Abstract
Abstract  741
741
We have previously shown that a costimulation blockade-containing regimen could provide effective protection against acute GvHD (aGvHD) in a non-human primate model. We therefore designed a first-in-disease trial of in vivo CD28:CD80/86-directed costimulation blockade with CTLA4-Ig (abatacept) to prevent aGvHD after unrelated-donor HSCT for patients > 12y. (Clinical Trials.Org #NCT01012492). In this trial, 10mg/kg abatacept was administered IV on day −1, +5, +14, +28 in addition to standard prophylaxis with cyclosporine + MTX. Enrollment of all patients is complete, and the study is evaluable for feasibility, toxicity, engraftment, and the primary immunologic outcome: the incidence of Grade III-IV aGvHD by d+100.
10 patients, with a median age of 44.5 y (17–74) were enrolled and treated. 6 patients received HLA-mismatched grafts (matched at 7/8 alleles) and 4 received 8/8 HLA-matched URD grafts. 8 received PBSCs and 2 received BM. All received high-intensity conditioning (Bu/Cy, TBI/Cy or Flu/Mel). With a median follow-up of 367 days (262–680), 7 patients are alive and in remission. 2 patients died of relapse (Day +121 and Day +147). One patient died, in remission, of multi-organ failure on day +453 post-transplant.
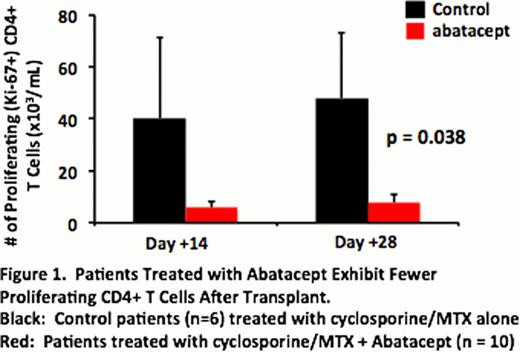
All 10 patients received all 4 scheduled abatacept doses, without infusion reactions. The average peak (230.9 +/− 7.4 mg/ml) and trough (45.9 +/− 2.8 mg/ml) abatacept levels, as well as the terminal T1/2 (19.6 +/− 1.9 days) were similar to that observed previously. Importantly, as has been previously established in vitro, patients receiving abatacept demonstrated significant inhibition of post-transplant CD4+ T cell proliferation (with >80% reduction in the accumulation of Ki-67+ proliferating CD4+ T cells at d+14 and +28 compared to a control cohort who received cyclosporine + methotrexate without abatacept, Figure 1). This data establishes, for the first time, the feasibility of giving abatacept to this new patient population, and that the PK and PD parameters in HSCT closely mirror those previously shown to effectively control immune-mediated diseases.
All patients achieved neutrophil engraftment (median d+16.5) and donor engraftment (100% CD33 chimerism at d+30). Lymphocyte recovery was rapid: Day +100 mean peripheral blood counts showed ALC = 1053 +/− 259 cells/ml, total T cells = 741 +/− 208 cells/ml, and CD8+ T cells = 381 +/− 99 cells/ml. The Day +100 CD4+ T cells = 285 +/− 105 cells/ml, not significantly different from historical controls (n = 43) that received CNI/MTX aGvHD prophylaxis without abatacept (262 +/−26 cells/ml).
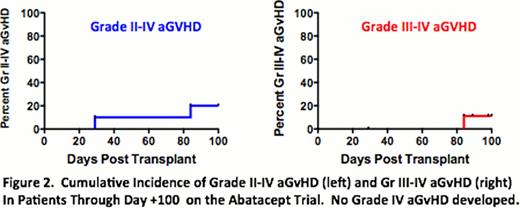
Patients receiving the abatacept-containing regimen had encouragingly low rates of early severe aGvHD: Two patients developed aGvHD before day +100, with one of these patients (Gr II) progressing to steroid-dependent cGvHD of the liver and one patient (Gr III) with complete resolution of aGvHD (and currently off steroids). The cumulative incidence of grade II-IV and III-IV aGvHD by day 100 was thus 20% and 10%, respectively (Figure 2). Importantly, there was no Gr IV aGvHD, no patient received salvage therapy for aGvHD, and there were no deaths from aGvHD. After day +100, one patient developed late acute GvHD (currently off all immunosuppression), and two patients developed overlap syndrome. Both had responsive disease and are currently either weaning or off corticosteroids. No other patient developed late aGvHD, and no other patient has developed moderate or severe cGvHD.
No life-threatening infections occurred. 5 patients developed CMV reactivation, all responsive to antivirals. No patient developed CMV disease. No EBV viremia >1000 copies/ml occurred. One patient developed EBV+ plasmacytic hyperplasia of the tongue, which resolved without intervention. No other EBV-related disease occurred.
This trial demonstrates, for the first time, the feasibility of adding in vivo T cell costimulation blockade with abatacept for aGvHD prevention. The decreased CD4+ T cell proliferation post-transplant and the encouragingly low rates of early, severe aGvHD suggest that costimulation blockade may be an effective agent for aGvHD prophylaxis and support the conduct of a larger, randomized phase 2 study.
Off Label Use: Abatacept was used in this trial. It is a costimulation blockade agent which was tested for its ability to prevent aGvHD.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal