Abstract
Using a uniform detection method for donor-specific anti-HLA antibodies (DSAs), we sought to determine the effect of preformed DSAs on outcomes in double umbilical cord blood transplantation. DSAs were associated with an increased incidence of graft failure (5.5% vs 18.2% vs 57.1% for none, single, or dual DSA positivity; P = .0001), prolongation of the time to neutrophil engraftment (21 vs 29 days for none vs any DSA; P = .04), and excess 100-day mortality or relapse (23.6% vs 36.4% vs 71.4% for none, single, or dual DSA positivity; P = .01). The intensity of DSA reactivity was correlated with graft failure (median of mean fluorescent intensity 17 650 vs 1 850; P = .039). There was inferior long-term progression-free and overall survival when comparing patients with DSAs against both umbilical cord blood units to those without DSAs (3-year progression-free survival, 0% vs 33.5%, P = .004; 3-year overall survival 0% vs 45.0%, P = .04). We conclude that identification of preformed DSAs in umbilical cord blood recipients should be performed and that the use of umbilical cord blood units where preformed host DSAs exist should be avoided.
Introduction
In North America, the use of double umbilical cord blood (UCB) transplantation has largely supplanted single UCB transplantation in adults because of a more reliable and shorter time to neutrophil engraftment. However, prediction of the dominantly engrafting UCB unit after double UCB transplantation is an inexact science. Factors such as total nucleated cell dose, CD34+ cell dose, HLA match, and order of UCB unit infusion have logically been associated with engraftment; however, none of these factors reliably predict the dominant engrafting unit.1-3
Anti-HLA antibodies may be observed in healthy individuals4,5 ; however, in patients with hematologic diseases, anti-HLA antibodies are more frequently detected because of the frequent use of transfusion therapy and alloimunization.6 In a small series, Gutman et al estimated the incidence of preformed anti-HLA antibodies to be 9% among individuals being considered for UCB transplantation.7 In solid organ transplantation, where crossing HLA barriers is routine, anti-HLA antibodies are linked to organ rejection and inferior outcomes.8,9 In hematopoietic stem cell transplantation, more limited data exist on the significance of these antibodies. Spellman et al, in a retrospective case-control study, demonstrated that the prevalence of donor-specific anti-HLA antibodies (DSAs) was higher in a group of mismatched unrelated donor recipients who suffered graft rejection than in a control group that engrafted.10 This analysis does not allow an accurate estimate of the impact of DSAs in individual patients because of the case-control design. In UCB transplantation, case reports have demonstrated that engraftment after single UCB transplantation can occur even in the presence of DSAs11,12 ; however, Takanashi et al have recently demonstrated a significant reduction in the cumulative incidence of neutrophil engraftment and inferior outcomes in the presence of DSAs after myeloablative single UCB transplantation.13,14
By examining all double UCB transplants at our institution, using a single standardized methodology for the detection of DSAs, we sought to determine whether the presence of DSAs had a detrimental effect on outcomes after double UCB transplantation.
Methods
UCB transplantation
All subjects underwent transplantation using sequentially administered double UCB units after either myeloablative or reduced intensity conditioning. Myeloablative conditioning consisted of fludarabine, cyclophosphamide, and total body irradiation, whereas reduced intensity conditioning consisted of fludarabine, melphalan, and antithymocyte globulin (ATG). GVHD prophylaxis was with cyclosporine and mycophenolate mofetil or tacrolimus and sirolimus. UCB products were administered between 1 and 6 hours apart. The unit with the higher total nucleated cell (TNC) count was administered first. All patients have been reported previously.15,16
UCB units had a minimum combined precryopreservation cell dose of 3.7 × 107 TNCs/kg, and each individual unit was required to have a minimum of 1.5 × 107 TNCs/kg before cryopreservation. UCB units were required to be a 4/6 match or better at the allele level for HLA-A, -B, and -DRβ1 with each other and with the recipient. The choice of UCB units, when multiple units were available, was hierarchically based on a higher cell dose, greater HLA compatibility, and a younger age of the cord blood unit. The presence of DSAs was not routinely considered in cord blood selection; however, DSA status might have been known to the treating physician.
Solid phase screening methodology to detect anti-HLA antibodies
All analyses were performed on cryopreserved pretransplantation patient serum that was examined for the presence of anti-HLA antibodies against HLA-A, -B, C, -DR, and -DQ. Cord units were not HLA typed at HLA-DP; therefore, this antigen was not considered. For general screening for anti-HLA antibodies, LABScreen Mixed microbeads (One Lambda) coated with purified class I or class II HLA antigens were used. We incubated 5 μL of microbeads with 20 μL of pretransplantation patient serum for 30 minutes, and then we washed the beads 8 times. Next, we added 100 μL of anti–human phycoerythrin to each well. After 30 minutes and 3 washes, the assay was run on a Luminex100 IS System instrument (Luminex) to detect fluorescent-tagged binding of human IgG. To determine the presence or absence of class I and II antibodies, results used the normalized ratio calculated by HLA Visual software (One Lambda). Positive screening samples were subsequently tested for anti-HLA antibodies against individual class I and II antigens. Assays with 1000 mean fluorescent intensity (MFI) above baseline were considered positive for defining the presence of anti-HLA antibodies. Anti-HLA antibodies were only considered for analysis if they were directed against class I or II HLA specificities found in the transplanted cord blood units.
Statistical analysis
Patient baseline characteristics and UCB unit characteristics were reported descriptively. Fisher exact test or Wilcoxon rank sum test were used for 2-group comparisons. The Cochran-Armitage test was used to test a trend in proportions of events (graft failure, early death, or relapse) for multiple group comparisons. Neutrophil engraftment was defined as the first of 3 consecutive days with neutrophil recovery to at least 0.5 × 109cells/L with UCB hematopoiesis measured by short-tandem repeat chimerism assessment. Platelet engraftment was defined as the first day of a platelet count of 20 × 109/L, without supporting transfusion in the prior 3 days. Graft failure was defined as the absence of neutrophil engraftment by day 42 from double UCB transplantation or loss of UCB chimerism by day 100 without malignant relapse. Time to engraftment was calculated reflecting death or relapse without engraftment as a competing risk. Malignancy relapse risk was defined by standard Center for International Blood and Marrow Transplant Research criteria.
Cumulative incidence curves for acute and chronic GVHD were constructed reflecting death or relapse as competing risks. Cumulative incidence curves for nonrelapse mortality (NRM) and relapse with or without death were constructed reflecting time to relapse and time to NRM as competing risks. Time to relapse and time to NRM were measured from the date of stem cell infusion. Overall survival (OS) was defined as the time from transplantation to death from any cause, whereas progression-free survival (PFS) was defined as the time from transplantation to progression or death from any cause. Surviving patients were censored at their date of last known follow-up. The log-rank test was used for comparisons of Kaplan-Meier curves; a Gray test17 was used for comparisons of cumulative incidence curves. Potential prognostic factors for OS, PFS, relapse, NRM, and engraftment were examined in proportional hazards models as well as in competing risks regression models.18 Because of the small number of graft failures, potential prognostic factors for graft failure were examined using univariable exact logistic regression analysis. To explore whether DSA intensity, measured as MFI, predicted graft failure, an analysis of receiver operator characteristics was performed. All P values are 2-sided. Significance was defined at the P = .05 level. All analyses were performed using SAS 9.2 (SAS Institute) and R 2.10.1 (R Foundation for Statistical Computing).
Results
We analyzed 73 patients who underwent double UCB transplantation between 2004 and 2008. DSAs were detected in 18 patients: 9 patients had DSAs directed against the first infused UCB unit, 2 patients had DSAs directed against the second infused UCB unit, and 7 patients had DSAs directed against both UCB units. Four patients had multiple DSAs directed against cord blood units. Baseline characteristics of these patients are shown in Table 1, UCB graft characteristics are shown in Table 2, and the specificity and intensity of the DSAs with engraftment outcomes are shown in Table 3. During the time period examined, an additional 26 cord blood transplants were performed. Reasons for not being included in this analysis include second transplantation (7), single UCB transplantation (1), death before stem cell administration (1), and lack of banked serum (17). Of the 17 patients with no samples for analysis, 14 patients successfully engrafted, whereas 3 patients expired before day 30 without engraftment.
Graft failure
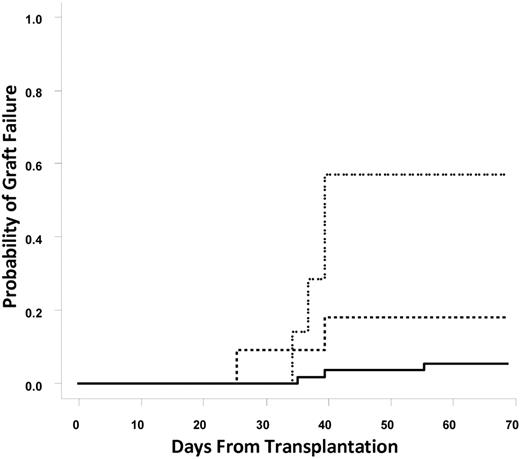
In total, 9 patients (12.3%) suffered from graft failure. The incidence of graft failure in patients without DSAs, with DSAs directed against a single UCB unit (n = 11), or with DSAs directed against both UCB units (n = 7) was 5.5%, 18.2%, and 57.1%, respectively (Cochran-Armitage trend test, P = .0001; Figure 1). Individual odds ratios (ORs) for graft failure comparing patients with DSAs against one UCB unit, DSAs against one or both UCB units, or DSAs against both UCB units to patients without DSAs were 3.85 (95% confidence interval [CI], 0.56%-26.4%; P = .19), 8.67 (95% CI, 1.89%-39.68%; P = .0055), and 23.1 (95% CI, 3.5%-153.9%; P = .002), respectively. These statistical associations were maintained when patients who underwent reduced intensity conditioning (n = 53) were examined. The incidence of graft failure in reduced intensity patients without DSAs (n = 40), with DSAs directed against a single UCB unit (n = 7), or with DSAs directed against both UCB units (n = 6) was 2.5%, 14%, and 67%, respectively (Cochran-Armitage trend test, P < .001). Overall, 6 of 18 patients with any DSAs experienced graft failure. It is notable that 3 of 4 patients with DSAs directed against multiple HLA antigens on a single cord blood unit experienced graft failure.
Cumulative incidence of graft failure. The solid line represents no DSAs, the dashed line represents DSAs against one unit, and the dotted line represents DSAs against both units.
Cumulative incidence of graft failure. The solid line represents no DSAs, the dashed line represents DSAs against one unit, and the dotted line represents DSAs against both units.
Also notable is that among the 11 patients with a single DSA (9 against unit 1, 2 against unit 2), 3 failed to engraft and an additional patient died by day 100, leaving 7 patients for evaluation for day 100 chimerism and engraftment analysis. Of these 7 patients, 2 engrafted with the unit against which there was only a low level of DSAs (MFIs 1100 and 1200), 3 engrafted with the unit without DSAs (MFIs against the nonengrafting unit, 5500, 7500, and 18 000), and 2 had prolonged (> 1 year) persistence of both units (intermediate MFIs, 1800 and 2300). Only 1 subject with DSAs against both units had persistence of a graft at day 100. The MFI in this case was 1200 and was directed against a common HLA-B antigen shared by both cord units.
Univariable exact logistic regression analysis identified only the presence of a DSA as a significant predictor of graft failure (exact OR, 8.32; 95% CI, 1.53%-58.88%; P = .01). Nonsignificant factors tested in this model included recipient age and sex, donor-recipient sex mismatch, malignancy risk status, myeloid versus lymphoid malignancy, prior stem cell transplantation, the use of sirolimus as GVHD prophylaxis, and CD34+ and TNC doses infused. Multivariable regression analysis could not be performed because of the small number of events.
The intensity of DSAs measured by MFI correlated with the occurrence of graft failure. The median MFI among 12 DSA patients without graft failure was 1850, whereas it was 17 650 in the 6 DSA patients who experienced graft failure (P = .039). Five of 6 DSA patients with MFI values greater than 7000 experienced graft failure, compared with 1 of 12 patients with values below 7000 (positive predictive value, 83%; sensitivity, 83%; area under the curve, 0.81).
Time to engraftment
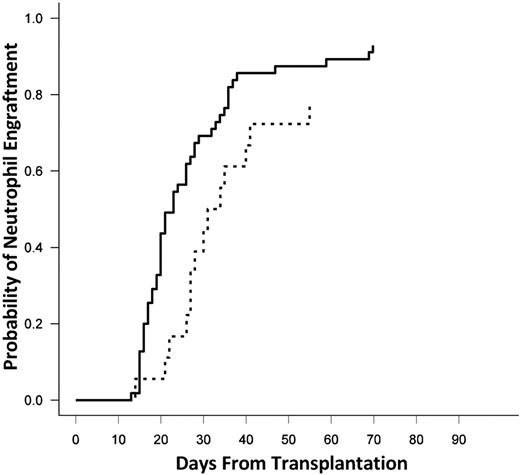
The median time to engraftment of neutrophils was 23 days (range, 13-70 days) in the entire cohort. When stratified by the presence or absence of DSAs, the median time to neutrophil engraftment was 21 days without DSAs and 29 days in the presence of a DSA (P = .04; Figure 2). There was no difference in the time to engraftment in patients with DSAs against a single versus both UCB units (30 vs 27 days; P = NS).
Cumulative incidence of neutrophil engraftment. The solid line represents no DSAs, and the dashed line represents DSAs against one or both units.
Cumulative incidence of neutrophil engraftment. The solid line represents no DSAs, and the dashed line represents DSAs against one or both units.
The median time to an unsupported platelet count was 42 days for the entire cohort. In patients with any DSAs, the median time to platelet engraftment was 50 days, compared with 42 days in patients without DSAs (P = 1.0). In patients without DSAs, the cumulative incidence of platelet engraftment by day 180 was 75%. The corresponding figures for patients with DSAs against one or both UCB units were 64% and 29%, respectively (P = .23); however, it is worth noting that the majority of patients with DSAs died early after transplantation.
In multivariable competing risk regression analyses examining the impact of DSAs on the cumulative incidence of neutrophil engraftment, the presence of any DSA (P = .01) or DSAs against both UCB units (P = .015) was associated with a reduction in the cumulative incidence of engraftment, whereas factors such as age, donor-recipient sex mismatch, conditioning intensity, year of transplant, malignancy risk, or myeloid versus lymphoid malignancy were not statistically associated with engraftment. When either CD34+ dose or TNC dose was included in the models, the presence of any DSA retained statistical significance (P = .019 and .013, respectively). When CD34+ dose was included in the models examining the impact of DSAs against both UCB units, there was a trend associating DSAs and engraftment (P = .053), whereas the association remained statistically significant when TNCs were included in the model (P = .016). Neither CD34+ nor TNC dose independently attained statistical significance in any of the models.
GVHD, early mortality, and long-term survival
The cumulative incidence of grade II to IV acute GVHD for the entire cohort was 17.8%. However, none of the patients with DSAs developed acute GVHD, whereas the cumulative incidence of acute GVHD in the group without DSAs was 23.6% (P = .025). This result is related to the small number of patients in the DSA group who were at risk of acute GVHD, because 14 of these 18 patients either relapsed or died within 100 days of transplant. No differences in the rates of chronic GVHD were noted in patients surviving beyond day 100 (23.3% overall).
