Abstract
Abstract  740
740
Graft-versus-host disease (GVHD) precludes an effective utilization of allogeneic hematopoietic stem cell transplantation (HSCT). Histone deacetylase inhibitors (HDACi) are an important class of anti-cancer agent and have recently been shown by us and others to attenuate experimental GVHD in multiple murine models. They suppress proinflammatory cytokine production, modulate antigen presenting cells (APCs), and enhance T regulatory cell (Treg) numbers and functions. We have translated our experimental observations from murine models in a first-in-human clinical trial to test the hypothesis that HDAC inhibition will be safe and reduce the severity of GVHD in patients undergoing matched related donor (MRD) reduced intensity conditioning (RIC) allogeneic HSCT.
From March 2009 through June 2012, we enrolled 45 patients at two centers, the University of Michigan and Washington University, in a phase I/II trial that combined the HDACi, vorinostat, with standard GVHD prophylaxis regimen consisting of tacrolimus and mycophenolate mofetil (MMF) and compared them with historical controls. The primary endpoint was the cumulative incidence of grade 2–4 acute GVHD (aGVHD) with a target risk of 25%, which would represent a statistically significant improvement over the 42% risk experienced by historical controls, assuming a type I error rate of 5%. The controls comprised of patients who underwent MRD RIC allogeneic HSCT and received standard GVHD prophylaxis with tacrolimus and MMF. Study eligibility included adult patients with a hematological malignancy for which RIC HSCT was considered appropriate and had an available 7/8 or 8/8-HLA-MRD. Specifically, patients with CLL and lymphoma must have been in CR, PR or had stable disease. Patients with myelodysplasia, acute leukemia or CML must have had < 20% blasts on the bone marrow examination. Study subjects received fludarabine 40 mg/m2 for 4 days, busulfan 3.2 mg/kg for 2 days, MMF for 29 days, and tacrolimus levels were maintained between 8–12 ng/mL through day (D) 56, and then tapered off by D180 (in the absence of GVHD). The investigational agent, vorinostat, was administered orally from D-10 to D100.
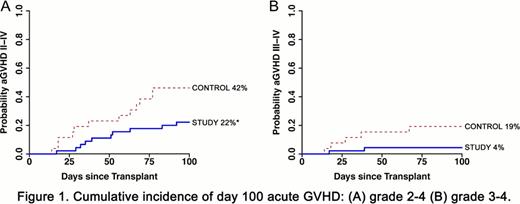
The median age of the study patients (vorinostat-treated) was 58 years (range, 43–69 years). There have been no cases of graft failure or excessive toxicity or death attributable to vorinostat. The median time to neutrophil and platelet engraftment was similar between study and controls (12 vs 11 days, respectively, for both groups). Bone marrow chimerism analyses for study patients showed the percent of myeloid (CD33+) engraftment to be 94%, 100% and 100%, and whole T cell (CD3+) engraftment to be 74.5%, 75%, and 100% at D30, D100 and D365, respectively. In the 45 study patients, the cumulative incidence of D100 grade 2–4 aGVHD was significantly lower compared to controls (22% vs 42%, respectively, P=0.04). Severe grade 3–4 aGVHD at D100 (4% vs 19% controls) and transplant-related mortality at 1 year (13% vs 19% controls) were also reduced. The incidence of aGVHD was globally diminished in all target organs, and all but one patient responded rapidly to standard steroid therapy. The incidence of relapse was similar between the study and controls (17% vs 20%, respectively). Infectious complications were not different between the groups. We performed several correlative analyses with patient samples to understand the putative mechanisms based on murine studies. Peripheral blood mononuclear cells (PBMCs) from study patients demonstrated enhanced histone (H3) acetylation at D30 compared with PMBCs from controls (P<0.03). Consistent with experimental data, there was greater acetylation of STAT-3 and increased percent and total numbers of CD4+CD25+CD127− Tregs in PBMCs in study patients compared to control or normal healthy patients (P=0.03 and P=0.04, respectively). Ex vivo stimulation of PBMCs from the study patients with TLR agonist (LPS) demonstrated reduced secretion of proinflammatory cytokines (TNF-α and IL-6) when compared with controls or healthy volunteers.
The data herein demonstrate translation of our experimental observations into a proof of concept first-in-human phase I/II clinical trial in prevention of GVHD. More importantly, the data suggest that administration of vorinostat in MRD RIC allogeneic HSCT may be a safe and effective strategy to reduce aGVHD.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract