Key Points
Mixed, atypical, and warm immunoglobulin G plus C AIHA (∼30% of cases) more frequently have a severe onset (Hb ≤6 g/dL) and require multiple therapy lines.
Infections, particularly after splenectomy, acute renal failure, Evans syndrome, and multitreatment, were predictors of fatal outcome.
Abstract
The clinical outcome, response to treatment, and occurrence of acute complications were retrospectively investigated in 308 primary autoimmune hemolytic anemia (AIHA) cases and correlated with serological characteristics and severity of anemia at onset. Patients had been followed up for a median of 33 months (range 12-372); 60% were warm AIHA, 27% cold hemagglutinin disease, 8% mixed, and 5% atypical (mostly direct antiglobulin test negative). The latter 2 categories more frequently showed a severe onset (hemoglobin [Hb] levels ≤6 g/dL) along with reticulocytopenia. The majority of warm AIHA patients received first-line steroid therapy only, whereas patients with mixed and atypical forms were more frequently treated with 2 or more therapy lines, including splenectomy, immunosuppressants, and rituximab. The cumulative incidence of relapse was increased in more severe cases (hazard ratio 3.08; 95% confidence interval, 1.44-6.57 for Hb ≤6 g/dL; P < .001). Thrombotic events were associated with Hb levels ≤6 g/dL at onset, intravascular hemolysis, and previous splenectomy. Predictors of a fatal outcome were severe infections, particularly in splenectomized cases, acute renal failure, Evans syndrome, and multitreatment (4 or more lines). The identification of severe and potentially fatal AIHA in a largely heterogeneous disease requires particular experienced attention by clinicians.
Introduction
Autoimmune hemolytic anemia (AIHA) is a relatively uncommon disorder caused by autoantibodies directed against self–red blood cells, with an estimated incidence of 1 to 3 per 105/year and a prevalence of 17:100 000. It is usually classified as warm (WAIHA), cold agglutinin disease (CAD), or mixed, according to the thermal range of the autoantibody. Clinically, AIHA may be idiopathic/primary (50% of cases) or secondary to lymphoproliferative syndromes (20%), autoimmune diseases (20%), infections, and tumors.1,2 The disease is greatly heterogeneous, ranging from cases developing gradually or fully compensated to patients with fulminant presentation and rapid onset of life-threatening anemia. The major determinants of clinical heterogeneity are the type of hemolysis (intravascular and/or extravascular), which is related to the autoantibody class, thermal amplitude and efficiency in activating complement, and efficacy of the erythroblastic response.1 Diagnosis of AIHA is usually simple, based on the direct antiglobulin test (DAT), which is typically positive with anti-immunoglobulin G (IgG) antisera in WAIHA and anti-C3d in CAD. However, diagnostic pitfalls are recognized with increasing frequency, due to IgA autoantibodies (not detectable by most polyspecific antiglobulin reagents), warm IgM, low-affinity IgG, or IgG below the threshold of sensitivity. In fact, despite the availability of various DAT techniques (microcolumn, solid phase, enzyme linked, flow cytometry, dual DAT, and mitogen-stimulated [MS] DAT3-5 ), about 10% of AIHA remains DAT negative. In these atypical cases, the diagnosis, made after extensive laboratory workup and exclusion of other causes of hemolysis, is frequently deferred, with consequent delay in therapy.1,2,5-7 Treatment of AIHA is still not evidence based and is mainly accomplished according to expert opinions and individual experience,8-11 with few prospective therapeutic trials12-15 and large but dated comprehensive clinical studies.16 Moreover, the temporal sequence of second-line options is still controversial, and no predictors of outcome are available. Considering the great clinical heterogeneity of the disease, both in terms of clinical presentation and response to treatment, in this multicenter study, we investigated a large number of primary AIHA cases, with the aim to correlate the serological characteristics and the severity of anemia at onset with the clinical outcome, in particular the response to treatments and the occurrence of acute complications.
Materials and methods
Patients
The study population included 308 consecutive patients with primary AIHA, studied from 1978 to 2013 at 8 Italian hematologic centers and followed for a median of 33 months (range 12-372) until December 2013; 157 cases (51%) were followed at a single center by 3 physicians, and 2 other centers provided an additional 100 patients (33%). The study protocol was approved by the Ethical Committee of Human Experimentation, and patients gave informed consent in accordance with the Declaration of Helsinki. Primary AIHA was defined by hemolytic anemia and positive DAT, in the absence of underlying lymphoproliferative, infectious, autoimmune, or neoplastic diseases. None of the patients had a drug-induced AIHA (see supplemental Methods available at the Blood Web site). According to DAT results and to the thermal characteristics of the autoantibody, patients were classified as WAIHA (DAT+ for IgG only or IgG plus C3d), CAD (DAT+ for C3d only, with cold agglutinins of I specificity at titer of 64 or higher), mixed (DAT+ for IgG and C3d, with coexistence of warm autoantibodies and high-titer cold agglutinins), or atypical AIHA (DAT negative, DAT+ for IgA only, warm IgM, MS-DAT positive only). The anemia at onset was defined as very severe (hemoglobin [Hb] ≤6 g/dL), severe (Hb 6.1-8), moderate (Hb 8.1-10), or mild (Hb >10).
