Abstract
Acute GVHD (aGVHD) remains a major source of morbidity after allogeneic hematopoietic cell transplantation. CD30 is a cell-surface protein expressed on certain activated T cells. We analyzed CD30 expression on peripheral blood T-cell subsets and soluble CD30 levels in 26 patients at the time of presentation of aGVHD, before the initiation of treatment, compared with 27 patients after hematopoietic cell transplantation without aGVHD (NONE). Analysis by flow cytometry showed that patients with aGVHD had a greater percentage of CD30 expressing CD8+ T cells with the difference especially pronounced in the central memory subset (CD8+CD45RO+CD62L+): GVHD median 12.4% (range, 0.8%-33.4%) versus NONE 2.1% (0.7%, 17.5%), P < .001. There were similar levels of CD30 expression in naive T cells, CD4+ T cells, and regulatory (CD4+CD127lowCD25+) T cells. Plasma levels of soluble CD30 were significantly greater in patients with GVHD: median 61.7 ng/mL (range, 9.8-357.1 ng/mL) versus 17.4 (range, 3.7-142.4 ng/mL) in NONE (P < .001). Immunohistochemical analysis of affected intestinal tissue showed many CD30+ infiltrating lymphocytes present. These results suggest that CD30 expression on CD8+ T-cell subsets or plasma levels of soluble CD30 may be a potential biomarker for aGVHD. CD30 may also represent a target for novel therapeutic approaches for aGVHD.
Introduction
Acute GVHD (aGVHD) remains a major source of morbidity and mortality after allogeneic hematopoietic cell transplantation (HCT). Initial treatment remains the administration of systemic corticosteroids, which can achieve response rates of approximately 50% as reported in large registry studies,1,2 with < 40% of patients maintaining a durable remission.3,4 There is no standard second-line therapy for patients who do not respond to the initial treatment with steroids although many agents are used, including antithymocyte globulin (ATG),5 sirolimus,6 etanercept,7 denileukin diftitox,8 and mycophenolate mofetil.9 Novel therapeutic approaches are needed, and the ideal therapy would be able to specifically suppress aGVHD without increasing a patient's susceptibility to opportunistic infections as well as maintaining an intact graft-versus-malignancy effect.
CD30 is a cell-surface molecule in the TNF receptor family and is displayed on a subset of activated T cells.10 Preliminary evidence has suggested that CD30 is up-regulated on T cells when exposed to allogeneic antigens and that these CD30+ T cells produce IL-5 and IFN-γ and exhibit enhanced B-cell helper activity.11,12 CD30 is an attractive target for therapy, given the demonstrated safety and efficacy of brentuximab vedotin (Adcetris; Seattle Genetics),13 an antibody-drug conjugate composed of an anti-CD30 monoclonal antibody covalently linked to the microtubulin toxin monomethylauristatin E. Brentuximab vedotin recently has gained approval from the Food and Drug Administration for use in the treatment of patients with relapsed/refractory Hodgkin lymphoma and anaplastic large cell lymphoma.14,15
In this study, we compared the expression of CD30 on specific peripheral blood T-cell subsets and levels of soluble CD30 (sCD30) from patients with aGVHD with patients without aGVHD after allogeneic HCT and also analyzed the expression of CD30 in the biopsies of affected tissues from patients with intestinal aGVHD. We believe these results suggest that CD30 may be a biomarker for diagnosis but, more importantly, a novel target for the therapy of aGVHD.
Methods
Samples and patients
Blood samples from patients after allogeneic HCT at Massachusetts General Hospital and Dana-Farber Cancer Institute had been previously collected and frozen under protocols approved by the Institutional Review Board at Dana-Farber Harvard Cancer Center. Blood samples from patients with aGVHD used in this analysis were collected at the time of presentation of symptoms of aGVHD and before the initiation of systemic treatment with corticosteroids (n = 26). The diagnosis of aGVHD was determined by the clinical and pathologic evaluation of the patient by his or her respective treating physicians, who did not have access to any of the data presented here. aGVHD was graded according to previously published standard criteria.16 Patients in the aGVHD group had either skin GVHD or gut GVHD because samples used in this analysis originally were collected in a protocol in which we compared differences in T-cell surface molecules between skin and intestinal disease. Patients with skin GVHD did not have any other organ involvement, whereas patients with gut disease were allowed to have skin or hepatic involvement, although their disease was predominantly in the gut. No patients with isolated hepatic GVHD were enrolled.
The control group for comparison was composed of samples collected from patients who had symptoms suggestive of aGVHD but in whom subsequent evaluation confirmed other etiologies (n = 8) and from patients after allogeneic HCT who had no evidence of aGVHD but had samples collected between days 50 and 100 after HCT (n = 19). All grafts were from peripheral blood stem cells and were obtained from either HLA-matched related (MRD) or HLA-matched unrelated donors (MUD). All patients received calcineurin inhibitor (either cyclosporine or tacrolimus dosed to target serum levels) based on aGVHD prophylaxis. All samples of blood were collected in EDTA tubes from patients at the time of presentation of aGVHD symptoms. Mononuclear cells were isolated by density gradient centrifugation and were washed before flow cytometry analysis.
Flow cytometry
Flow cytometry was analyzed by individuals blinded to the clinical characteristics and course of all patients, and the results of CD30 expression were not available to treating physicians. Cells were stained with the use of an antibody panel consisting of conjugated antibodies against CD3, CD4, CD8, CD45RO, CD62L, CD25, CD127, and CD30. These reagents were purchased from Beckman Coulter. The cells were analyzed with a BD FACSCanto II (BD Biosciences). T-cell subsets were defined as follows: CD45+CD3+CD4+CD45RO− (naive CD4+ T cells), CD45+CD3+CD4+ CD45RO+CD62L− (effector memory CD4+ T cells), CD45+CD3+CD4+ CD45RO+CD62L+ (central memory CD4+ T cells), CD45+CD3+CD8+ CD45RO− (naive CD8+ T cells), CD45+CD3+CD8+CD45RO+CD62L− (effector memory CD8+ T cells), and CD45+CD3+CD8+CD45RO+ CD62L+ (central memory CD8+ T cells). Flow cytometry results were analyzed with BD FACSDiva software (BD Biosciences). CD30 positivity within the PE channel was determined with a PE mouse IgG1-κ isotype control (BD Biosciences).
sCD30 levels
Plasma from patient samples had been collected and stored with each sample of PBMCs. Corresponding plasma samples were located and thawed. Analysis for sCD30 was performed on each plasma sample with the Human sCD30 Instant ELISA kit (eBioscience).
Immunohistochemistry
Many patients included in this study had undergone diagnostic tissue biopsies at the time of aGVHD evaluation. Samples of intestinal biopsies were obtained from the pathology archives of MGH and were subjected to immunohistochemical analysis for CD30 expression. In summary, paraffin-embedded tissue was sectioned, deparaffinized, and stained with CD30 antibody (Clone JCM182; Leica) on a Leica Bond-III immunostainer. CD30+ cells were enumerated by a pathologist who was blinded to the clinical characteristics and course of the patients.
Statistical analysis
Descriptive statistics, including median and ranges of continuous variables, as well as percents and frequencies for categorical parameters, are presented. For group comparisons, we used the Wilcoxon rank-sum test and χ2 test to assess the group difference for continuous and categorical data, respectively. Logistic regression was used to assess whether the expression of CD30 was associated with the development of aGVHD when we adjusted for donor type and the intensity of the conditioning regimen. Spearman correlation was used to analyze potential correlations between time of sample collection and CD30 expression or sCD30 levels as well as any relationship between CD30 expression and sCD30 levels. Data were analyzed with the use of R 2.14.1 statistical software packages.
Results
Patient and sample characteristics
Clinical characteristics of the 53 patients whose samples were analyzed are shown in Table 1; no significant differences were found between the 2 groups. A total of 22 patients were treated with a myeloablative conditioning regimen, whereas 31 patients underwent reduced-intensity HCT. Both groups were similar with regard to underlying hematologic conditions. A total of 30 patients received stem cells from MRD, whereas 23 patients underwent transplantation from a MUD. All patients underwent transplantation via the use of peripheral blood stem cells. All patients received calcineurin inhibitor (either cyclosporine or tacrolimus) based on GVHD prophylaxis, with most receiving additional methotrexate, sirolimus, or a combination. A total of 13 patients received ATG, and 2 patients received alemtuzumab during conditioning.
The onset of aGVHD for patients in the GVHD group varied between day 23 and day 183 after transplantation (or donor leukocyte infusion, DLI) with a median time to development of aGVHD of 47 days after HCT or DLI. Two patients had blood and tissue samples collected when developing aGVHD in the context of receiving DLI. In terms of the 26 patients who were diagnosed with clinical aGVHD, 12 patients had gut-predominant disease, and 14 patients had primarily cutaneous disease. Of the gut-GVHD patients, 3 had grade 4 disease, 8 had grade 2, and 1 had grade 1, with all patients graded on the basis of lower intestinal symptoms. In the patients with GVHD of the skin, 6 had grade 2 disease, and 8 had grade 3 disease. In terms of hepatic disease, no patient with skin GVHD had any liver involvement, and 2 patients with intestinal disease had grade 3 and 4 hepatic disease, respectively. The incidence of CMV reactivation and EBV viremia was not significantly different between the 2 groups around the time samples were collected (data not shown).
Of the patients who did not have GVHD, 8 patients presented with symptoms suspicious for GVHD but the evaluation proved otherwise, and 19 patients were randomly chosen patients whose samples had been collected between days 50 and 100 after HCT. In the 8 patients who presented with symptoms, 2 presented with a skin rash, and both were diagnosed as having drug reactions. The other 6 patients presented with diarrhea and were diagnosed with 2 cases of viral gastroenteritis and one case each of gastrointestinal bleeding from gastritis, antibiotic-associated diarrhea, Clostridium difficile colitis, and narcotic withdrawal.
Surface expression of CD30 on peripheral T-cell subsets after HCT
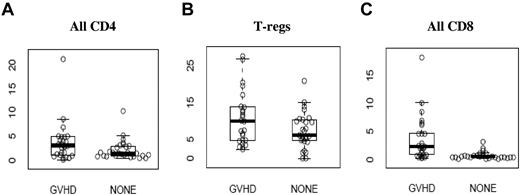
Flow cytometry analysis of surface expression of CD30 on peripheral T-cell subsets is shown in Table 2, expressed as the percentage of cells in each subset expressing CD30. Expression of CD30 on all CD4+ T cells (and subsets) and regulatory T cells (CD4+CD127lowCD25+) was not significantly different between the 2 groups, but there was a significantly greater percentage of CD8+ T cells expressing CD30 in the GVHD group compared with the NONE group (Figure 1). Specifically, a median of 2.30% (range, 0.30-18.2) of all CD8+ T cells in the GVHD group expressed CD30, compared with a median of 0.6% (range, 0.2-3.2) in the NONE group (P < .0001).
CD30 expression on T-cell subsets (A) CD4+ T cells, (B) regulatory T cells (T-regs), and (C) CD8+ T cells. Values on the y-axis are the percentage of the cells in each specific T-cell subset expressing CD30. Exact values and ranges are listed in Table 2.
CD30 expression on T-cell subsets (A) CD4+ T cells, (B) regulatory T cells (T-regs), and (C) CD8+ T cells. Values on the y-axis are the percentage of the cells in each specific T-cell subset expressing CD30. Exact values and ranges are listed in Table 2.
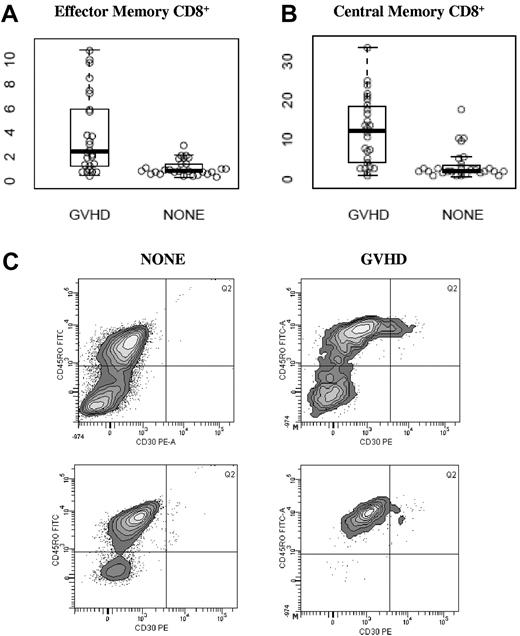
As shown in Figure 2, CD30 expression was especially increased on CD8+ effector memory (CD45RO+, CD62L−) and CD8+ central memory (CD45RO+, CD62L+) subsets. In the CD8+ effector memory subset, a median of 2.40% (range, 0.40%-10.80%) of cells expressed CD30 in the GVHD group compared with a median of 0.80% (range, 0.40%-2.90%) in the NONE group, P < .001; Figure 2A). In the CD8+ central memory subset, a median of 12.35% (range, 0.80%-33.4%) of cells expressed CD30 in the GVHD group compared with 2.1% (range, 0.7%-17.5%, P < .001; Figure 2B). Within the NONE group, there was no difference in CD30 expression in any subset studied between patients who presented with symptoms and those who did not (data not shown). Within the GVHD group, there was no difference in CD30 expression in any subset studied between patients who presented with skin-predominant GVHD versus intestinal-predominant GVHD (data not shown). A representative histogram gated on the CD8+ effector memory subset is shown in Figure 2C.
CD30 expression on memory CD8+ T-cell subsets. Values along the y-axis are percentage of cells in each subset that express CD30. Median and ranges are displayed in Table 2. (A) Effector memory CD8+ T cells, (B) central memory CD8+ T cells, and (C) representative histograms gated on CD8+CD62L− analyzing CD30 expression in effector memory CD8+ T cells by flow cytometry in 2 patients without (NONE) and 2 patients with aGVHD. Percentages reported are the percent CD30+ within CD8+CD45RO+CD62L−.
CD30 expression on memory CD8+ T-cell subsets. Values along the y-axis are percentage of cells in each subset that express CD30. Median and ranges are displayed in Table 2. (A) Effector memory CD8+ T cells, (B) central memory CD8+ T cells, and (C) representative histograms gated on CD8+CD62L− analyzing CD30 expression in effector memory CD8+ T cells by flow cytometry in 2 patients without (NONE) and 2 patients with aGVHD. Percentages reported are the percent CD30+ within CD8+CD45RO+CD62L−.
Because there was heterogeneity in several clinical variables as well as the time after HCT when samples were collected, as determined by when patients presented with symptoms, a linear regression model was used to illustrate the significant correlation between aGVHD and CD30 expression even after we adjusted for these variables. Results showed that time after transplantation, the intensity of the conditioning regimen (myeloablative vs reduced intensity), donor type (MRD vs MUD), and GVHD prophylaxis regimen (use of ATG or alemtuzumab containing vs other) had no significant correlation with CD30 expression on effector or central memory CD8+ T cells. However, the presence of GVHD had a clear correlation with CD30 expression in both subsets (P < .0001).
Measurement of sCD30 levels
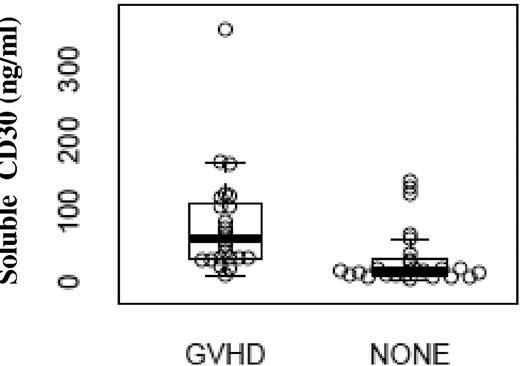
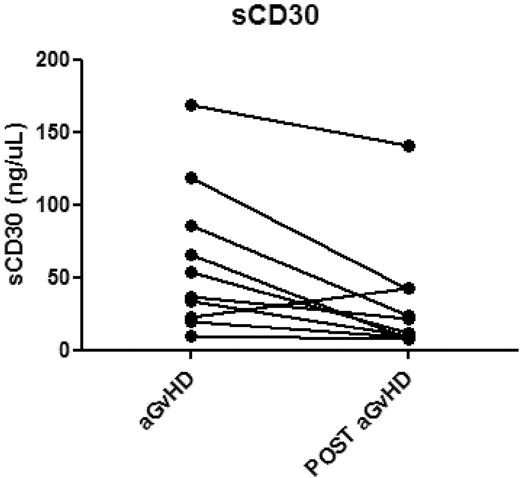
Plasma samples corresponding to 24 of the aGVHD and 25 of the NONE blood samples were identified and analyzed. As illustrated in Figure 3, plasma levels of sCD30 were significantly greater in patients with aGVHD (median, 61.7 ng/mL; range, 9.8-357.1) compared with sCD30 levels from the group without GVHD (median, 17.4 ng/mL; range, 3.7-142.4 ng/mL), P < .001. There was no correlation of sCD30 levels with time of sample collection (P = .47). In addition, there appeared to be significant correlations between sCD30 levels and expression of CD30 on CD8+ effector memory T cells (data not shown, P = .04) and CD8+ central memory T cells (data not shown, P = .05). A total of 10 patients with GVHD who had responded to treatment had additional samples collected anywhere from 91 to 138 days after the date of initial sample collection. sCD30 levels were also measured in these later samples. A total of 9 of 10 patients had decreases in sCD30 levels after successful treatment of GVHD with a mean difference of 30 ng/μL (Figure 4).