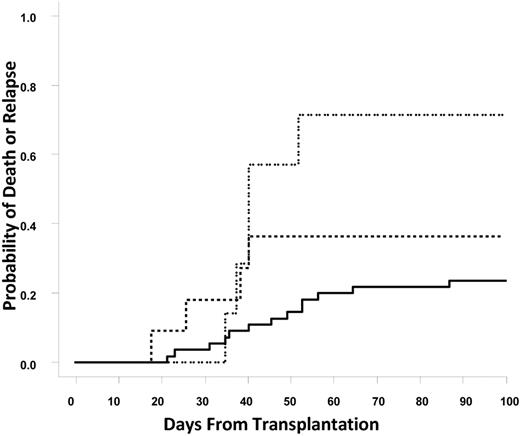
Similar to graft failure, death or relapse within 100 days of transplantation was correlated with the presence of DSAs. The rates of death or relapse within 100 days for the group of patients without DSAs, with DSAs against a single UCB unit, or DSAs against both UCB units were 23.6%, 36.4%, and 71.4%, respectively (Cochran-Armitage trend test, P = .01; Figure 3). Relapse with or without death within 100 days of transplantation was significantly associated with DSA status (14.5%, 18.2%, and 57.1%; Cochran-Armitage trend test P = .017); however, nonrelapse mortality within 100 days of transplantation was not significantly different between no DSA and DSA patients (9% vs 17%; P = NS). Causes of death among patients with DSA included sepsis or infection (4), relapse (3), veno-occlusive disease (1), GVHD (1), and other (1).
Cumulative incidence of early death or relapse. The solid line represents no DSAs, the dashed line represents DSAs against one unit; and the dotted line represents DSAs against both units.
Cumulative incidence of early death or relapse. The solid line represents no DSAs, the dashed line represents DSAs against one unit; and the dotted line represents DSAs against both units.
Univariable exact logistic regression analysis identified only the presence of DSAs against both UCB units as a significant predictor of early death or relapse (exact OR, 6.98; 95% CI, 1.03%-79.69%; P = .046). The presence of DSA against a single UCB unit approached statistical significance (exact OR, 3.17; 95% CI, 0.91%-11.31%; P = .07). Nonsignificant factors in this model included recipient age and sex, donor-recipient sex mismatch, malignancy risk status, myeloid versus lymphoid malignancy, prior stem cell transplantation, the use of sirolimus as GVHD prophylaxis, and the CD34+ and TNC doses infused. In multivariable exact logistic regression analysis, the presence of DSAs against both UCB units retained statistical significance for early death or relapse (P = .03). In a parallel multivariable model, there was a trend associating the presence of DSAs against a single UCB unit and early death or relapse (P = .08). When TNC or CD34+ dose was added to these models, the presence of DSAs against both UCB units retained statistical significance (P = .012 when TNC was added, P = .021 when CD34+ was added). When either TNC or CD34+ cell count was added to the model examining the influence of only a single DSA, the presence of DSA was predictive of early death or relapse (P = .039 for TNC, P = .029 for CD34+). CD34+ cell count or TNC was not independently predictive of early death or relapse in any model construct.
The PFS and OS at 3 years from transplantation was 30% (95% CI, 19.5%-41.4%) and 43.3% (30.6%-55.4%) for the entire cohort, with a median follow-up among survivors of 33.8 months. Three-year PFS and OS were nonsignificantly shorter in patients with any DSA compared with those without DSAs (PFS: 18.5% vs 33.5%, P = .14; OS: 40.0% vs 44.8%, P = .81). However, when comparing patients with DSAs directed against both UCB units with those without any DSA, both PFS and OS were significantly shorter (3-year PFS: 0% vs 33.5%, P = .004; 3-year OS: 0% vs 45.0%, P = .04; Figure 4A-B). When examined in a Cox proportional hazards model, the presence of DSAs against both UCB units and malignancy risk status were both significantly correlated with PFS and OS (P < .05 for all outcomes). The presence of a single DSA itself did not attain statistical significance, whereas malignancy risk was correlated with both PFS (P = .05) and OS (P = .06) in a parallel model.
Progression-free and overall survival. (A) PFS. (B) OS. The solid line represents no DSAs, and the dashed line represents DSAs against both units.
Progression-free and overall survival. (A) PFS. (B) OS. The solid line represents no DSAs, and the dashed line represents DSAs against both units.
Discussion
In this retrospective analysis, we have demonstrated that the presence of preformed DSAs directed at transplanted UCB units can have important clinical consequences. In patients with preformed DSAs against transplanted UCB units, there is an increased incidence of graft failure, a delay in the time to neutrophil and platelet engraftment, excess early mortality, and decreased long-term OS. These findings demonstrate the necessity to screen for DSAs before the selection of UCB units for eventual transplantation.
The mechanism of DSA-mediated cord blood rejection is unknown. In solid organ transplantation, the presence of DSAs (either against HLA or other antigens) leads to the deposition of terminal complement factors in the transplanted tissue and is associated with chronic allograft injury and dysfunction.19,20 The specific role of T cells in this process is unknown; however, in allogeneic stem cell graft rejection, it is likely that T cells play an important role. If T cells are involved, then determining the origin of these T cells is of critical importance.
It is difficult to elucidate the individual roles of residual host and donor T cells in the rejection process, because processes such as in vivo T-cell depletion, associated with increased rates of graft failure, simultaneously depletes both host and donor T cells. Ex vivo T-cell depletion, which removes donor T cells only, also is associated with increased rates of graft failure, arguing against the role of donor T cells in graft failure. Although the number of T cells delivered with the graft is low, it is clear that these T cells are immunologically active. Recently, Gutman et al21 have identified a UCB versus UCB CD8+ T-cell response that predicted UCB dominance after double UCB transplantation in 9 of 10 patients examined. Whether preformed DSAs primed the T-cell response in these cases is unknown. In addition, other cellular mediators, such as NK cells, may be involved. One factor relevant to our analysis is that > 70% of our patients underwent reduced intensity conditioning, which includes ATG, and functionally depletes both recipient and some amount of donor T cells. Because the majority of patients underwent reduced intensity conditioning transplantation, the influence of ATG use could not be tested in the multivariable models. In addition, because the presence of DSAs may have been known to the treating physician, this could have influenced unit selection, and the overall incidence of graft failure.
If preformed DSAs are pathogenic, then a strategy to circumvent the ill effects of these antibodies should be sought. Unfortunately, strategies to reduce the production of antibody, either through depletion of B cells (with monoclonal antibodies such as rituximab) or depletion of plasma cells (with proteosome inhibitors such as bortezomib), are unlikely to be successful unless the continued production of DSAs is implicated in the graft rejection process. If complement-mediated pathways are involved in stem cell graft rejection, then strategies that include the use of the terminal complement inhibitor eculizumab could be useful; however, evidence supporting the role of complement activation in stem cell graft rejection is lacking. In solid organ transplantation, plasmapheresis or plasma exchange is used frequently to reduce the titer of anti-donor antibodies, although the titer threshold in stem cell transplantation is unknown, as is the effectiveness of this procedure.
One interesting observation in this study was that no patient with DSAs developed acute GVHD, whereas 23.6% of patients without DSAs had grade II to IV acute GVHD. The incidence of acute GVHD in double UCB transplantation is between 40% and 60%,22,23 whereas it is much lower in recipients of single UCB transplantation.24,25 One hypothesis linking our findings to the disparities in GVHD incidence is that patients with DSAs more frequently had hematopoiesis derived solely from one of the 2 transplanted UCB units. This theory also would explain the delay in the time to neutrophil engraftment noted in the presence of DSAs in our study, because single UCB transplantation is associated with longer time to engraftment compared with double UCB transplantation. An alternative hypothesis is that some, but not all, of the stem cell progenitors are eliminated by DSAs, leading to delayed but dual engraftment of both transplanted UCB units. Even though our sample size was too small for statistical testing, it was interesting to note that among patients with DSAs against only unit, those with the highest DSA titers had engraftment by the other unit, whereas patients with the lowest titers had engraftment with affected unit, suggesting that the intensity of the DSA is relevant.
Why there was an excess of graft failure in patients with DSAs against only one UCB unit also remains an unanswered question. If host-derived T cells are important, then perhaps spreading of immune responses against other mismatched HLA antigens or non-HLA antigens, under the influence of other HLA factors is possible.26 It is also possible that DSAs were transferred with the other UCB unit,27 leading to a priming of donor-derived of cotransplanted T cells. Finally, it is feasible that the cotransplanted unit without DSAs simply was insufficient alone to sustain long-term hematopoiesis.
Until recently, in vitro crossmatching was used to determine compatibility between donors and recipients. The relationship between a positive crossmatch and graft rejection in allogeneic transplantation is well established.28 Gutman et al have demonstrated that there is a relationship between the presence of preformed DSAs and a positive crossmatch.7 With this in mind, they altered their UCB search strategy to avoid the use of these units and therefore were unable to correlate the presence of DSAs and clinical graft failure. However, in contrast to their study, we used a much lower threshold for the detection of DSAs (1000 vs 4000 MFI), and we demonstrate that even low-titer antibodies are relevant in this setting for the prediction of graft failure. When examining an even lower threshold for the detection of DSAs, Brunstein et al29 were recently unable to detect a statistical difference in the rate of graft failure, despite a small difference in the rate of engraftment between those with DSAs and those without (78% vs 86%; P = NS). This is consistent with our results that demonstrate that a higher MFI was associated with an increased rate of failure to engraft. Although our cutoff value of 1000 MFI can be used for future studies, further research in a larger sample size is required to determine the optimal cutoff value for cord blood selection.
Previous research in unrelated donor transplantation10 and in single-unit UCB transplantation13,14 has demonstrated a correlation between the presence of DSAs and graft rejection. This analysis demonstrates a correlation between the presence of DSAs and graft rejection, as well as other major transplantation outcomes, in double UCB transplantation. This has significant impact on search strategies for compatible UCB units for transplantation. Several factors must be considered when searching for the optimal unit,30 and we feel that the presence of DSAs should be an important factor in the selection algorithm. Units against which there are very intense antibody responses should be particularly avoided. Given the adverse effect on survival, the use of 2 UCB units with preformed DSAs in the recipient should be avoided as well.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This study was supported in part by the Patrick Carney Foundation. C.C. is supported by the Stem Cell Cyclists of the Pan-Mass Challenge.
Authorship
Contribution: C.C. designed the research, analyzed the data, contributed to patient care, wrote the manuscript, and reviewed the manuscript; H.T.K. analyzed the data and wrote and reviewed the manuscript; L.S. analyzed the data and reviewed the manuscript; D.S. performed the research and reviewed the manuscript; B.G. contributed to patient care and reviewed the manuscript; P.A. contributed to patient care and reviewed the manuscript; J.K. contributed to patient care and reviewed the manuscript; V.H. contributed to patient care and reviewed the manuscript; E.A. contributed to patient care and reviewed the manuscript; K.B. reviewed the manuscript; J.R. performed the research and reviewed the manuscript; R.J.S. contributed to patient care and reviewed the manuscript; E.M. performed the research and reviewed the manuscript; and J.H.A. designed the research and reviewed the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Corey Cutler, Dana-Farber Cancer Institute, 450 Brookline Ave, Boston, MA 02115; e-mail: corey_cutler@dfci.harvard.edu.