Treatment and outcome assessment
The following lines of treatment were considered: (1) steroids (either by mouth or IV), alone or in combination with IV immunoglobulin; (2) immunosuppressant/cytotoxic drugs; (3) rituximab, administered either at the standard dose of 375 mg/m2 per week for 4 weeks or at the fixed low dose (LD) of 100 mg/wk for 4 weeks12 ; and (4) splenectomy. In addition, transfusion requirement, plasma exchange, and erythropoietin administration were also recorded. Response to the different treatments was defined as complete response (CR) (Hb ≥12 g/dL and normalization of all hemolytic markers), partial response (PR) (Hb ≥10 g/dL or at least 2 g/dL increase in Hb, and no transfusion requirement), as reported elsewere.14 Transfusion efficacy was defined as an increase in Hb levels of at least 1 g/dL per blood unit transfused, stable for at least 3 days. Clinical outcomes included death and occurrence of infections (according to Common Terminology Criteria for Adverse Events Version 4.0, http://evs.nci.nih.gov), thrombotic events, and renal failure. Finally, we assessed the time to next treatment because of lack of response or relapse defined as Hb ≤10 or at least 2 g/dL decrease in Hb with or without increase in lactate dehydrogenase (LDH) serum levels.
Statistical analysis
Student t and χ2 test were used to analyze continuous and categorical variables, respectively. To evaluate the risk of relapse, we calculated cumulative incidence, taking death from any cause as a competing risk.17 We then fitted Cox and Fine and Gray models to calculate the hazard ratios (HRs) and subdistribution HRs of relapse and 95% confidence intervals (95% CI), adjusted for gender and age (entered as a continuous variable).14 All the analyses were performed with Stata 13.18
Results
Clinical and serological characteristics of AIHA patients at onset
The clinical and serological characteristics of the AIHA patients studied are shown in Table 1. The majority of cases were WAIHA, followed by CAD and a small fraction of mixed and atypical (1 DAT positive for IgA only, 6 MS-DAT positive only, and 9 DAT negative). Hb values were significantly different among these categories (P = .0001), being lower in mixed (median 5.8, range 2-10.7 g/dL), atypical (median 6.2, range 3-9), and in IgG+C3 WAIHA (median 6.9. range 2.9-11.5), whereas reticulocytes and LDH showed no differences among groups (supplemental Table 1). Splenomegaly was present in 32% of cases, regardless of the serological AIHA type and the severity of anemia at onset. Twenty-one subjects (7%) were diagnosed with Evans syndrome, the majority of them WAIHA (P = .046), with a severe onset (Hb <6 g/dL in 13/21 and Hb 6.1-8 g/dL in 3/21, P = .002).
Clinical characteristics of primary AIHA patients
| . | N* . |
|---|---|
| Patients, N | 308 |
| Male/female | 111 (36)/197 (64) |
| Median age at onset, y (range) | 58 (0-95) |
| <18 y | 10 (3) |
| 18-45 y | 73 (24) |
| 45-65 y | 104 (34) |
| >65 y | 121 (39) |
| AIHA serological type** | |
| Warm, DAT positive for IgG | 131 (43) |
| Warm, DAT positive for IgG+C | 52 (17) |
| CAD | 84 (27) |
| Mixed | 24 (8) |
| Atypical | 16 (5) |
| Median follow-up, mo (range) | 33 (6-372) |
| Alive/dead at time of study† | 221 (72)/63 (21) |
| Died of AIHA | 11/63 (17) |
| . | N* . |
|---|---|
| Patients, N | 308 |
| Male/female | 111 (36)/197 (64) |
| Median age at onset, y (range) | 58 (0-95) |
| <18 y | 10 (3) |
| 18-45 y | 73 (24) |
| 45-65 y | 104 (34) |
| >65 y | 121 (39) |
| AIHA serological type** | |
| Warm, DAT positive for IgG | 131 (43) |
| Warm, DAT positive for IgG+C | 52 (17) |
| CAD | 84 (27) |
| Mixed | 24 (8) |
| Atypical | 16 (5) |
| Median follow-up, mo (range) | 33 (6-372) |
| Alive/dead at time of study† | 221 (72)/63 (21) |
| Died of AIHA | 11/63 (17) |
Values are n (%) unless otherwise indicated.
One patient of 308 studied was diagnosed with paroxysmal cold hemoglobinuria and not included in the subsequent analysis.
A total of 23 cases (7%) cases were lost at follow-up.
Relationship among clinical severity at onset, AIHA serological type, and number of therapy lines
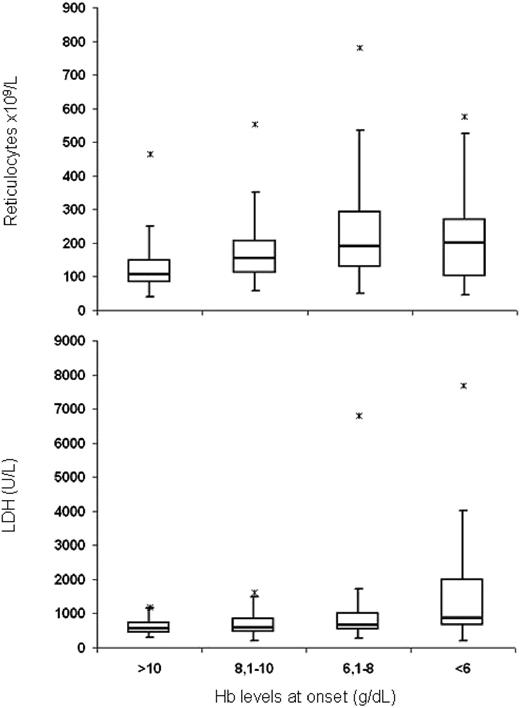
Considering the degree of anemia at onset, 27% of cases had Hb levels ≤6, 36% had Hb levels 6.1-8, 24% had Hb levels 8.1-10, and 13% had Hb levels >10 g/dL; the most severe cases were mainly mixed and atypical forms, whereas only a small fraction of CAD was characterized by a severe onset (P = .0001) (Table 2). Moreover, a severe onset was associated with younger age (P = .0039). As shown in Figure 1, reticulocytes were differently distributed according to clinical severity (P = .0001); in particular, the mean reticulocytes progressively increased with the worsening of anemia, except for cases with Hb <6 g/dL, which showed an erythropoietic response clearly inadequate to compensate anemia and therefore possibly contributing to the clinical severity. Reticulocytes <100 × 109/L (18% of cases) were not associated with AIHA serological type. As expected, LDH levels increased from mild to more severe forms (P = .0001).
Relationship between AIHA serological type and clinical severity at onset
| AIHA serological type . | Hb at onset (g/dL) . | |||
|---|---|---|---|---|
| <6 . | 6.1-8 . | 8.1-10 . | >10 . | |
| Warm (n = 183) | ||||
| IgG (n = 131) | 38 (29%) | 46 (35%) | 33 (25%) | 14 (11%) |
| IgG+C (n = 52) | 16 (31%) | 23 (44%) | 10 (19%) | 3 (16%) |
| Cold (n = 84) | 8 (9.5%) | 29 (34.5%) | 27 (32%) | 20 (24%) |
| Mixed (n = 24) | 15 (63%) | 6 (25%) | 2 (8%) | 1 (4%) |
| Atypical (n = 16) | 8 (50%) | 5 (31%) | 3 (19%) | 0 |
| AIHA serological type . | Hb at onset (g/dL) . | |||
|---|---|---|---|---|
| <6 . | 6.1-8 . | 8.1-10 . | >10 . | |
| Warm (n = 183) | ||||
| IgG (n = 131) | 38 (29%) | 46 (35%) | 33 (25%) | 14 (11%) |
| IgG+C (n = 52) | 16 (31%) | 23 (44%) | 10 (19%) | 3 (16%) |
| Cold (n = 84) | 8 (9.5%) | 29 (34.5%) | 27 (32%) | 20 (24%) |
| Mixed (n = 24) | 15 (63%) | 6 (25%) | 2 (8%) | 1 (4%) |
| Atypical (n = 16) | 8 (50%) | 5 (31%) | 3 (19%) | 0 |
Box-and-whisker plot displaying reticulocyte and LDH values in AIHA patients divided according to the clinical severity at onset. Median values are indicated; *maximum outlier values.
Box-and-whisker plot displaying reticulocyte and LDH values in AIHA patients divided according to the clinical severity at onset. Median values are indicated; *maximum outlier values.
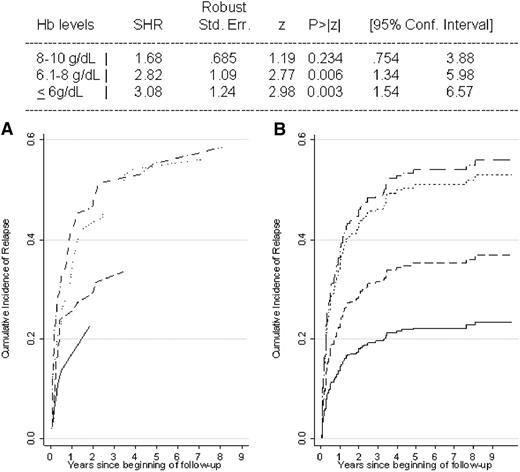
Considering therapy, 145 patients (47%) were treated with 1 line of therapy only, 80 (26%) with 2 lines, 40 (13%) with 3 lines, and 12 (4%) with ≥4 lines (supplemental Table 2). The number of therapy lines was significantly associated with AIHA serological type and with the severity of anemia at onset (P = .0001 for both). In particular, the great majority of patients who had never been treated were CAD, the majority of WAIHA (60%) received first-line steroid therapy only, and patients with IgG+C WAIHA, mixed, and atypical forms were more frequently treated with ≥2 therapy lines (P < .0001). As shown in Figure 2, the crude and gender- and age-adjusted cumulative incidences of relapse were significantly increased in more severe cases, and the adjusted HRs of relapse were increased in AIHA categories with lower Hb values at onset (test for linear trend, P < .001). Even considering Hb as a continuous variable, each gram of reduction yielded a 14% increased risk of relapse (95% CI, 7% to 20%; P < .001). Moreover, an increased risk of relapse was associated with younger age at diagnosis (11% every 10 years; 95% CI, 4% to 19%; P = .004), whereas gender and serological type were irrelevant. Cox models gave results similar to those obtained with Fine and Gray analyses (data not shown).
Cumulative incidence and adjusted HRs of relapse in AIHA patients according to the clinical severity at onset. Crude data (A) and data adjusted for gender and age with the Fine and Gray model (B). Patients were divided according to Hb levels at onset: >10 g/dL (solid line), 8.1-10 g/dL (dashed line), 6.1-8 g/dL (dotted line), and ≤6 g/dL (dashed-dotted line). SHR, subdistribution HR.
Cumulative incidence and adjusted HRs of relapse in AIHA patients according to the clinical severity at onset. Crude data (A) and data adjusted for gender and age with the Fine and Gray model (B). Patients were divided according to Hb levels at onset: >10 g/dL (solid line), 8.1-10 g/dL (dashed line), 6.1-8 g/dL (dotted line), and ≤6 g/dL (dashed-dotted line). SHR, subdistribution HR.
Response to the different therapies
First line.
Steroid therapy was mostly administered in WAIHA, mixed, and atypical cases. As shown in Table 3, an overall response (OR) was observed in ∼75% of cases and CR mostly in mild cases (P = .002) and in WAIHA; 50% of them required no further treatment. At variance, CR was lower in CAD and generally observed at high steroid dosages. The median time of steroid administration was 8.5 months (1-284) with a time to PR and CR of 15 days (6-166) and 40 days (8-187), respectively. In the majority of patients (70%), steroids were administered by mouth only; in 30% of patients (mostly severe cases; 57% with Hb levels ≤6 g/dL and 24% with Hb 6.1-8 g/dL), oral steroids were preceded by IV administration. In 56 cases (almost all severe forms), IV immunoglobulin was administered along with steroids; of note, 33% of patients were unresponsive to this treatment.