sCD30 levels (ng/mL) in patients with aGVHD compared with patients without (NONE). Patients with aGVHD had a median of 61.7 ng/mL (range, 9.8-357.1) of sCD30 compared with a median of 17.4 ng/mL (3.7-142.4) in the control group, P < .001.
sCD30 levels (ng/mL) in patients with aGVHD compared with patients without (NONE). Patients with aGVHD had a median of 61.7 ng/mL (range, 9.8-357.1) of sCD30 compared with a median of 17.4 ng/mL (3.7-142.4) in the control group, P < .001.
sCD30 levels measured at the diagnosis of GVHD and several months later in 10 individual patients who responded to treatment. Post aGVHD samples ranged from 91 to 138 days after the date of the original sample collection. The mean change was a decrease of 30 ng/μL.
sCD30 levels measured at the diagnosis of GVHD and several months later in 10 individual patients who responded to treatment. Post aGVHD samples ranged from 91 to 138 days after the date of the original sample collection. The mean change was a decrease of 30 ng/μL.
CD30 expression in tissue biopsies
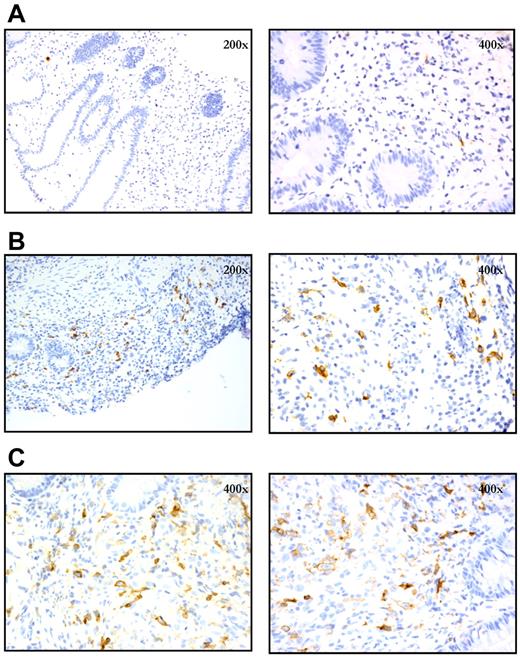
Paraffin-embedded tissues from diagnostic intestinal biopsy samples obtained from patients with suspected aGVHD were subjected to immunohistochemical analysis for CD30 expression. In total, 9 rectal biopsies from patients with clinical intestinal aGVHD were analyzed, and 8 showed a significant infiltrate of CD30+ cells (≥ 1 CD30+ cells per 400× high-power field, 10 fields counted), with a mean of 2.3 CD30+ cells per 400× field for all 9 affected biopsies. The majority of the CD30+ cells were large lymphocytes, morphologically, with prominent nucleoli (Figure 4). In contrast, 3 rectal biopsies from 3 patients obtained after allogeneic HCT who did not have aGVHD had few CD30+ cells (all with an average of < 1 CD30+ cells per 400× field), with a mean of 0.4 cells per 400× field. To evaluate a sufficient number of samples to achieve statistical significance, a more comprehensive analysis with comparison to additional control patients and normal colon samples is planned. Representative images are depicted in Figure 5.
Expression of CD30 in diagnostic intestinal biopsy specimens as assessed by immunohistochemistry. (A) Patient without aGVHD who was diagnosed with C difficile colitis; CD30+ cells are rare (mean 0.4 per 400× field). (B) Patient with clinical grade 2 intestinal aGVHD, with numerous CD30+ cells (mean 4.7 per 400× field). (C) Patient with clinical grade 4 intestinal aGVHD, with numerous CD30+ cells (mean 3.5 per 400× field).
Expression of CD30 in diagnostic intestinal biopsy specimens as assessed by immunohistochemistry. (A) Patient without aGVHD who was diagnosed with C difficile colitis; CD30+ cells are rare (mean 0.4 per 400× field). (B) Patient with clinical grade 2 intestinal aGVHD, with numerous CD30+ cells (mean 4.7 per 400× field). (C) Patient with clinical grade 4 intestinal aGVHD, with numerous CD30+ cells (mean 3.5 per 400× field).
Discussion
CD30 has been previously shown to be a cell-surface molecule expressed on subsets of activated T cells. It is also known to be expressed by certain malignant cells, including Reed-Sternberg cells in classic Hodgkin lymphoma and in malignant cells of anaplastic large cell lymphoma. In this study, we investigated the expression of CD30 in the context of aGVHD. Specifically, we analyzed CD30 expression on peripheral blood T-cell subsets, plasma levels of sCD30, and CD30 expression in biopsies of affected intestinal tissue from patients with aGVHD after allogeneic HCT. Our results showed that there was increased expression of CD30 on CD8+ T cells in patients who had aGVHD compared with patients after allogeneic HCT who did not have aGVHD. This difference was clearly because of increased expression of CD30 on the effector memory and central memory CD8+ subsets. In addition, plasma sCD30 levels were significantly increased in patients with aGVHD, and CD30-expressing cells were present throughout diagnostic biopsies of aGVHD. To our knowledge, this is the first study investigating surface CD30 expression in the context of aGVHD in human patients after allogeneic HCT. Although limited in relative numbers of samples, our study collected samples all at the time of presentation of symptoms and before any treatment with corticosteroids or other immunosuppressant drugs.
Previously, Chan et al had suggested that CD30 expression identified the predominant proliferating T-cell population induced by activation through contact with allogeneic antigens.17 Specifically, they showed that mixed-lymphocyte reactions involving HLA-mismatched pairs generated a population of CD30+ T cells that coexpressed CD45RO and CD25, whereas activation without the presence of allogeneic cells induced significantly less CD30 expression. Analysis of cytokines showed that these mixed-lymphocyte reactions induced the production of both IL-5 and IFN-γ. In addition, this CD30+ T-cell population appeared to be the predominant proliferating cell population induced in response to allogeneic antigens. The actual function of CD30, however, remains unclear.
CD30 has also been previously investigated in murine models of GVHD. Blazar et al used a murine model of BM transplantation to assess the importance of CD30 and CD153 (CD30 ligand) in GVHD.18 Interestingly, their results using CD30−/− donor mice showed a minimal effect on CD8+-mediated GVHD, whereas CD4+-mediated GVHD appeared to be reduced. In addition, blocking antibodies against CD30L appeared to reduce mortality from CD4+-mediated GVHD as well as CD4+ T-cell migration into target organs. Although CD30 did not appear to play a major role in GVHD mediated by CD8+ T cells in this model, this may reflect the high degree of MHC mismatch in this model. CD8+ T cells may play a more important role in human aGVHD, especially when recipients and donors are HLA matched. In another study, Zeiser et al showed that regulatory (CD4+CD25+) T cells from CD30−/− mice were not able to suppress GVHD as effectively as regulatory T cells that expressed CD30.19 Furthermore, use of an anti-CD153 antibody reduced regulatory T-cell protection from proinflammatory cytokine accumulation.
The significance of variations in levels of sCD30 in the first 120 days after allogeneic HCT was explored in a small prospective study of 30 patients. Analysis showed that there was a great variety of sCD30 levels at baseline before HCT and greater sCD30 levels at the time of engraftment correlated with the subsequent development of severe aGVHD. Furthermore, all patients who developed grade 3-4 aGVHD had a clear increase in sCD30 before clinical manifestations and sCD30 levels appeared to decrease if patients responded well to treatment.20 Our data are similar to these findings, that is, showing significantly greater levels of sCD30 in patients with aGVHD and corresponding decreases in patients responding to therapy.
Brentuximab vedotin (anti-CD30–microtubulin toxin monomethylauristatin E antibody drug conjugate) is approved for use in patients with relapsed/refractory Hodgkin lymphoma or anaplastic large cell lymphoma. In a pivotal phase 2 study, Chen et al treated 102 patients with relapsed/refractory Hodgkin lymphoma with 1.8 mg/kg every 3 weeks and showed that 95% had evidence of tumor shrinkage and 83% experienced relief from B symptoms.14 Shustov et al treated 30 patients with relapsed/refractory anaplastic large cell lymphoma and showed an overall response rate of 87% (complete response rate 57%).15 Brentuximab vedotin is now being tested in earlier phases of treatment for CD30+ lymphomas as well as being incorporated into other settings, such as maintenance therapy after autologous stem cell transplantation (www.clinicaltrials.gov ID NCT01100502) and conditioning/maintenance regimens with allogeneic HCT. On the basis of the preliminary data presented here and the safety data from the aforementioned trials, we have initiated a phase 1 trial of brentuximab vedotin for the treatment of steroid-refractory aGVHD at our institutions.
In conclusion, CD30 expression appears to be selectively increased on effector and central memory CD8+ T cells in patients after allogeneic HCT at the time of clinical manifestations of aGVHD. In concordance, sCD30 levels also are elevated in patients presenting with aGVHD. In addition, CD30+ lymphocytes were shown to infiltrate affected intestinal biopsies of patients with acute intestinal GVHD. The data presented here provide preliminary evidence to stimulate further investigation for potential clinical applications of CD30 in aGVHD: (1) a predictive, diagnostic, or prognostic biomarker; and (2) a novel target for therapy. Further analysis in a large number of patients with time-matched controls is needed to confirm whether CD30 expression can serve as a sensitive and specific biomarker for aGVHD and whether expression levels can be prognostic or potentially followed serially as a correlate of response to therapy. Such a study has just finished accrual at our institutions and analysis is planned in the near future. In addition, a larger study on serial plasma samples is needed to confirm whether sCD30 can potentially serve as a diagnostic or correlative biomarker as well. Future clinical trials will determine whether CD30 is a viable target for the treatment and prophylaxis of aGVHD.
Preliminary results of this study were presented in abstract form at the 2012 American Society of Blood and Marrow Transplantation Tandem meetings, San Diego, CA, February 4, 2012.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported in part by Seattle Genetics, the Rogers Family Foundation, the Ted and Eileen Pasquarello Research Fund, and PO1 CA142106.
National Institutes of Health
Authorship
Contribution: Y.-B.C. was involved in the data collection, conception, design of analysis, and interpretation of the data, drafting of the article, preparation of the manuscript, and final approval of the version to be published; S.M. was involved in conception, performing analyses, and final approval of the manuscript; R.H. was involved in performing analyses, preparation of the manuscript, and final approval of the manuscript; H.C. was involved in statistical analysis and critical revision for intellectual content and final approval of the manuscript; E.C. and C.I. were involved in data collection and final approval of the manuscript; I.S.P. was involved in performing analyses; and M.J., T.R.S., C.S.C., R.J.S., and J.R. were involved in interpretation of the data, critical revision for important intellectual content, and final approval of the manuscript.
Conflict-of-interest disclosure: Y.-B.C. has received consulting fees and funding to conduct clinical trials from Seattle Genetics. The remaining authors declare no competing financial interests.
Correspondence: Yi-Bin Chen, MD, Bone Marrow Transplant Unit, Department of Hematology/Oncology, Cox 106, Massachusetts General Hospital, 55 Fruit St, Boston, MA 02114; e-mail: ychen6@partners.org.