In this issue of Blood, Cutler and colleagues present evidence that donor-specific anti-HLA antibodies are associated with graft failure in double umbilical cord blood transplantation (CBT).1 Engraftment of donor cells is the first important step in successful transplantation and, until recently, the causes of engraftment failure remained elusive.
Improvement in anti-HLA antibody detection using preparations of single HLA antigen allows precise antibody detection and quantitation, and has provided new insights in a significant fraction of graft rejection cases.2 The article by Cutler et al adds to the recently published data that show that anti-HLA antibodies directed against the mismatched HLA antigen of the donor (or donor-specific anti-HLA antibodies [DSAs]) have a deleterious effect on engraftment of donor cells in patients receiving HLA mismatch grafts.3-5
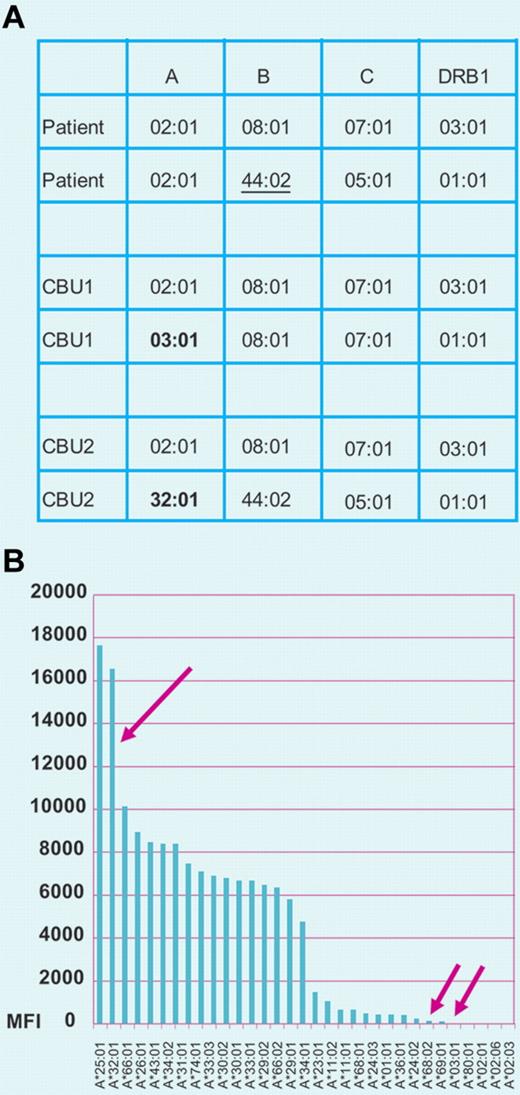
(A) HLA alleles of patient receiving a double cord blood transplantation and of the 2 CBU infused. Patient and CBU1 present 2 mismatches in HLA-A and HLA-B loci; the mismatches at these loci occur in the HvG (HLA-A*03:01) and GvH (B*44:02) vectors, respectively, because the patient and donor are homozygous at these loci. CBU2 and the patient present only one mismatch (only in the HvG vector; A*32:01). These units present single mismatches in HLA-A in the HvG vector (A*03:01 in CBU1 and A*32:01 CBU2). (B) Evaluation of anti-HLA antibodies in the patient's serum. The patient's serum shows strong reactivity against the antigen preparation of A*32:01 present in CBU 2 and shows negligible reactivity against A*03:01 present in CBU1 and the patient's self–HLA-A antigen A*02:01. These test results indicate that CBU2 is at high risk of rejection and CBU1 is likely to engraft. Professional illustration by Paulette Dennis.
(A) HLA alleles of patient receiving a double cord blood transplantation and of the 2 CBU infused. Patient and CBU1 present 2 mismatches in HLA-A and HLA-B loci; the mismatches at these loci occur in the HvG (HLA-A*03:01) and GvH (B*44:02) vectors, respectively, because the patient and donor are homozygous at these loci. CBU2 and the patient present only one mismatch (only in the HvG vector; A*32:01). These units present single mismatches in HLA-A in the HvG vector (A*03:01 in CBU1 and A*32:01 CBU2). (B) Evaluation of anti-HLA antibodies in the patient's serum. The patient's serum shows strong reactivity against the antigen preparation of A*32:01 present in CBU 2 and shows negligible reactivity against A*03:01 present in CBU1 and the patient's self–HLA-A antigen A*02:01. These test results indicate that CBU2 is at high risk of rejection and CBU1 is likely to engraft. Professional illustration by Paulette Dennis.
Solid phase assays allow the detection of the presence of HLA antibodies reactive with donor antigens. Panel A of the figure shows the HLA phenotypes of a patient and 2 cord blood units (CBUs); panel B shows results of a solid phase assay that identifies antibody reactivity against HLA antigens present in one of the infused units. Anti-HLA antibodies have been associated with graft failure in single-unit CBT.6 In contrast, in double cord transplantation a direct relationship between DSAs and graft rejection has been more difficult to demonstrate. In double cord transplants, although both grafts can initially be detected, only one of them achieves long-term engraftment and the engraftment rate is higher than that observed for transplants using the infusion of single unit.
The study conducted by Cutler and colleagues confirms a strong association between the presence of anti-HLA antibodies and graft failure. This study elegantly demonstrates a major effect of DSAs in double umbilical CBT, with an increase in day-100 treatment-related mortality, and inferior survival of patients receiving double umbilical CBT with DSAs against both units.1 DSAs remained signficiant in multivariate analysis even when cell doses were considered.1 Moreover, the median fluorescence intensity (MFI) of antibody levels was significantly higher in patients who experienced graft failure compared with those who did not.1 An intriguing aspect of double umbilical CBT was that DSAs directed against 2 or more mismatched antigens may pose a higher risk of rejection; in the study discussed here, although numbers were small, the authors note that 3 of 4 patients with DSAs against multiple HLA antigens on a single CBU experienced graft failure.1
While it is becoming widely accepted that anti-HLA antibodies should be routinely evaluated before transplant it remains unclear what antibody levels should be considered significant. In the present study, Cutler et al identified a level of 1000 MFI as associated with a higher risk of graft failure in double umbilical CBT; however, different levels could apply to different types of transplantation. In studies of different types of transplantation we have found a higher risk of graft failure in T cell–depleted haplo-identical stem cell transplantation (using CD34-selected grafts)3 and lower risk in matched unrelated donor transplantation. In the latter, in addition to hematopoietic stem cells, the graft contains other cells that express HLA class I antigens and has variable expression of class II HLA antigens. It could be postulated that these cells also bind and absorb anti-HLA antibodies thereby passively reducing the titer and decreasing the risk of stem cell rejection.6 Although this hypothesis is plausible, it is also possible that donor-derived T lymphocytes play an active role in the protection of the graft.
The mechanism by which graft failure occurs remains unclear. An important hypothesis advanced by Cutler and collaborators involves complex mediated cell lysis. A T cell–mediated mechanism is still possible but less likely and may not play a primary role. These factors prevent adhesion to the stem cell niche (figure panel B). Animal studies suggest that direct binding rather than primed T cells are the primary mechanism of graft rejection.7,8
The most important cause of allo-sensitization remains intrauterine exposure of the fetus to paternal HLA antigens. Accordingly, the problem is most common in multiparous women, while transfusion of blood products may also contribute to the problem.3,5,6 The frequency of allo-antibodies can be as high as 20% in patients being evaluated as candidates for allogeneic hematopoietic stem cell transplantation.6 Given the potentially catastrophic impact of HLA allo-sensitization against the donor, screening for the presence of anti-HLA antibodies before mismatched transplantations is warranted.
Going forward, prospective studies of treatment strategies for allo-sensitized patients is needed. In addition, selection of units or donors based on a recipient's anti-HLAantibody specificities is likely to minimize the risk of graft rejection.
Conflict-of-interest disclosure: The authors declare no competing financial interests. ■