhances AMD3100-induced mobilization (Figure 6A, middle shadowed panels; Figure 6B). Moreover, AMD3100, a Cxcr4 antagonist,40 also augments G-CSF-induced mobilization in Ext1-deficient mice (Figure 6C), suggesting the role of heparan sulfate in HSPC retention is independent of the Cxcl12–Cxcr4 axis.
Pharmacological competitive inhibition of heparan sulfate modulates Vcam1-dependent adhesion. (A-B) Vcam1 inhibition abrogates heparin-induced mobilization. (A) PB circulating progenitor cells measured by CFU assay in animals receiving vehicle, AMD3100, or Vcam1 neutralizing antibody alone or in combination with heparin. Gray shadowed area is intended to highlight the fact that coadministration of heparin enhances AMD3100-induced mobilization. (B) Representative image of the CFU assay quantified in A. (C) Relative PB reconstitution, 16 weeks after transplantation, of recipient CD45.1 mice transfused with PB from Ext1 control or mutant mice (CD45.2) receiving G-CSF alone or in combination with AMD3100. Recipient mice were lethally irradiated and received CD45.1 cells for radioprotection. Data are representative of at least 2 independent experiments; n = 5 to 8 mice per genotype per experiment. In mobilization experiments, PB was collected from at least 5 mice per experimental group. Data are represented as mean ± SD or median ± interquartile range. *P < .05; **P < .01; ***P < .001.
Pharmacological competitive inhibition of heparan sulfate modulates Vcam1-dependent adhesion. (A-B) Vcam1 inhibition abrogates heparin-induced mobilization. (A) PB circulating progenitor cells measured by CFU assay in animals receiving vehicle, AMD3100, or Vcam1 neutralizing antibody alone or in combination with heparin. Gray shadowed area is intended to highlight the fact that coadministration of heparin enhances AMD3100-induced mobilization. (B) Representative image of the CFU assay quantified in A. (C) Relative PB reconstitution, 16 weeks after transplantation, of recipient CD45.1 mice transfused with PB from Ext1 control or mutant mice (CD45.2) receiving G-CSF alone or in combination with AMD3100. Recipient mice were lethally irradiated and received CD45.1 cells for radioprotection. Data are representative of at least 2 independent experiments; n = 5 to 8 mice per genotype per experiment. In mobilization experiments, PB was collected from at least 5 mice per experimental group. Data are represented as mean ± SD or median ± interquartile range. *P < .05; **P < .01; ***P < .001.
Mx1+ stromal cells control engraftment of transplanted HSCs and progenitor cells
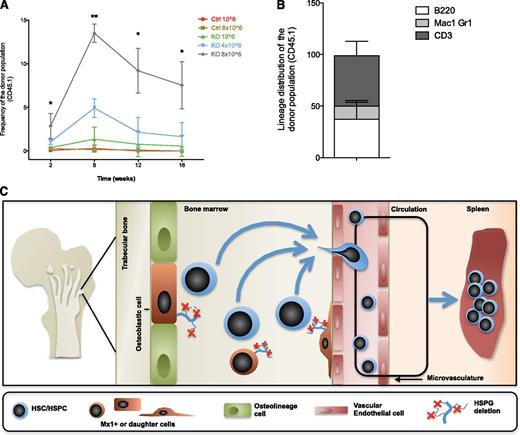
HSPC engraftment in the BM relies on the efficient evacuation of BM niches, which is often achieved by means of total body irradiation and high-dose chemotherapy.41 These conditioning methods are toxic and undesirable for patients who do not suffer from a malignancy requiring irradiation or high-dose chemotherapy as part of the treatment plan. We evaluated whether abrogation of Ext1 in Mx1+ stromal cells may enable engraftment without cytotoxic conditioning. Three weeks after pIpC induction, control and mutant mice were transplanted with congenic CD45.1 BM cells and followed for 16 weeks. Notably, although control animals failed to engraft independent of the cell dose transplanted, mutant mice showed a significant increase in engraftment in a dose-dependent manner throughout the course of the transplant (Figure 7A). Secondary transplantation to further define the enhanced HSC engraftment is the subject of future work. The engrafting cells demonstrated long-term multilineage reconstitution (Figure 7B). Hence, Mx1+ stromal cell production of HSPGs modulates the process of HSPC engraftment in the BM, suggesting that targeting heparan sulfate may provide a means of enabling engraftment without cytotoxic preconditioning.
Mx1+ stromal cells control engraftment of transplanted HSPCs. (A) Total PB reconstitution of nonconditioned control recipient mice (infused with 106 and 8 × 106 CD45.1 congenic BM cells) and mutant recipient mice (infused with 106, 4 × 106, and 8 × 106 CD45.1 congenic BM cells). (B) PB analysis 16 weeks after nonconditioned transplantation of 8 × 106 CD45.1 congenic BM cells into Ext1 mutant recipient mice showing the contribution to B cells (B220), myeloid cells (Mac1 and Gr1), and T cells (CD3). (C) Proposed model for the functional role of Ext1/HSPG in the niche. Data are representative of 2 independent experiments; n = 5 to 8 mice per genotype. Data are represented as mean ± SD. *P < .05; **P < .01.
Mx1+ stromal cells control engraftment of transplanted HSPCs. (A) Total PB reconstitution of nonconditioned control recipient mice (infused with 106 and 8 × 106 CD45.1 congenic BM cells) and mutant recipient mice (infused with 106, 4 × 106, and 8 × 106 CD45.1 congenic BM cells). (B) PB analysis 16 weeks after nonconditioned transplantation of 8 × 106 CD45.1 congenic BM cells into Ext1 mutant recipient mice showing the contribution to B cells (B220), myeloid cells (Mac1 and Gr1), and T cells (CD3). (C) Proposed model for the functional role of Ext1/HSPG in the niche. Data are representative of 2 independent experiments; n = 5 to 8 mice per genotype. Data are represented as mean ± SD. *P < .05; **P < .01.
Discussion
Secreted and membrane-bound HSPGs have been implicated in numerous biological processes in organisms from Drosophila to mammals, creating the microenvironment necessary for heterologous cell interactions to create and maintain tissues.5,42 In hematopoiesis, several prior reports have indicated the importance of HSPG. For example, heparan sulfate mimetics were shown to induce HSPC mobilization,13-15 whereas overexpression of the heparan sulfate-cleaving enzyme heparanase resulted in HSPCs accumulating in the BM.16 Moreover, glypican 3, a cell surface HSPG, inhibits the extracellular dipeptidyl peptidase CD26,17 which affects HSPC homing and egress from the BM.18
Here, we present evidence that skeletal stem/progenitor cell production of heparan sulfate control HSPC BM retention and can be inhibited to mobilize more potent HSPC, even in settings of mobilization resistance, and may be targeted to enable nontoxic conditioning for HSPC engraftment. Through these data, we show that Mx1-expressing stromal cells and/or their descendants make up a population of cells that participate in a niche for HSCs (Figure 7C). We regard these cells as most likely the BM-localized mesenchymal cells we previously defined.20 However, it cannot be excluded that other populations of Mx1-expressing cells such as those in the liver or endothelial cells outside the BM also contribute to the phenotype observed here. We note that this is a consideration for most, if not all, studies using gene modification in stromal elements, as the promoters used for the recombinase are not constrained to a single cell type in vivo. The possible role of nonstromal, non-BM cells may be particularly relevant for the leukocytosis observed here, as adhesion molecules such as VCAM-1 on microvessels could have been affected. We do not consider homing to be the basis for the HSPC changes. Rather, given the imaging studies and homing analyses presented here, we think these are most likely a result of altered retention.
The data presented indicate that manipulation of endogenous HSPGs produced by Mx1-expressing cells can alter localization sufficiently to be of relevance to clinically important issues. First, coadministration of G-CSF and heparin mobilized qualitatively distinct HSPC with more rapid hematopoietic reconstitution and increased secondary transplant ability. These data suggest combined use of these agents may favor the collection of highly potent HSPCs for transplantation. Further definition of the distinct subsets mobilized is the subject of future work, but the gene expression analysis presented may provide testable methods of discriminating the populations differentially mobilized when heparin was involved. Second, heparin or Ext1 deletion enhanced G-CSF-induced HSPC mobilization, even in the setting of G-CSF resistance induced by a diabetic phenotype. This suggests a potential clinical setting in which a combination approach may be both testable and, if validated, of clinical utility. Parenthetically, the pathophysiologic basis for the diabetic mobilization phenotype includes neuropathy.26 Because the deletion of Ext1 prevents the development of the mobilization defect, it is intriguing to consider whether the biochemical activities of the enzyme play a role in the processes compromising neurologic function in diabetes. Our data do not address this experimentally, but Ext1 has been shown to be of significance in neurologic development and may be worth evaluating in this setting of neural dysfunction.43
Finally, Ext1 deletion provided a context in which infused HSPC could engraft and achieve levels of chimerism meaningful for some nonmalignant clinical conditions. It should be noted that the level of chimerism was more than double that seen when AMD3100 was used as a conditioning regimen by others using fivefold more BM cells.44 The ability of infused cells to engraft without cytotoxic therapy is intriguing and may reflect some hematopoietic cell autonomous changes in endogenous cells or that the dynamics of entry to and exit from the niche are altered sufficiently to enable infused cells to compete favorably with endogenous cells. The basis for this is unclear but is likely a result of the retention defects we observed. Because the induction of Ext1 deletion in animals did not compromise the apparent well-being of the animals during the 3-week interval before infusion of the cells, inhibiting Ext1 function may be a strategy to accomplish HSPC engraftment without cytotoxic conditioning. This issue is of increasing importance with the improved success of genetically modifying HSPC for nonmalignant diseases.45,46 Achieving levels of engraftment similar to those observed here with gene-modified cells may be sufficient for a clinical effect, particularly in settings in which gene-modified cells may have a competitive advantage.
The online version of this article contains a data supplement.
There is an Inside Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank J. LaVecchio, G. Buruzula, and S. Ionescu from the Harvard Stem Cell and Regenerative Biology Department flow cytometry core as well as L. Prickett, K. Folz-Donahue, and S. Lahiri from the Harvard Stem Cell Institute/Massachusetts General Hospital Center for Regenerative Medicine flow core. We are grateful to Lexicon Genetics and the Mutant Mouse Regional Resource centers for kindly providing the Ext1 mouse model.
This work was supported by National Institutes of Health, National Heart, Lung, and Blood Institute grants HL044851, HL97794, and HL097748 (to D.T.S.) and HL100402 and HL099997 (to A.J.W. and D.T.S.). B.S. is a recipient of the American Society of Hematology Scholar Award and a former Scholar of the Chamber of Industry and Commerce of Navarra.
Authorship
Contribution: B.S. conceived the study, designed and conducted the experiments, analyzed the data, and wrote the manuscript; F.F. designed and conducted the experiments and analyzed the data. R.Z.Y. designed and conducted the experiments, analyzed the data, and wrote the manuscript; C.M.C., V.W.C.Y., A.P.-S., S.M.S., R.P., A.S., S. Lotinun, S. Lympery, S.M.-F., R.d.T., R.D., R.V., and S.S.A. conducted the experiments; Y.Y., R.B., and C.P.L. contributed vital new reagents and or analytical tools; A.J.W. designed the experiments; and D.T.S. conceived the study, designed the experiments, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current affiliation for S. Lotinun is Department of Physiology, Faculty of Dentistry, Chulalongkorn University, Bangkok, 10330, Thailand.
Correspondence: David T. Scadden, 7 Divinity Ave, Sherman Fairchild Building 258B, Cambridge, MA 02138; e-mail: david_scadden@harvard.edu.
References
Author notes
B.S. and F.F. contributed equally to this study.