Response rates to different therapies according to AIHA serological type
| Therapy (no. of patients) . | Warm IgG (n = 123) . | Warm IgG+C (n = 51) . | Cold (n = 64) . | Mixed (n = 24) . | Atypical (n = 15) . |
|---|---|---|---|---|---|
| Steroids (277) | CR 50%, PR 32% (n = 123) | CR 49%, PR 27% (n = 51) | CR 27%, PR 42% (n = 64) | CR 37.5%, PR 25% (n = 24) | CR 40%, PR 40% (n = 15) |
| Immunosuppressants (76) | CR 57%, PR 30% (n = 30) | CR 27%, PR 53% (n = 15) | CR 28%, PR 39% (n = 18) | CR 11%, PR 56% (n = 9) | CR 25%, PR 75% (n = 4) |
| Rituximab standard dose (55) | CR 45%, PR 19% (n = 11) | CR 43%, PR 50% (n = 14) | CR 45%, PR 35% (n = 20) | CR 50%, PR 33% (n = 6) | CR 0%, PR 100% (n = 1) |
| Rituximab LD (19) | CR 80%, PR 0% (n = 5) | CR 100%, PR 0% (n = 2) | CR 37.5%, PR 37.5% (n = 8) | CR 100%, PR 0% (n = 2) | CR 100%, PR 0% (n = 2) |
| Splenectomy (32) | CR 75%, PR 0% (n = 16) | CR 70%, PR 10% (n = 10) | CR 33%, PR 0% (n = 3) | CR 100%, PR 0% (n = 1) | CR 100%, PR 0% (n = 2) |
| Therapy (no. of patients) . | Warm IgG (n = 123) . | Warm IgG+C (n = 51) . | Cold (n = 64) . | Mixed (n = 24) . | Atypical (n = 15) . |
|---|---|---|---|---|---|
| Steroids (277) | CR 50%, PR 32% (n = 123) | CR 49%, PR 27% (n = 51) | CR 27%, PR 42% (n = 64) | CR 37.5%, PR 25% (n = 24) | CR 40%, PR 40% (n = 15) |
| Immunosuppressants (76) | CR 57%, PR 30% (n = 30) | CR 27%, PR 53% (n = 15) | CR 28%, PR 39% (n = 18) | CR 11%, PR 56% (n = 9) | CR 25%, PR 75% (n = 4) |
| Rituximab standard dose (55) | CR 45%, PR 19% (n = 11) | CR 43%, PR 50% (n = 14) | CR 45%, PR 35% (n = 20) | CR 50%, PR 33% (n = 6) | CR 0%, PR 100% (n = 1) |
| Rituximab LD (19) | CR 80%, PR 0% (n = 5) | CR 100%, PR 0% (n = 2) | CR 37.5%, PR 37.5% (n = 8) | CR 100%, PR 0% (n = 2) | CR 100%, PR 0% (n = 2) |
| Splenectomy (32) | CR 75%, PR 0% (n = 16) | CR 70%, PR 10% (n = 10) | CR 33%, PR 0% (n = 3) | CR 100%, PR 0% (n = 1) | CR 100%, PR 0% (n = 2) |
CR, Hb ≥12 g/dL; PR, Hb ≥10 g/dL. Rituximab at standard dose, 375 mg/m2 per week for 4 weeks; LD, fixed dose of 100 mg/wk for 4 weeks.
Second line.
Splenectomy was performed in 32 cases (10%; mostly WAIHA or cases with Hb levels ≤8 g/dL; P = .04) after a median of 25 months from diagnosis (range 11-190). The OR was 75%, irrespective of Hb levels at onset, but splenectomy was ineffective in 2 out of 3 CAD patients. Among the 24 responsive cases, 8 (33%) relapsed after a median of 41 months (range 20-331).
Medical second-line therapies were generally administered in association with steroids, during tapering and as steroid-sparing agents, making the evaluation of response less clear-cut. Considering immunosuppressants, azathioprine was administered in 31, cyclophosphamide in 40, and cyclosporine in 12; 7 patients received >1 drug. The OR to azathioprine was 71% (29% CR and 42% PR), and the best responses were observed in WAIHA (57% CR); cyclophosphamide induced an OR in 72% of cases (38% CR and 38% PR), irrespective of serological type and severity of anemia; cyclosporine was administered in 12 patients (almost all WAIHA), inducing a CR and PR in 42% and 17% of cases, respectively. Three patients (2 mixed and 1 CAD) were treated with mycophenolate mofetil, with a CR in 2. Of note, among the 60 cases responsive to immunosuppressants, 8 (13%) relapsed after a median of 11 months (range 4-36).
Rituximab was administered in 55 cases at conventional doses and in 19 at LDs, the former mostly in cold and mixed forms and in cases with Hb levels ≤8 g/dL (P = .04). Conventional doses induced an OR in 80% of cases (44% CR and 36% PR), which compared favorably with lower doses (84% OR, 68% CR and 16% PR), except for CAD, which showed greater PR. Predictors of response to LD rituximab were WAIHA (P = .02), younger age (P = .02), and shorter interval between diagnosis and rituximab therapy (P = .06). At variance, OR to conventional doses occurred irrespectively of age, serological type, clinical severity at onset, and disease duration. Relapses were recorded in 2 out of 42 (1 CAD) responders to standard doses, compared with 6 out of 16 (5 CAD) responsive to lower doses (P = .005). Relapses occurred mostly within the first year after treatment. The drug was administered as second-line therapy in 61% of patients, as third-line therapy after splenectomy in 4%, and after immunosuppressants in 45%, with fairly better responses in second compared with further lines (88% vs 79%).
Last-option treatments.
“Last-option treatments”9 were given in 4 cases: 2 patients (both WAIHA with severe onset) were treated with high-dose cyclophosphamide, vincristine, rituximab, and prednisone; 1 recovered and 1 underwent splenectomy followed by a successful autologous peripheral stem cell transplant. One patient with CAD was ineffectively treated with eculizumab as a fifth line of therapy (after steroids, 2 immunosuppressors, and rituximab). One case (IgG+C WAIHA with Hb 3.9 g/dL at onset) was treated with bortezomib (1.9 mg × 3 every 3 days) and eculizumab (1200 mg weekly for 4 weeks) as fifth and sixth lines of therapy, after steroids plus IV immunoglobulin, cyclophosphamide, rituximab, and plasma exchange; the patient recovered, although the contribution of the single drugs is difficult to establish. Plasma exchange was performed in 5 cases (4 with Hb at onset ≤6 g/dL); it was ineffective in 1 CAD and 1 atypical and effective in 2 IgG+C WAIHA and 1 mixed, even if associated with other therapies.
Supportive treatments.
Transfusions were given to 115 patients irrespective of serological type. One-third of patients (38/115) were unresponsive, mainly those with a severe onset (43% Hb ≤6 g/dL and 25% Hb 6.1-8 g/dL, P = .004). Unresponsiveness to transfusions was not reasonably related to the presence of alloantibodies (supplemental Results). Finally, erythropoietin was administered in 14 cases, mostly severe forms (Hb at onset ≤6 g/dL in 5, and 6.1-8 g/dL in 7 cases) and effective in 13, although associated with other treatments.
Thrombotic, infectious, and acute complications.
A thrombotic event was recorded in 33 patients (11%); in detail, 11 cases of pulmonary embolisms, 13 deep venous thrombosis, 5 splanchnic thrombosis, 1 disseminated intravascular coagulation, 3 strokes, 2 transient ischemic attacks, and 3 cardiac ischemic events (5 patients experienced >1 event); in 1 out of 3 cardiac ischemic events, the role of concomitant anemia was reported as relevant. These events were associated with a severe onset (79% in cases with Hb ≤8 g/dL, P = .024) and with higher median LDH levels (837 U/L, range 346-9149 vs 750 U/L, range 201-12028, P = .006). Moreover, a thrombotic event was more frequent in patients who had undergone splenectomy (8/33, 24% vs 24/274, 8.75%, P = .014). The positivity for anti-cardiolipin antibodies or lupus anticoagulant (13% of cases) was not associated with a thrombotic event.
Infectious complications (grade ≥3) occurred in 26 cases (mostly pneumonia), of whom 10 had grade 3, 11 had grade 4 (8 with associated severe respiratory insufficiency, and 3 with septic shock), and 5 had grade 5 (all have died for severe respiratory insufficiency and septic shock). The causative agent was identified in 11 of 26 cases (4 Pneumocystis jirovecii, 2 Mycoplasma pneumoniae, 1 Staphylococcus epidermidis, 1 Pseudomonas aeruginosa, 1 Varicella Zoster Virus, 1 Candida albicans, and 1 Plasmodium vivax reactivation), without differences between splenectomized and nonsplenectomized patients. Severe infectious events were not associated with serological AIHA type and severity at onset or with the number of therapy lines (particularly immunosuppressants and rituximab), although the small number of cases may weaken the statistical analysis. On the contrary, infectious complications grade ≥3 were observed more frequently in splenectomized cases (27% vs 9%, P = .01). Acute renal failure was recorded in 6 cases and was not associated with AIHA clinical or serological characteristics.
At the time of the analysis, 149 patients (48%) were still in follow-up; for the 72 cases who had recovered and were no longer regularly followed, clinical charts were available and their health condition was assessed by a telephone call. Sixty-three patients (21%) have died, 11 because of AIHA (3.6%). These fatal outcomes, directly followed by the physicians involved in the study, were also due to concomitant infection in 5, myocardial infarction in 1, pulmonary embolism in 1, and multiorgan failure in 4. The analysis showed that death was not associated with the severity of anemia at onset or with the serological type of AIHA (7 WAIHA, 2 CAD, and 2 mixed); at variance, it was associated with infections (HR 11.47; 95% CI, 3.43-38.4; P = .0004), acute renal failure (HR 17.99; 95% CI, 4.73-68.40; P = .001), Evans syndrome (HR 6.8; 95% CI, 1.99-23.63; P = .0074), previous splenectomy (HR 3.21; 95% CI, 0.92-11.25), and multitreatment (≥4 lines of therapy) (HR 9.1; 95% CI, 2.41-34.36; P = .0076). Death was not associated with thrombotic events or with the type of treatment, in particular immunosuppressants or rituximab.
Discussion
AIHA is usually thought to be a benign disease, often disregarded or poorly considered by oncohematologists. However, a small number of patients become a medical challenge because of diagnostic pitfalls, refractoriness to therapy, or occurrence of life-threatening acute complications, above all in the absence of evidence-based guidelines. In this study, we retrospectively evaluated a large number of primary AIHA patients to identify predictors of outcome related to the clinical and serological characteristics of AIHA. We confirm a great heterogeneity in clinical presentation, with about one-third of cases with a very severe onset, a fraction probably overestimated because some of the involved institutions are reference centers for AIHA. Reticulocytopenia, found in about 20% of patients as already reported19-21 and possibly related to immune-mediated destruction or apoptosis of red cell precursors,22 is mostly observed in severe cases and may represent a clinical emergency.23
The serological characteristics of our population were similar to those already reported,1,2 ie, mostly warm cases, followed by CAD and a small fraction of mixed forms; the higher relative frequency of CAD than found in other studies18 may be due to the different criteria for the exclusion of underlying lymphoproliferative disorders. Of note, a fraction of WAIHA displayed a DAT positive for IgG+C, suggesting the binding and activation of complement by some IgG autoantibodies and/or the existence of small or undetectable amounts of IgM. Hb values were lower in IgG+C and in mixed forms, consistent with the known 10-fold greater degree of red blood cell destruction by IgM-induced intravascular hemolysis compared with IgG-mediated extravascular hemolysis.1,2 We describe about 5% of DAT-negative cases, a percentage rather lower than previously reported (∼10% to 20%1,2 ), possibly due to the use of more sensitive tests, such as MS-DAT, which allowed the diagnosis in some DAT-negative cases.3,4 These cases showed a more severe pattern and may represent a diagnostic puzzle, causing troublesome delays in the start of therapy. This notion is clinically important, because an earlier onset of therapy has been related with lower probability of relapse.1,2 Of note, we observed a case of paroxysmal cold hemoglobinuria, an ultrarare disease in adults but still reported in children and usually postinfective.16,20
Concerning treatment, first-line therapy with steroids was administered in almost all cases, with the exception of mild asymptomatic CAD, which generally requires only protection against cold exposure and occasional transfusions during the winter.1,2,10,24 Responses were better in warm AIHA and in milder cases, being able to ensure recovery in about half of patients for the period of observation. It is not known how many adult patients are cured by steroids alone, but it is estimated that a complete and persistent remission occurs in <20% of patients.1,2,7 The rather better responses observed in our series, besides the duration of the follow-up, may be related to a longer steroid administration (∼8 months). In fact, although a rapid steroid discontinuation is tempting, patients receiving LDs for >6 months have a lower incidence of relapse than those discontinuing the medication earlier.25 In contrast to what recently recommended,10 steroids were frequently given in CAD but obtained lower response rates (mainly PRs) at unacceptably high doses.1,2,10,20,26,27
Splenectomy was performed in a small proportion of cases (10%), probably reflecting the general withdrawal of this option due to the availability of other treatments, with a 75% OR in warm and mixed forms, but it was poorly effective in CAD,10,27 which is mainly characterized by extravascular hepatic hemolysis.1,28,29 The rate of splenectomy in adults is not known8 ; in a large pediatric series of 256 AIHA cases (99 with Evans syndrome), splenectomy had been performed in 13.9%.20 In our series, two-thirds of cases required no further treatment, a proportion greater than the presumed cure rate reported of about 20%,8,30,31 although the small number of cases and the duration of follow up do not allow definite conclusions.
Rituximab was administered in about one-fourth of patients, mostly cold and mixed forms and in severe cases, with an 80% OR (half of them complete), in line with the literature.32,33 The drug was preferentially given at conventional doses, although the OR to LD compared favorably with standard doses, mainly in WAIHA.14,34 At variance, cold forms showed lower response and increased relapse rates after LD rituximab, suggesting that standard doses are the best treatment of CAD, in line with the more recent reports11,12,35 and with a possible activity against a small undetectable pathogenic B-cell clone.10,11,27,35 The drug is now recommended as first-line treatment of CAD, alone or in combination with fludarabine10 ; however, the good performance and safety observed in warm and mixed forms suggest that rituximab should be put forward, even before splenectomy.
Immunosuppressants were administered in one-fourth of our cases with “good” responses, possibly attributable in part to the simultaneous administration of steroids, but was sustained in a considerable proportion of subjects. Of note, few cases were successfully treated with mycophenolate, which has raised increasing interest because of proven effectiveness in ∼90% of refractory immune cytopenias in children36 and in post–hematopoietic stem cell transplant AIHA together with rituximab.31,37 Thus, notwithstanding the availability of other new and/or more effective treatments, such as rituximab and splenectomy, our findings indicate that immunosuppressants are still used to a certain extent in the clinical practice and may still have a place in the therapeutic arsenal of AIHA, mostly as steroid-sparing agents.
Plasma exchange was performed in a small number of patients in whom the anemia could not be stabilized with steroids and transfusions alone, as a temporizing measure.1,2 Its reported efficacy is controversial, with generally short-lived favorable effects, and it represents an “heroic or last-ditch effort on behalf of a patient”.38,39 Regarding last-option treatments, the uncertain response to bortezomib and eculizumab in our patients and in the literature40,41 awaits confirmation from ongoing trials. Moreover, 2 case was successfully treated with autologous peripheral stem cell transplant, which is reported effective in 20% to 40% of cases in small series, mostly Evans syndromes.1,2,42,43 Finally, we confirmed the utility of erythropoietin, particularly in the presence of reticulocytopenia,44 suggesting that this drug may overcome the inadequate bone marrow compensation, as now established for thrombopoietin agonists in primary immune thrombocytopenia.45
Altogether, our results showed that one-fourth of primary AIHA patients require at least 2 lines of therapy, about 13% require 3, and a small fraction (4%) require ≥4 lines; the most severe cases and the mixed and atypical forms had a 3-fold increased risk of relapse or unresponsiveness to therapy. Younger age was also associated with a severe clinical presentation and an increased risk of relapse and refractoriness, probably due to a more aggressive immune reactivity.
The occurrence of thrombotic events has never been assessed in large series, nor has it been correlated with AIHA serological type, clinical severity, and therapy. The association of thrombosis and anti-phospholipid antibodies has been reported in small series, including patients with systemic lupus erythematosus, without a definite causative link.46 Thrombotic complications were mostly related to a severe onset and intravascular hemolysis, regardless of the presence of anti-cardiolipin antibodies or lupus anticoagulant. Prospective studies are required to clarify the role of this prothrombotic factor, among the several reported in the literature, and the utility of anticoagulant prophylaxis. At variance, infectious episodes occurred irrespective of the serological AIHA type, severity at onset, and type of medical therapy lines, including immunosuppressants and rituximab. Both thrombotic and infectious complications were mainly observed in splenectomized patients, as reported previously,47-49 but only the latter were related to a fatal outcome, confirming that the most-feared complication after splenectomy is overwhelming sepsis,49,50 although vaccination against pneumococci, meningococci, and hemophilus was performed in all cases.
In conclusion, the novelty of our study is the effort to identify risk factors for infectious and thrombotic complications and to elucidate predictors of outcome, including refractoriness to therapy and death. This was carried out 308 cases of primary AIHA, which at present is one of the largest studies in the literature, given the rarity of this disease. We showed that cases with a severe onset (mostly mixed and atypical forms) are frequently refractory to different therapies. Although the conclusions of a retrospective/observational study about the efficacy of various treatments are less straightforward than those obtained prospectively, we suggest to put forward rituximab among the second-line options, given its efficacy and safety. In addition, standard rituximab doses should be preferred in CAD, whereas lower doses may be equally effective in WAIHA and mixed forms. Finally, we suggest to defer splenectomy after rituximab, given the increased risk of thromboembolism, infections, and fatal outcome in splenectomized patients. Notwithstanding the availability of some predictors of outcome and the improvement of diagnostic tools and clinical management of AIHA, this disease remains largely heterogeneous, with sometimes a rapid fatal outcome, thus requiring particular experienced attention by clinicians.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by research funding from Fondazione Istituto di Ricovero e Cura a Carattere Scientifico Ca’ Granda Ospedale Maggiore Policlinico (RC 2014) and by Ministry of Health (RF 2010) Convention N. 141/RF-2010-2303934.
Authorship
Contribution: W.B. designed research, collected, analyzed, and interpreted data and wrote the manuscript; B.F. collected, analyzed, and interpreted data and wrote the manuscript; A.Z. collected data for statistical analysis and partially wrote the manuscript; T.R., I.N., E.D.B., M.L., C.T., F.A., A.F., A.P.L., P.N., C.B., M.C., L.S., P.D.F., and G.T. followed patients, collected data, and critically revised the manuscript; N.R. and M.A.V. performed serological investigation; D.C. performed statistical analysis and contributed to the interpretation of data; G.G., F.R., and A.C. critically revised the manuscript; and A.Z. contributed to study design, interpreted data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Wilma Barcellini, UOC Oncoematologia, UOS Fisiopatologia delle Anemie - Padiglione Granelli, Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico, Via F. Sforza 35, 20122 Milano, Italy; e-mail wbarcel@policlinico.mi.it.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal