Abstract
T-cell immunoglobulin mucin-3 (Tim-3) is expressed on pathogenic T cells, and its ligand galectin-9 (gal-9) is up-regulated in inflamed tissues. When Tim-3+ T cells encounter high gal-9 levels, they are deleted. Tim-3 is up-regulated on activated T cells during GVHD. Inhibition of Tim-3/gal-9 binding by infusion of a Tim-3-Ig fusion protein or Tim-3−/− donor T cells increased T-cell proliferation and GVHD lethality. When the Tim-3/gal-9 pathway engagement was augmented using gal-9 transgenic recipients, GVHD lethality was slowed. Together, these data indicate a potential for modulating this pathway to reduce disease by increasing Tim-3 or gal-9 engagement. Paradoxically, when Tim-3/gal-9 was inhibited in the absence of donor T-regulatory cells (Tregs), GVHD was inhibited. GVHD reduction was associated with decreased colonic inflammatory cytokines as well as epithelial barrier destruction. CD25-depleted Tim-3−/− donor T cells underwent increased activation-induced cell death because of increased IFN-γ production. To our knowledge, these studies are the first to show that although the absence of Tim-3/gal-9 pathway interactions augments systemic GVHD, concurrent donor Treg depletion paradoxically and surprisingly inhibits GVHD. Thus, although donor Tregs typically inhibit GVHD, under some conditions, such Tregs actually may contribute to GVHD by reducing activation-induced T-cell death.
Introduction
GVHD remains the leading cause of morbidity and mortality after bone marrow transplantation (BMT). Patients are given immune suppressive therapy to prevent or diminish the severity of GVHD after allogeneic BMT that in turn increases the risk of infection and disease recurrence. Novel GVHD strategies remain a high priority.
The T-cell immunoglobulin mucin (TIM) family consists of 3 proteins (TIM-1, -3, and -4), homologous in mouse and human.1 Tim-3 was the first described member2 and has been the most well studied. Differentiated T-effector cells (Teffs) express Tim-3 with the highest density on T-helper (Th)1, lower density on Th17, and no expression on Th2 cells.3,4 The expression of galectin-9 (gal-9), identified as a ligand for Tim-3, is up-regulated in inflamed tissues.5-8 When Tim-3+ Teffs encounter high gal-9 levels, they are deleted.5,9-11 A major function of the Tim-3/gal-9 pathway is to limit immune responses under conditions of tissue inflammation and injury. In vivo blockade of Tim-3/gal-9 interaction or the use of Tim-3 knockout (−/−) mice increases Th1 cells within inflamed tissues.2,12,13
When Tim-3 binds with gal-9, Th1 responses are inhibited and peripheral tolerance is induced.5,12,13 In vivo blocking strategies relying on monoclonal anti–Tim-3 antibody and Tim-3-Ig fusion protein showed exacerbation of experimental autoimmune encephalomyelitis and autoimmune diabetes.2,12 Transplant tolerance induced by donor-specific transfusion and anti-CD154 treatment was impaired.13 Thus, Tim-3/gal-9 signaling acts to dampen a Th1 immune response, whereas signaling blockade results in an amplified Th1 response and increased disease. These results were solidified when gal-9 was discovered to be the ligand for Tim-3 and caused cells to aggregate and undergo apoptosis in vitro.5 Hence, a major function of the Tim-3/gal-9 pathway is to limit adaptive Th1 responses. GVHD effects are largely mediated by Th1 Teffs, making the Tim-3/gal-9 pathway an attractive target for regulating GVHD lethality.
Although there is evidence for a negative regulatory function of the Tim-3/gal-9 pathway in autoimmunity, its role in acute GVHD is unclear. We show that during acute GVHD, donor T-cells rapidly up-regulate Tim-3 and nonhematopoietic cells up-regulate gal-9. Allogeneic T-cell proliferation was increased on inhibition of Tim-3. Tim-3 inhibition with Tim-3-Ig or use of Tim-3−/− donor T cells accelerated GVHD lethality. Conversely, gal-9 transgenic (Tg) recipients had a significantly reduced rate of GVHD. These results suggest that Tim-3/gal-9 signaling negatively regulates T cells during GVHD and inhibiting Tim-3/gal-9 increases Teffs and GVHD lethality. Paradoxically and surprisingly, when Tim-3 was inhibited in the absence of donor Tregs, GVHD lethality was significantly reduced. This result was explained by an increased level of IFN-γ secretion that leads to increased activation-induced cell death (AICD). Recipients of Treg-depleted Tim-3−/− donor T cells had less damage to the epithelial layer of the colon as well as a reduced percentage of inflammatory cytokine secretion. These results suggest that increased levels of IFN-γ can lead to protection of the colon from GVHD and reduce the lethality rate.
Methods
Mice
C57BL/6 (H2b) and BALB/c (H2d) mice were purchased from the National Institutes of Health. B6D2F1 (H2b/d) mice were purchased from The Jackson Laboratory. Mice expressing gal-9 under the β-actin promoter and TIM-3−/− mice are on the BALB/c background and were described previously.12,14 B6-L2G85 (luc+) express luciferin under the β-actin promoter were kindly provided by Dr Robert Negrin (Stanford University, Palo Alto, CA).15 TEa CD4+ Tg T cells express a TCR that recognizes the peptide ASFEAQGALANIAVDKA in the context of I-Ab and were described previously.16 TEa Tg mice (kindly provided by Dr Alexander Rudensky, Sloan-Kettering Institute, New York, NY) were crossed with B6-L2G85 mice to produce cells that were TEa+luc+. Mice were bred and housed in a specific pathogen-free facility in microisolator cages and used at 6 to 16 weeks of age. All experiments were approved by the Institutional Animal Care and Use Committee of the University of Minnesota.
Bone marrow transplantation
Mice were lethally irradiated by an x-ray source on day1. In total, 1 × 107 bone marrow (BM) cells with or without purified T cells were infused on day 0. For GVHD induction, T cells were isolated from lymph nodes and purified by incubation with phycoerythrin-labeled antibodies to CD19, γδ-TCR, and DX5 or NK1.1 (eBioscience); incubated with anti–phycoerythrin beads; and depleted on magnetic column (Miltenyi Biotec). Flow cytometric phenotyping demonstrated > 95% purity. Mice were monitored daily for survival, weighed twice weekly, and examined for clinical GVHD. Where indicated, mice were clinically scored for GVHD as described previously.17
Frozen tissue preparation
Tissues including colon, small intestine, liver, lung, and spleen were taken at indicated days after transplantation, embedded in Optimal Cutting Temperature compound (Miles), snap-frozen in liquid nitrogen, and stored at −80°C.
Immunofluorescence
Cryosections (6 μm) were fixed in acetone and incubated with monoclonal rat anti–gal-9 (Biolegend) overnight at 4°C, and with anti–CD45-FITC (eBioscience) for 30 minutes at room temperature. Fluorochrome-labeled secondary antibodies to rat (Jackson ImmunoResearch Laboratories) were incubated for 30 minutes at room temperature. Slides were mounted with 4,6-diamidino-2-phenylindole SlowFade Gold (Invitrogen). Confocal images were acquired on a FluoView 500 confocal laser scanning microscope under 40×/0.9 oil-immersion objective lens using FluoView 3.2 software (Olympus) and then processed with Photoshop CS3 (Adobe Systems).
CFSE experiments
T cells purified from lymph nodes were labeled for 15 minutes with 5μM carboxyfluorescein diacetate succinimidyl diester (CFSE; Invitrogen) at room temperature followed by quenching with fetal bovine serum. Labeled purified T cells (1 × 107) were infused into lethally irradiated recipients. Spleens were harvested on indicated days, and single cell suspensions were made. Cells were surface stained and acquired on an FACS LSRII Fortessa flow cytometer (BD Biosciences). Analysis was performed using FlowJo Version 9.4.11 software (TreeStar).
Flow cytometry
Single-cell suspensions were stained with the following monoclonal antibodies (mAbs): Tim-3, CD8a, CD4, CD45.1, H2Kd, IFN-γ, IL-2, IL-17, granzyme-B, Ki-67, annexin V, and activated caspase-3. For cytokine detection, cells were stimulated in vitro with cell stimulation cocktail and protein transport inhibitor cocktail (eBioscience) for 5 hours. Cells were then surface stained with appropriate surface antibodies followed by fixing and permeabilizing with the Fix/Perm permeabilization kit (Invitrogen). Finally, cells were labeled with the appropriate intracellular antibodies. Cell apoptosis and death were measured by staining cells with annexin V staining kit and 7-AAD (eBioscience). Phenotypic acquisition of cells was performed on the LSRII Fortessa flow cytometer (BD Biosciences) and analyzed with FlowJo software (TreeStar).
FITC-dextran assay
FITC-dextran assay was used to evaluate mucosal integrity. Mice were given 400 μL of FITC-dextran orally (Sigma-Aldrich) at a concentration of 40 mg/mL in PBS. Plasma was collected from peripheral blood, mixed 1:1 with PBS, and analyzed on a plate reader at excitation/emission wavelength of 485/535 nm.
Antibodies and reagents
Hybridoma clone 5D1218 was used to produce αTIM-3 mAb. Protein was purified over a protein G column and brought to a concentration of 2 mg/mL in PBS. In some studies, Tim-3-Ig (100 μg/dose) or control human IgG was injected intraperitoneally every day from days 1 to 5 and 3 times per week until day 28. Where indicated, transplanted mice received αIFN-γ (100 μg/dose; National Cancer Institute) or rat IgG intraperitoneally twice per week from days 1 to 28.
T-cell isolation
Spleens were processed into a single-cell suspension followed by red cell lysis. Colons were harvested, cut longitudinally, and then into 5-mm pieces. Gut pieces were incubated with 15.4 mg/mL dithiothreitol in calcium- and magnesium-free phosphate-buffered saline with 10% serum (30 minutes at 37°C) followed by treatment with 5mM EDTA in RPMI 1640 with 10% serum (15 minutes at 37°C). Tissue was incubated 3 times with 1 mg/mL collagenase D (Roche) in RPMI 1640 with 5% serum (45 minutes at 37°C). Lymphocytes were purified on a 44/67% Percoll gradient (800g at 20°C for 20 minutes).
Treg suppression assay
Tregs were isolated from spleens and of BALB/c or Tim-3−/− mice. T cells were labeled with 1μM CFSE. T-cell/natural killer (NK)–depleted splenocytes from BALB/c mice were used as APCs. CFSE-labeled T-responder cells (4 × 105) were stimulated with 4 × 105 APCs in RPMI-c and 0.25 μg/mL purified α-CD3. Four days later, cells were harvested and proliferation was determined by CFSE dilution.
Bioluminescence imaging studies
The IVIS imaging system (Xenogen) was used for bioluminescence imaging studies. Firefly luciferin substrate (0.1 mL; 30 mg/mL) was injected intraperitoneally, and imaging was performed immediately after substrate injection. Data were analyzed and are presented as photon counts per area.
Statistics
The Kaplan-Meier product-limit method was used to calculate survival. Differences between groups were determined using log-rank statistics. Group comparisons were analyzed by Student t test or 1-way ANOVA with a Tukey multiple comparison test. P values ≤ .05 were considered significant.
Results
Tim-3 expression is up-regulated on donor T cells during acute GVHD
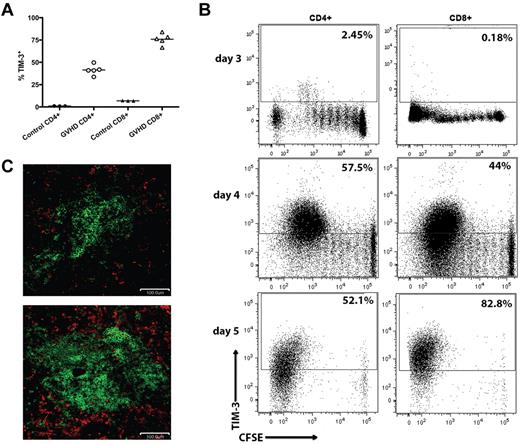
Tim-3 can be expressed on subsets of differentiated T cells that have undergone 2 to 3 rounds of cell division.12 To determine whether Tim-3+ Teffs are induced during acute GVHD generated in fully conditioned recipients, C57BL/6 (B6; H2b) recipients were lethally irradiated followed by an infusion of BALB/c (H2d) BM with or without purified donor T cells from BALB/c mice to induce GVHD. Whereas Tim-3 was expressed on an average of 41% of CD4+ and 76% of CD8+ donor T cells on day 7 of the GVHD response, few naive T cells were Tim-3+. To examine the in vivo kinetics of Tim-3 up-regulation on donor T cells, we performed an in vivo mixed leukocyte reaction (MLR). B6 recipients were lethally irradiated and given 107 BALB/c-purified CFSE-labeled donor T cells. On days 3, 4, and 5 after transfer, splenocytes were analyzed for CFSE dilution and Tim-3 expression. Donor T cells began up-regulating Tim-3 on day 3 and by day 5 > 50% of donor CD4+ and > 80% of donor CD8+ T cells express Tim-3 after several rounds of division (Figure 1B). Although gal-9 expression was not observed on CD4+ or CD8+ T cells by flow cytometry (data not shown), gal-9 expression was up-regulated in the spleen on day 7. Figure 1C shows immunofluorescent staining of spleen from the BM control and GVHD mice on day 7 after BMT. Gal-9+ (shown in red) cells were CD45− but surrounded CD45+ cells (shown in green) in the spleen, indicating that nonhematopoietic, stromal cells strongly expressed gal-9 in GVHD recipients.
Tim-3/gal-9 expression is up-regulated during acute GVHD. (A) B6 mice were lethally irradiated (11.0 Gy) and infused with 107 MHC-mismatched BALB/c NTCD BM and 3 × 106 BALB/c purified T cells. Recipient mice were killed on day 7, along with 3 naive BALB/c mice for control, and the spleens were examined by flow cytometry for Tim-3 expression. Cells were gated on CD4 or CD8 positive, H-2Kd positive events. (B) CFSE-labeled BALB/c purified T cells (107) were transferred into lethally irradiated B6 recipients. Spleens were harvested and analyzed by flow cytometry for CFSE dilution and Tim-3 expression on days 3, 4, and 5. Data shown are representative of 4 mice per group per day. Cells were gated on CD4 or CD8 positive, H2Kd positive events. Numbers indicate percentage of Tim-3+ cells. (C) B6 mice were lethally irradiated and infused with 107 BALB/c NTCD BM and 3 × 106 BALB/c purified T cells and killed on day 7. Cryosections from the spleen of BM only recipients (top) and T-cell recipients (bottom) were stained for CD45 in FITC (shown in green) and gal-9 (shown in red). Original magnification ×20. Pictures are representative of 2 experiments, with 3 to 4 mice/group.
Tim-3/gal-9 expression is up-regulated during acute GVHD. (A) B6 mice were lethally irradiated (11.0 Gy) and infused with 107 MHC-mismatched BALB/c NTCD BM and 3 × 106 BALB/c purified T cells. Recipient mice were killed on day 7, along with 3 naive BALB/c mice for control, and the spleens were examined by flow cytometry for Tim-3 expression. Cells were gated on CD4 or CD8 positive, H-2Kd positive events. (B) CFSE-labeled BALB/c purified T cells (107) were transferred into lethally irradiated B6 recipients. Spleens were harvested and analyzed by flow cytometry for CFSE dilution and Tim-3 expression on days 3, 4, and 5. Data shown are representative of 4 mice per group per day. Cells were gated on CD4 or CD8 positive, H2Kd positive events. Numbers indicate percentage of Tim-3+ cells. (C) B6 mice were lethally irradiated and infused with 107 BALB/c NTCD BM and 3 × 106 BALB/c purified T cells and killed on day 7. Cryosections from the spleen of BM only recipients (top) and T-cell recipients (bottom) were stained for CD45 in FITC (shown in green) and gal-9 (shown in red). Original magnification ×20. Pictures are representative of 2 experiments, with 3 to 4 mice/group.
Tim-3/gal-9 pathway inhibition leads to increased allogeneic proliferation in vitro and in vivo and accelerated GVHD lethality
Previously, it has been shown that alloreactive T cells have increased expansion after αTim-3 blockade.12,19 After we determined that Tim-3 expression was highly up-regulated during acute GVHD, we sought to determine whether allogeneic T cells would increase their proliferation when the Tim-3 pathway was blocked. Initially, we performed an in vitro MLR by mixing B6-purified T cells with irradiated BALB/c stimulators (1:1) and adding monoclonal αTim-3 blocking antibody in 3 different concentrations. Figure 2A shows that αTim-3 mAb at 5 μg/mL resulted in significantly increased proliferation on day 5 through day 8 compared the irrelevant IgG control. The increased proliferation in vitro was dose dependent.
Inhibiting Tim-3 increases allogeneic T-cell proliferation. (A) MLR was performed by mixing B6-purified T cells with irradiated BALB/c stimulators (1:1) and αTim-3. These cultures were pulsed with [3H]thymidine on the indicated days and harvested 16 hours later. Proliferation was determined as a measure of radioactive uptake. One-way ANOVA with Tukey multiple comparison test (P < .001, n = 5 per group). ■ indicates control IgG; and ○, αTim-3 5D12 mAb. (B) CFSE-labeled WT or Tim-3−/− BALB/c purified T cells (107) were transferred into lethally irradiated CD45.1+ congenic B6 recipients. Spleens were harvested and analyzed by flow cytometry for CFSE dilution and on days 3 and 4. Representative histograms shown are representative of 4 mice per group per day. Filled histogram, WT donor; open histogram, Tim-3−/− donor. Cells were gated on CD45.1+, H2Kd positive, CD4 or CD8 positive events.
Inhibiting Tim-3 increases allogeneic T-cell proliferation. (A) MLR was performed by mixing B6-purified T cells with irradiated BALB/c stimulators (1:1) and αTim-3. These cultures were pulsed with [3H]thymidine on the indicated days and harvested 16 hours later. Proliferation was determined as a measure of radioactive uptake. One-way ANOVA with Tukey multiple comparison test (P < .001, n = 5 per group). ■ indicates control IgG; and ○, αTim-3 5D12 mAb. (B) CFSE-labeled WT or Tim-3−/− BALB/c purified T cells (107) were transferred into lethally irradiated CD45.1+ congenic B6 recipients. Spleens were harvested and analyzed by flow cytometry for CFSE dilution and on days 3 and 4. Representative histograms shown are representative of 4 mice per group per day. Filled histogram, WT donor; open histogram, Tim-3−/− donor. Cells were gated on CD45.1+, H2Kd positive, CD4 or CD8 positive events.
An in vivo assay was used to establish the average number of divisions of divided cells (proliferative capacity) of Tim-3−/− versus wild-type (WT) T cells. B6 mice were transplanted with 107 allogeneic T cells from BALB/c donors labeled with CFSE. On days 3 and 4, splenocytes were analyzed for CFSE dilution. Donor T cells had very low division on day 3, and there was no significant difference between the groups. By day 4, Tim-3−/− versus WT donor T cells had significantly increased proliferative capacity (Figure 2A). These results indicate that inhibition of Tim-3 increases allogeneic T-cell proliferation compared with controls.
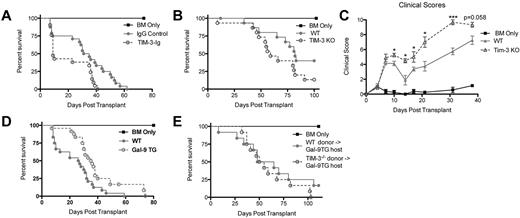
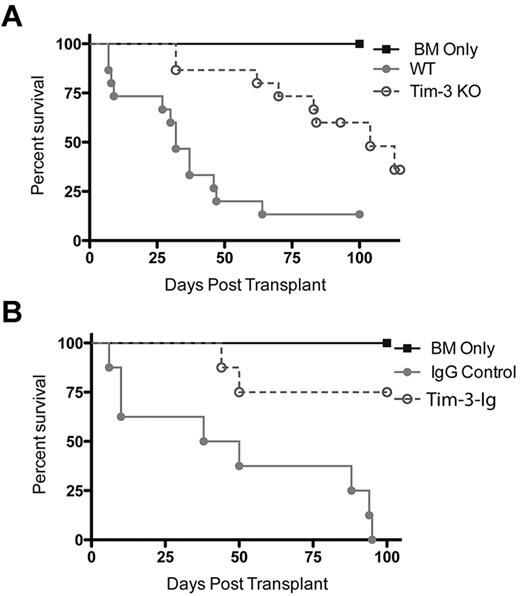
Because of the increased expression of Tim-3 during acute GVHD and increased proliferative capacity of allogeneic donor T cells on Tim-3 blockade, we reasoned that GVHD would be augmented on Tim-3/gal-9 pathway inhibition. Soluble Tim-3-Ig fusion protein (Tim-3-Ig) binds gal-9, precluding Tim-3 engagement. To test its effect on GVHD, B6 recipients were lethally irradiated, given BALB/c BM with or without BALB/c splenocytes (15 × 106) and Tim-3-Ig or IgG at doses of 100 μg intraperitoneally every day from days 1 to 5 and then 3 times per week until day 21. Figure 3A shows recipients of Tim-3-Ig had a significant increase in GVHD lethality, with a median survival time (MST) of only 9 days versus 33 days for recipients of IgG (P = .012).
Inhibiting Tim-3 results in accelerated lethality while increasing gal-9 expression results in decreased lethality. (A) B6 mice were lethally irradiated and infused with 107 BALB/c NTCD BM and 15 × 106 BALB/c splenocytes. Survival plot of IgG control (closed circle) versus Tim-3-Ig treatment (open circle) is shown (P = .012, n = 21-24/group). (B) B6 mice were lethally irradiated and infused with 107 BALB/c NTCD BM and 1 × 106 BALB/c or 1 × 106 Tim-3−/−–purified T cells. Survival plot of BALB/c (closed circle) versus Tim-3−/− (open circle) donor is shown (P = .083, n = 16/group). (C) B6 mice were lethally irradiated and infused with 107 BALB/c NTCD BM and 1 × 106 BALB/c or 1 × 106 Tim-3−/−–purified T cells. Mice were analyzed for clinical scores (n = 8/group). (D) BALB/c or gal-9 Tg mice were lethally irradiated and given 107 B6 NTCD BM and 2 × 106 B6-purified T cells. Survival plot of BALB/c versus gal-9 Tg recipients is shown (P < .01, n = 24/group). (E) gal-9 Tg mice were lethally irradiated and given 107 BALB/c NTCD BM and 3 × 106 BALB/c or 3 × 106 Tim-3−/−–purified T cells. Survival plot of BALB/c versus Tim-3−/− donors is shown (P = .217, n = 12/group).
Inhibiting Tim-3 results in accelerated lethality while increasing gal-9 expression results in decreased lethality. (A) B6 mice were lethally irradiated and infused with 107 BALB/c NTCD BM and 15 × 106 BALB/c splenocytes. Survival plot of IgG control (closed circle) versus Tim-3-Ig treatment (open circle) is shown (P = .012, n = 21-24/group). (B) B6 mice were lethally irradiated and infused with 107 BALB/c NTCD BM and 1 × 106 BALB/c or 1 × 106 Tim-3−/−–purified T cells. Survival plot of BALB/c (closed circle) versus Tim-3−/− (open circle) donor is shown (P = .083, n = 16/group). (C) B6 mice were lethally irradiated and infused with 107 BALB/c NTCD BM and 1 × 106 BALB/c or 1 × 106 Tim-3−/−–purified T cells. Mice were analyzed for clinical scores (n = 8/group). (D) BALB/c or gal-9 Tg mice were lethally irradiated and given 107 B6 NTCD BM and 2 × 106 B6-purified T cells. Survival plot of BALB/c versus gal-9 Tg recipients is shown (P < .01, n = 24/group). (E) gal-9 Tg mice were lethally irradiated and given 107 BALB/c NTCD BM and 3 × 106 BALB/c or 3 × 106 Tim-3−/−–purified T cells. Survival plot of BALB/c versus Tim-3−/− donors is shown (P = .217, n = 12/group).
To determine whether Tim-3 expression on donor T cells would impact GVHD lethality, lethally irradiated WT B6 mice were given BALB/c BM with or without purified T cells from BALB/c or Tim-3−/− mice to induce GVHD. Despite that Tim-3−/− T cells had increased proliferative capacity, T cell–mediated GVHD lethality was augmented, albeit more modestly than with using blocking Tim-3-Ig and WT cells. Figure 3B shows pooled survival data with Tim-3−/− donor T cells having a trend (P = .083) toward accelerated lethality (MST, 59 days) compared with WT (MST, 82 days). To strengthen these data, clinical scoring was performed. B6 mice were lethally irradiated and given BALB/c BM and 3 × 106 BALB/c or Tim-3−/−–purified T cells. Consistent with the survival data, Figure 3C shows that mice that received Tim-3−/− donor T cells had significantly increased clinical scores (days 10-31, P < .05). In summary, both using a blocking reagent and using Tim-3−/− donor cells resulted in augmented GVHD lethality.
Decreased lethality in gal-9 Tg recipients
It has been shown that gal-9 Tg mice have a reduced ability to prime a Th1 immune response and generate Teffs.21 Based on this premise, we sought to determine whether gal-9 Tg recipient mice could inhibit GVHD lethality. In these mice, gal-9 expression is driven by a ubiquitous promotor (β-actin), leading to high gal-9, especially in the small intestine, liver, and lung (supplemental Figure 1A, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Gal-9 Tg mice coexpress gal-9 on B220+, CD11b+, and CD11c+ APCs present in the spleen (supplemental Figure 1B). BALB/c or gal-9 Tg mice were lethally irradiated and given B6 BM and 2 × 106 B6-purified T cells. Figure 3D shows that gal-9 Tg recipients had a significant decrease in lethality rate (MST, 35.5 days) compared with WT recipients (MST, 26.5 days). To determine whether the decreased lethality rate was dependent on Tim-3 expression by donor T cells, gal-9 Tg mice were lethally irradiated and given BALB/c BM and 3 × 106 BALB/c or Tim-3−/−–purified T cells. Gal-9 Tg recipients that received Tim-3−/− donor T cells did not have the survival advantage seen with Tim-3 expressing BALB/c T cells (Figure 3E). This demonstrates that Tim-3 expression on donor T cells was necessary for the survival advantage in gal-9 Tg recipients.
Donor Treg depletion increases survival rates when the Tim-3/gal-9 pathway is blocked
We have shown that depletion of Tregs from donor T-cell grafts worsens GVHD, whereas donor Treg add-back diminishes GVHD lethality.21 To determine whether donor Treg-mediated GVHD suppression was diminished using Tim-3−/− versus WT T cells, we depleted the graft of Tregs. B6 recipients were lethally irradiated and given BALB/c BM ± Treg (CD25)–depleted donor-purified T cells from WT BALB/c or Tim-3−/− mice. Remarkably, and contrary to our predictions, mice that received Tim-3−/−–purified Treg-depleted T cells were protected against GVHD lethality compared with recipients of WT Treg-depleted T cells (P < .001), resulting in a MST of 104 days versus a MST of 32 days, respectively (Figure 4A). Clinical scoring showed significantly decreased scores for recipients of Tim-3−/− Treg-depleted T cells (data not shown). To determine whether these surprising findings were unique to the mode of Tim-3/gal-9 pathway blockade, we used Tim-3-Ig instead of Tim-3−/− donor T-cells. Lethally irradiated B6 recipients were given BALB/c BM ± CD25-depleted donor-purified T cells and Tim-3-Ig or IgG (100 μg days 0 to 5 then qod until day 28 after BMT). Recipients of WT Treg-depleted T cells and irrelevant IgG treatment had a rapid GVHD lethality course, with a MST of 32 days. Consistent with findings using Tim-3−/− Treg-depleted T cells, Figure 4B shows that recipients of Treg-depleted BALB/c T-cells and Tim-3-Ig treatment were protected against GVHD lethality compared with recipients of WT-purified Treg-depleted T cells (P < .01), resulting in 75% versus 0% long-term survival, respectively. Collectively, these data from 2 distinct but complementary approaches support the notion that blockade of the Tim-3/gal-9 pathway in the absence of donor Tregs inhibits, rather than augments, GVHD lethality.
Inhibiting Tim-3 in the absence of Tregs leads to decreased lethality. (A) B6 mice were lethally irradiated and infused with 107 BALB/c NTCD BM and 3 × 106 BALB/c or Tim-3−/−–purified CD25− T cells. Survival plot of BALB/c (closed circle) versus Tim-3−/− (open circle) donor is shown (P < .001, n = 15/group). (B) B6 mice were lethally irradiated and infused with 107 BALB/c NTCD BM and 106 BALB/c CD4+CD25− T cells. Survival plot of IgG control (closed circle) versus Tim-3-Ig (open circle) treatment is shown (P < .01, n = 8/group).
Inhibiting Tim-3 in the absence of Tregs leads to decreased lethality. (A) B6 mice were lethally irradiated and infused with 107 BALB/c NTCD BM and 3 × 106 BALB/c or Tim-3−/−–purified CD25− T cells. Survival plot of BALB/c (closed circle) versus Tim-3−/− (open circle) donor is shown (P < .001, n = 15/group). (B) B6 mice were lethally irradiated and infused with 107 BALB/c NTCD BM and 106 BALB/c CD4+CD25− T cells. Survival plot of IgG control (closed circle) versus Tim-3-Ig (open circle) treatment is shown (P < .01, n = 8/group).
To investigate whether the Tim-3 pathway had differential effects on Teffs versus Tregs, we performed in vitro studies to address expression and suppressive capacity. Naive Tregs were isolated from BALB/c and Tim-3−/− mice and activated and expanded in vitro. Neither the naive or activated Tregs expressed Tim-3 (supplemental Figure 2A). A Treg suppression assay was performed using naive Tregs isolated from the spleen of BALB/c and Tim-3−/− mice. Tregs were combined with CFSE-labeled CD25-depleted purified T cells and irradiated APCs in vitro and stimulated with αCD3. The cells were then harvested on day 4 and analyzed for CFSE dilution. There was no significant difference in the ability of WT Tregs to suppression Teff division compared with Tim-3−/− Tregs (supplemental Figure 2B).
Tim-3/gal-9 pathway blockade results in decreased gut injury in recipients of Treg-depleted donor grafts
To investigate the mechanism of increased survival, we sought to evaluate the gastrointestinal tract, a major GVHD target organ. B6 recipients were lethally irradiated and given BALB/c BM ± CD25-depleted donor-purified T cells from BALB/c or Tim-3−/− mice. To measure epithelial integrity of the GI tract, we used an FITC-dextran assay in which the loss of epithelial cell integrity results in leakage of FITC-dextran from the intestine into the peripheral blood. FITC-dextran was administered orally to mice on days 14 and 21, and serum levels were measured 4 hours later. On day 14, mice that received Tim-3−/− CD25-depleted donor T cells had a significantly reduced level of FITC-dextran in the serum (1.629 μg/mL) compared with recipients of WT CD25-depleted T cells (2.762 μg/mL), indicating increased epithelial integrity (Figure 5A; P = .046). On day 21, there was also a trend (P = .078) toward reduced levels in the serum of recipients of CD25-depleted Tim-3−/− T cells. On both days, there was no significant difference between the group that received BM only and the group that received CD25-depleted Tim-3−/− T cells. Histopathology scores of the gut on day 21 showed a trend (P < .07) toward reduced pathology in the colon of recipients of CD25-depleted Tim-3−/− T cells (data not shown).
Inhibiting Tim-3 in the absence of T-regs reduces gut pathology. (A) B6 mice were lethally irradiated and infused with 107 BALB/c NTCD BM and 3 × 106 BALB/c or Tim-3−/−–purified CD25− T cells. FITC-dextran (16 mg) was administered orally to mice on day 14 (P = .046, n = 4) and days 21(P = .0778, n = 4). Serum levels were measured 4 hours later. (B) B6 mice were lethally irradiated and infused with 107 BALB/c NTCD BM and 3 × 106 BALB/c or Tim-3−/−–purified CD25− T cells. Mice were killed on day 21, and lamina propria lymphocytes were analyzed for effector cytokines.
Inhibiting Tim-3 in the absence of T-regs reduces gut pathology. (A) B6 mice were lethally irradiated and infused with 107 BALB/c NTCD BM and 3 × 106 BALB/c or Tim-3−/−–purified CD25− T cells. FITC-dextran (16 mg) was administered orally to mice on day 14 (P = .046, n = 4) and days 21(P = .0778, n = 4). Serum levels were measured 4 hours later. (B) B6 mice were lethally irradiated and infused with 107 BALB/c NTCD BM and 3 × 106 BALB/c or Tim-3−/−–purified CD25− T cells. Mice were killed on day 21, and lamina propria lymphocytes were analyzed for effector cytokines.
The colon was examined for the presence of T cells expressing effector cytokines that cause tissue damage. B6 hosts were infused with BALB/c BM ± BALB/c CD25-depleted purified T cells from BALB/c or Tim-3−/− mice. On day 21, lamina propria lymphocytes (LPLs) were isolated, restimulated, and stained for the expression of IL-2, IFN-γ, and IL-17, known effector molecules involved in GVHD. Expression of IL-2, IFN-γ, and IL-17 in CD4+ and CD8+ T cells was significantly decreased in recipients of CD25-depleted Tim-3−/− versus WT T cells (Figure 5B). These data suggest that in our model where Tim-3 is inhibited in the absence of donor Tregs, the gastrointestinal tract is better protected, which is associated with increased overall survival.
Elevated IFN-γ leads to activation-induced cell death
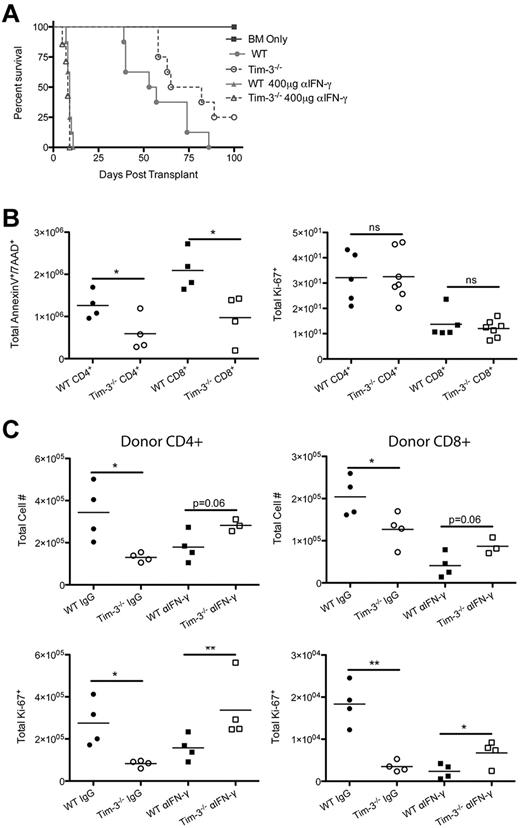
One explanation for the paradoxical results seen with the Tim-3/gal-9 blockade in recipients of unmanipulated versus Treg-depleted donor T cells could be the over exuberant production of IFN-γ, resulting in donor T-cell AICD in the latter case. To examine this possibility, B6 recipients were lethally irradiated and given BALB/c BM and WT or Tim-3−/−–purified T cells that were either unmanipulated or CD25 depleted. On day 7 after BMT, splenocytes were restimulated in vitro in the presence of brefeldin A, and then T cells were analyzed for IFN-γ expression by intracellular cytokine staining. Figure 6 shows T cells obtained from recipients of Tim-3−/− compared with WT donor T cells had significantly increased IFN-γ expression in both donor CD4+ (2.4 × 105 vs 1.2 × 105) and CD8+ (1.8 × 105 vs 1.1 × 105) T cells. Along with IFN-γ, we also found that T cells isolated from recipients of Tim-3−/− had increased levels of the effector molecule granzyme-B (CD4+, 3.0 × 105 vs 1.2 × 105; CD8+, 5.4 × 105 vs 2.1 × 105). Ki-67 staining was used to determine that Tim-3−/− donor T cells were also proliferating more than WT T cells (CD4+, 1.1 × 105 vs 3.0 × 104; CD8+, 1.3 × 105 vs 4.1 × 104). These results indicate that simultaneously inhibiting Tim-3/gal-9 and depleting donor Tregs leads to increased donor T-cell effector molecule secretion and increased proliferation.
Elevated IFN-γ levels lead to AICD. (A) B6 mice were lethally irradiated and infused with 107 BALB/c NTCD BM and 3 × 106 BALB/c or Tim-3−/−–purified CD25− T cells. Mice were killed on day 7, and splenocytes were analyzed for IFN-γ (n = 4), granzyme-B (n = 8), and Ki-67 (n = 8; *P < .05, ***P < .001). (B) B6 mice were lethally irradiated and infused with 107 BALB/c NTCD BM and 3 × 106 BALB/c or Tim-3−/−–purified CD25− T cells. Mice were killed on day 7, and splenocytes were analyzed for Annexin V/7-AAD (n = 4) and activated caspase-3 (P < .05, n = 8). (C) B6D2F1 mice were lethally irradiated and infused with 107 B6 NTCD BM, 5 × 105 B6-purified T cells, and 2.5 × 104 TEa monoclonal luciferase–enriched T cells.
Elevated IFN-γ levels lead to AICD. (A) B6 mice were lethally irradiated and infused with 107 BALB/c NTCD BM and 3 × 106 BALB/c or Tim-3−/−–purified CD25− T cells. Mice were killed on day 7, and splenocytes were analyzed for IFN-γ (n = 4), granzyme-B (n = 8), and Ki-67 (n = 8; *P < .05, ***P < .001). (B) B6 mice were lethally irradiated and infused with 107 BALB/c NTCD BM and 3 × 106 BALB/c or Tim-3−/−–purified CD25− T cells. Mice were killed on day 7, and splenocytes were analyzed for Annexin V/7-AAD (n = 4) and activated caspase-3 (P < .05, n = 8). (C) B6D2F1 mice were lethally irradiated and infused with 107 B6 NTCD BM, 5 × 105 B6-purified T cells, and 2.5 × 104 TEa monoclonal luciferase–enriched T cells.
The increased activation state that is observed in Tim-3−/− donor T cells compared with WT donor T cells supports the possibility that these cells are undergoing AICD, reducing the number of donor alloreactive T cells, and ultimately leading to an increased survival rate. To examine AICD of donor T cells, B6 mice were lethally irradiated and infused with BALB/c BM and BALB/c or Tim-3−/−–purified CD25− donor T cells. Splenocytes were analyzed on day 7 after transplantation. First, we analyzed the number of cells that were expressing annexin V/7-AAD (indicating dead cells). There were significantly increased numbers of Tim-3−/− donor T cells that were annexin V+/7-AAD+ compared with WT donor T cells (Figure 6B). There were significantly increased numbers of Tim-3−/− compared with WT donor T cells that were active-caspase 3+, a more specific cell-death marker. Cumulatively, these data suggest that recipients of Tim-3−/− Treg-depleted donor T cells are more activated leading to increased AICD, which was associated with decreased GVHD lethality.
To further analyze AICD, we sought to track the T-cell expansion and contraction in vivo using a monoclonal tracer system.22 B6D2F1 mice were lethally irradiated and infused with B6 NTCD BM, 5 × 105 B6-purified T cells, and 2.5 × 104 TEa monoclonal luciferase–enriched T cells (Tim-3-Ig vs IgG treatment). Initially, mice that received Tim-3-Ig had a significant increase in T-cell expansion followed by a contraction that resulted in a significantly smaller T-cell pool at later time points in the assay (days 20-24).
Blocking IFN-γ eliminates the promotion of AICD
To further analyze the role of IFN-γ in promoting AICD, we sought to inhibit IFN-γ to determine whether this cytokine was responsible for the survival differences between the recipients of CD25-depleted WT or Tim-3−/− donor T-cells. B6 recipients received BALB/c BM ± CD25-depleted donor purified T cells from WT BALB/c or Tim-3−/− mice and were treated with either IgG or α-IFN-γ antibody (400 μg twice per week). Whereas recipients of CD25-depleted Tim-3−/− T cells had an MST of 74 days, the lethality rate was significantly higher (P = .046) in those receiving CD25-depleted WT T cells, with an MST of 55 days. The groups that received anti–IFN-γ mAb treatment had no statistically significant difference between the survival curves with recipients of either CD25-depleted WT or Tim-3−/− experiencing 100% lethality by 2 weeks after BMT. In summary, IFN-γ is necessary for the increased survival that is observed when Tim-3/gal-9 is inhibited in the absence of donor Tregs.
Further studies were done to analyze the effect of IFN-γ blockade. B6 recipients were infused with BALB/c BM ± CD25-depleted donor-purified T cells from WT BALB/c or Tim-3−/− mice and were treated with either IgG or αIFN-γ antibody. Contrary to Figure 6, blocking IFN-γ resulted in a significantly decreased number of Tim-3−/− donor T cells that were annexin V+/7-AAD+ compared with WT T cells and eliminated any difference in Ki-67+ at day 7 after transplantation (Figure 7). LPLs were isolated on day 21 after transplant. Significantly fewer cells were found in recipients of Tim-3−/− versus WT donor T cells. Blocking with αIFN-γ resulted in a trend (P < .06) toward increased cells in recipients of Tim-3−/− T cells compared with WT. When we analyzed the number of proliferating LPLs, Ki-67+ cell number in recipients of Tim-3−/− versus WT T cells was decreased. Blocking IFN-γ resulted in a significant increase in the number of Ki-67+ cells in recipients of Tim-3−/− T cells compared with WT cells.
Blocking IFN-γ eliminates the promotion of AICD. (A) B6 mice were lethally irradiated and infused with 107 BALB/c NTCD BM and 3 × 106 BALB/c or Tim-3−/−–purified CD25− T-cells. Recipients of Tim-3−/− CD25-depleted T cells had a significantly (P = .046) higher survival than those given WT CD25-depleted T cells. Survival plot of IgG control versus αIFN-γ treatment is shown (n = 8/group). (B) B6 mice were lethally irradiated and infused with 107 BALB/c NTCD BM and 3 × 106 BALB/c or Tim-3−/−–purified CD25− T-cells. Mice were killed on day 7, and splenocytes were analyzed by flow cytometry for annexin V (n = 4) and Ki-67 (n = 5-8; *P < .05). (C) B6 mice were lethally irradiated and infused with 107 BALB/c NTCD BM and 3 × 106 BALB/c or Tim-3−/−–purified CD25− T cells for IgG control treatment and 1 × 106 BALB/c or Tim-3−/−–purified CD25− T-cells for αIFN-γ treatment. Mice were killed on day 21, and lamina propria lymphocytes were analyzed for total CD4+ and CD8+ cell numbers (*P < .05) and total Ki-67 (*P < .05, **P < .01).
Blocking IFN-γ eliminates the promotion of AICD. (A) B6 mice were lethally irradiated and infused with 107 BALB/c NTCD BM and 3 × 106 BALB/c or Tim-3−/−–purified CD25− T-cells. Recipients of Tim-3−/− CD25-depleted T cells had a significantly (P = .046) higher survival than those given WT CD25-depleted T cells. Survival plot of IgG control versus αIFN-γ treatment is shown (n = 8/group). (B) B6 mice were lethally irradiated and infused with 107 BALB/c NTCD BM and 3 × 106 BALB/c or Tim-3−/−–purified CD25− T-cells. Mice were killed on day 7, and splenocytes were analyzed by flow cytometry for annexin V (n = 4) and Ki-67 (n = 5-8; *P < .05). (C) B6 mice were lethally irradiated and infused with 107 BALB/c NTCD BM and 3 × 106 BALB/c or Tim-3−/−–purified CD25− T cells for IgG control treatment and 1 × 106 BALB/c or Tim-3−/−–purified CD25− T-cells for αIFN-γ treatment. Mice were killed on day 21, and lamina propria lymphocytes were analyzed for total CD4+ and CD8+ cell numbers (*P < .05) and total Ki-67 (*P < .05, **P < .01).
Discussion
Here, we demonstrate the important role for the Tim-3/gal-9 pathway in GVHD in MHC-disparate recipients. Tim-3 was found to be highly up-regulated on infused donor T cells, suggesting a role in GVHD regulation. Inhibition of Tim-3/gal-9 binding led to an increase in proliferation of donor T cells both in vitro and in vivo. Blocking the Tim-3/gal-9 pathway with Tim-3-Ig resulted in significant augmentation of GVHD lethality. Importantly, to our knowledge, we have shown for the first time that depletion of Tregs in the absence of Tim-3 resulted in the amelioration of GVHD lethality. Tim-3−/− donor T cells that were CD25-depleted resulted in decreased gut GVHD as evidenced by increased mucosal integrity (decreased FITC-dextran serum levels) as well as decreased effector cytokine production by donor T cells. We found that this paradoxical result was caused by the increased levels of IFN-γ causing AICD of the donor T cells. Blocking IFN-γ eliminated lethality differences between WT and Tim-3−/− CD25-depleted donor T-cell infusion, suggesting that IFN-γ levels are critical for regulating GVHD.
Previously, we showed that negative regulatory pathways play an important role in regulating GVHD.23-25 This study examined the role of a novel negative regulator Tim-3 and its expression and role in regulating acute GVHD. Infused donor T cells rapidly up-regulated Tim-3 and caused striking up-regulation of the ligand gal-9 on nonhematopoietic, stromal cells. Gal-9 is up-regulated by IFN-γ, which is increased during acute GVHD.8,26 Tim-3 ligand is detected on CD4+ and to a much lesser extent CD11c+ cells by Tim-3-Ig fusion protein staining.12,13 However, a recent finding showed that a monoclonal α-Gal9 antibody did not stain human resting CD4+ cells.27 It is possible that the Tim-3L protein used in previous studies was binding to alternative Tim-3 ligands other than gal-9, as was discussed by Cao et al.28,29
In the absence of Tim-3, donor T cells were able to proliferate more rapidly. These data are consistent with other studies involving a Th1 response and showed that effector Th1 cells express Tim-3 and that proliferation was increased when the Tim-3 pathway was blocked with Tim-3-Ig.12,13 The Tim-3-Ig fusion protein was used to show that mice were more susceptible to autoimmune diseases such as experimental autoimmune encephalomyelitis and diabetes. To evaluate the effect of Tim-3 on GVHD, we first used the Tim-3-Ig to block the pathway. As expected, Tim-3 pathway blockade with Tim-3-Ig resulted in significantly increased lethality compared with controls that received human IgG1. These data confirm and extend those of Oikawa et al who showed in a nonirradiated parent-into-F1 model of a graft-versus-host response that Tim-3 expression was up-regulated on CD8+ T cells, albeit not CD4+ T cells, and a blocking anti–Tim-3 mAb caused weight loss and increased the frequency of both CD4+ and CD8+ T cells expressing IFN-γ.6 As further evidence for the fact that the Tim-3-Ig fusion protein was blocking the Tim-3/gal-9 pathway, we also used Tim-3−/− donor T cells to inhibit the pathway. Compared with the Tim-3-Ig, the GVHD lethality observed in recipients of Tim-3−/− donor T cells was more modestly augmented. This could be because of the binding of Tim-3-Ig to receptors other than gal-928,29 or compensatory suppressive mechanisms that have overlapping functions with Tim-3. With respect to the latter, Tim-3−/− mice do not develop spontaneous autoimmunity, even though it has been shown that Tim-3−/− mice are more susceptible to autoimmune disease induction.12
Our data are consistent with reduced Tim-3+ Teff deletion by gal-9+ cells because of Tim-3-Ig blockade and provide in vivo evidence that the Tim-3/gal-9 pathway is an important negative regulator of GVHD. As predicted from this hypothesis, gal-9 Tg versus WT recipients had significantly decreased GVHD lethality, which should help eliminate harmful Tim-3+ Teffs. Systemic administration of recombinant galectin-9 (rgal-9) has been shown to improve allogeneic cardiac as well as skin grafts.30,31 Increased allograft survival was consistent with deletion of CD4+Tim-3+ Th1 cells. Because of these promising findings, it may be possible to develop the use of reagents, such as rgal-9, for use in the clinic.
Our most surprising finding was the amelioration of GVHD lethality when Tim-3/gal-9 binding was inhibited in the absence of Tregs. Tregs have a known inhibitory role in GVHD and Treg deletion of donor T-cell grafts typically leads to a more aggressive GVHD lethality response.21 Initially, we sought to determine whether inadequate Treg-mediated suppression in the context of Tim-3/gal-9 pathway blockade contributed to augmented GVHD lethality. Because Tregs can express gal-9, blockade of Tim-3/gal-9 binding may directly reduce the suppression of Tim-3+ T-cells by gal-9+ Tregs, thereby augmenting alloreactive Teff responses.19,32 Contradictory to our expectations, inhibiting Tim-3/gal-9 binding using Tim-3−/− donor T cells or Tim-3-Ig in the absence of donor Tregs did not augment but rather actually reduced GVHD lethality.
The gut is the site of the largest immune compartment in the body and has a very complex immunologic environment. A significant amount of morbidity and mortality from GVHD is a consequence of damage to the gut leading to increased intestinal permeability. This allows for endotoxin to cross the epithelial barrier invoking a cytokine storm leading to even more tissue damage. Studies have shown that an increased level of cytokine production in the gut, mainly by donor infiltrating T cells, can mediate acute GVHD.33,34 Conversely, it has been shown that inhibiting cytokines (IFN-γ or IL-12) can inhibit acute GVHD in murine models.35,36 A reduced inflammatory response may be a mechanism responsible for the reduced epithelial damage and slowed GVHD lethality that we observed in recipients of Treg depleted Tim-3−/− donor T cells. Our results show a lower percentage of both CD4+ and CD8+ donor T cells were secreting inflammatory cytokines (IFN-γ, IL-2, and IL-17) in the lamina propria of the colon. This reduction in inflammatory cytokines could mean a reduction of gut epithelial damage as supported by our finding of reduced serum concentration of orally administered FITC-dextran. We concluded that recipients of Treg-depleted Tim-3−/− donor T cells had a decreased GVHD lethality rate because of a reduction of inflammatory cytokine secretion in the gut and increased integrity of the epithelial barrier.
Previously, we have reported that high IFN-γ production results in AICD in CD4+ and to a lesser extent, CD8+ T cells.37 IFN-γ has antiproliferative properties and has been shown to be a potent inducer of negative regulatory pathways that can limit GVHD injury. Consistent with this hypothesis, it has been shown that the loss of IFNγ via the use of IFN-γ−/− donor T cells or neutralizing anti–IFN-γ mAb markedly accelerated GVHD lethality.38 We revealed increased IFN-γ levels in the spleen during the early phases of GVHD (day 7) in recipients of Treg-depleted Tim-3−/− versus WT donor T-cells. IFN-γ was shown to be required for AICD of T lymphocytes.39 This early burst of IFN-γ could be responsible for an increased level of AICD in donor T cells. Granzyme B is capable of mediating AICD of T cells.40 Recipients of Treg-depleted Tim-3−/− donor T cells had significantly increased granzyme-B secretion. Increased cell death was detected by measuring levels of annexin V/7-AAD expression as well as levels of activated caspase-3. This provides a mechanism for the gut protection we observed that led to a reduced GVHD lethality rate.
In summary, the Tim-3/gal-9 pathway can act as a suppressor of acute GVHD. Tim-3 is up-regulated in the early stages of GVHD on Teffs where it can regulate T-cell proliferation and affect GVHD lethality. We linked the inhibition of the Tim-3/gal-9 pathway in the absence of donor Tregs to critical IFN-γ levels capable of ameliorating GVHD. It may be possible to translate our findings that enhanced signaling of the Tim-3/gal-9 pathway early after transplantation to dampen the Th1 response and reduce GVHD in the presence of a T-cell replete graft using reagents such as rgal-9 protein.
The online version of the article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Beverly Norris for purification of the αTim-3 monoclonal antibody and Nick Bade and Kazutoshi Aoyama for technical assistance.
This work was supported in part by National Institutes of Health grants R01 AI34495, R01 HL56067, and P01 AI056299.
National Institutes of Health
Authorship
Contribution: R.G.V. designed and performed research, analyzed the data, and wrote the paper; P.A.T. performed experiments, provided advice, and edited the paper; R.F. performed experiments and edited the paper, Q.Z. performed experiments; A.P.-M. and M.H. provided data; M.H., D.L., A.C.A., T.B.S., and V.K.K. provided essential reagents or mice, advice, and edited the paper; and B.R.B. designed, organized, and supervised research and edited the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Bruce R. Blazar, Department of Pediatrics; 420 Delaware St SE, MMC 109; University of Minnesota, Minneapolis, MN 55455; e-mail: blaza001@umn.edu.

![Figure 2. Inhibiting Tim-3 increases allogeneic T-cell proliferation. (A) MLR was performed by mixing B6-purified T cells with irradiated BALB/c stimulators (1:1) and αTim-3. These cultures were pulsed with [3H]thymidine on the indicated days and harvested 16 hours later. Proliferation was determined as a measure of radioactive uptake. One-way ANOVA with Tukey multiple comparison test (P < .001, n = 5 per group). ■ indicates control IgG; and ○, αTim-3 5D12 mAb. (B) CFSE-labeled WT or Tim-3−/− BALB/c purified T cells (107) were transferred into lethally irradiated CD45.1+ congenic B6 recipients. Spleens were harvested and analyzed by flow cytometry for CFSE dilution and on days 3 and 4. Representative histograms shown are representative of 4 mice per group per day. Filled histogram, WT donor; open histogram, Tim-3−/− donor. Cells were gated on CD45.1+, H2Kd positive, CD4 or CD8 positive events.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/120/3/10.1182_blood-2011-10-387977/4/m_zh89991294180002.jpeg?Expires=1767751076&Signature=skO3X7gwoX8fv7VgRJSRX0OWf14bP4vWP85WKoZEyg1wpvwacgc0xRghBkI90t-48pfIR8DeznDVOKpH~429WoBduBa1kMw4H-t84DxQaKZjwWiZEcsPJMD5bXej~6vluXorGewEHTX57BKW5KGAdmMpzfIsqSI4633eU1Mb4OBeU0YkOjAGJi~peTwiL9ana5Isc0qZyhhDbIfEhUSjGWRvrcPKr5X9PawTImW6HQExIx5KpW9IwBsasV2p~DBXxIDdY7PiajVN0x18p52rNxBNuk7Rv6uvO5iY7h1veUTqBflVDcjUz-ZZOmwJiygmMmv0us9OOLhr~J3AY4QPqw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal