Abstract
We designed a phase 1-2 study to evaluate the safety and the efficacy of increasing doses of bendamustine (160 mg/m2, 180 mg/m2, and 200 mg/m2 given on days −7 and −6) coupled with fixed doses of etoposide, cytarabine, and melphalan (BeEAM regimen) as the conditioning regimen to autologous stem cell transplantation for resistant/relapsed lymphoma patients. Forty-three patients (median age, 47 years) with non-Hodgkin (n = 28) or Hodgkin (n = 15) lymphoma were consecutively treated. Nine patients entered the phase 1 study; no patients experienced a dose-limiting toxicity. Thirty-four additional patients were then treated in the phase 2. A median number of 6 × 106 CD34+ cells/kg (range, 2.4-15.5) were reinfused. All patients engrafted, with a median time to absolute neutrophil count > 0.5 × 109/L of 10 days. The 100-day transplantation-related mortality was 0%. After a median follow-up of 18 months, 35 of 43 patients (81%) are in complete remission, whereas 6 of 43 relapsed and 2 of 43 did not respond. Disease type (non-Hodgkin lymphomas vs Hodgkin disease) and disease status at transplantation (chemosensitive vs chemoresistant) significantly influenced DFS (P = .01; P = .007). Remarkably, 4 of 43 (9%) patients achieved the first complete remission after receiving the high-dose therapy with autologous stem cell transplantation. In conclusion, the new BeEAM regimen is safe and effective for heavily pretreated lymphoma patients. The study was registered at European Medicines Agency (EudraCT number 2008-002736-15).
Introduction
Although advanced-stage Hodgkin disease (HD) and aggressive non-Hodgkin lymphomas (NHL) are considered chemotherapy-responsive tumors, many patients either relapse or never achieve a remission.1-6 The prognosis is generally poor when conventional chemoradiotherapy salvage regimens are used.1-6 However, high-dose therapy (HDT) followed by stem cell rescue has been shown to improve disease-free survival (DFS) compared with salvage chemotherapy alone in chemosensitive relapsed patients.7-12 High-dose chemotherapy followed by autologous stem cell transplantation (ASCT) may thus be considered the treatment of choice for relapsed/refractory lymphomas.7-12
Various conditioning regimens have been used as chemotherapy treatment before ASCT, with DFS and overall survival (OS) rates ranging from 34% to 60% and 26% to 46%, respectively.7-17 To date, few comparative randomized trials have been performed, and no regimen has demonstrated superiority to another. Furthermore, little is known regarding the comparative toxicity and efficacy of various HDT regimens applied in lymphomas.7-17 Advances in the conditioning regimens and supportive care have reduced transplantation-related mortality (TRM) to < 10%. However, the commonly used conditioning regimens have pros and cons, and new conditioning strategies are required and considered by several Investigators.
Bendamustine (Ribomustin/Levact; Mundipharma International Ltd, and Treanda; Cephalon) was first synthesized in the early 1960s in the former German Democratic Republic. It combines the alkylating activity of the mustard group with the antimetabolite activity of the purine analog structure. Data emerging from in vitro experiments presented convincing evidence that bendamustine has a very different pharmacologic profile from alkylating agents. These data provided clear evidence that activation of apoptotic pathways is a key element of the activity of bendamustine. Bendamustine is also very effective when apoptotic pathways were dysfunctional (eg, p53-independent cytotoxic effects) by causing a mitotic catastrophe in malignant cell lines and has activity in multiresistant cell lines that do not respond to treatment with other alkylating agents.18
These findings may be related to the unique chemical structure of bendamustine, and therefore the compound may be of particular clinical value for treating diseases refractory to alkylators and other compounds. Bendamustine was then studied in several entities of B-cell neoplasms and demonstrated significantly superior efficacy compared with standard therapies in the treatment of relapsed chronic lymphocytic leukemia (CLL) and indolent NHL.19-22 We therefore planned a phase 1-2 study to test the safety and the efficacy of increasing doses of bendamustine, coupled with fixed doses of etoposide, cytarabine, and melphalan (ie, BeEAM), in the conditioning regimen to ASCT for resistant/relapsed lymphoma patients.
Methods
Biologic studies
Carmustine (BCNU), melphalan, etoposide, and cytarabine were obtained from Sigma-Aldrich Inc, whereas bendamustine was provided by Mundipharma International Ltd. MEC-1 and JEKO-1 cell lines were purchased from the German Collection of Microorganisms and Cell Cultures (DSMZ). MEC-1 and JEKO-1 cells were cultured in RPMI-1640 medium containing 10% FBS, 2 × 10-3M glutamine, 100 units/mL penicillin, and 100 μg/mL streptomycin at 37°C in a 5% CO2/95% air, humidified atmosphere.
Cell viability of the combinations tested were studied with the use of the colorimetric MTT assay as described elsewhere.23 Results of the experiments are expressed as the percentage of cells surviving compared with the untreated control. The synergistic effect between the drug association was evaluated with the Calcusyn Version 2.0 software (Biosoft) based on Chou-Talalay method.24 This equation calculates a combination index (CI) that quantities the potency of the combination and indicates whether it is antagonistic, additive, or synergistic.
Patient population
Forty-three patients with resistant/relapsed NHL (n = 28) or HD (n = 15) lymphoma were consecutively enrolled in the study between August 2008 and June 2010. The characteristics of patients are listed on Table 1. All patients with NHL received at least one rituximab-containing regimen before salvage therapy. The study was conducted in accordance with the principles of the Declaration of Helsinki, Good Clinical Practice, and the current National Rules for conducting clinical studies. The protocol was approved by the Marche Nord Hospital Institutional Ethics Committee. All subjects provided written informed consent. The study was registered with the European Medicines Agency (EMA) with EudraCT number 2008-002736-15.
Conditioning regimen
Increasing doses of bendamustine coupled with fixed doses of etoposide, cytarabine, and melphalan were administered in an inpatient setting as a preparative regimen for ASCT. In the phase 1 study, 3 cohorts of 3 patients each were treated starting with bendamustine 160 mg/m2 given on days −7 and −6. The dose of bendamustine was then escalated according to the modified Fibonacci increment rule until the onset of severe adverse events and/or the attainment of the expected maximum tolerated dose but not > 200 mg/m2. The authors of previous studies have shown doses of 280 mg/m2 to have dose-limiting toxicities in solid tumors, which comprised 3 cases of cardiac toxicity grade 2 when given as one dose every 3 weeks.25 The dose-limiting toxicity after 2 days' administration was 180 mg/m2 per day when 2 of 3 patients experienced a grade 4 thrombocytopenia.26 Nonhematologic toxicity, including cardiac toxicity, was not dose-limiting with this schedule. Therefore, we considered safe a dose escalation of 20 mg/m2 up to a maximum dose of 200 mg/m2. Moreover, to exclude cardiac toxicity at the higher level of bendamustine, 3 additional patients received the maximum dose of 200 mg/m2.
Etoposide, cytarabine, and melphalan were administered at the conventional doses. All patients were therefore conditioned with the new BeEAM chemotherapy regimen consisting of bendamustine on days −7 and −6 (dose finding); cytarabine, 400 mg/m2 intravenously daily, from day −5 to day−2; etoposide, 200 mg/m2 intravenously daily, from day −5 to day −2; and melphalan, 140 mg/m2 intravenously on day −1 before reinfusion of autologous stem cells.
Supportive care and clinical monitoring
All patients received 5μ g/kg/d of filgrastim starting on day +6 from autograft. Patients were nursed in single or double rooms in reverse isolation and received antimicrobial prophylaxis that consisted of oral nystatin, fluconazole, and either ciprofloxacin 500 mg twice daily or levofloxacin 500 mg daily. Packed RBCs and platelet transfusions were administered to maintain a hemoglobin level > 8 g/dL and a platelet count > 10 × 109/L. Patients were treated with broad-spectrum antibiotics when fever developed, and the absolute neutrophil count was < 0.5 × 109/L. Liposomal amphotericin B at a dosage of 1 mg/kg/d (AmBisome; Gilead Sciences Ltd) was added if patients had persistent fever after 4-7 days of intravenous antimicrobial therapy. Patients underwent daily assessment of symptoms and physical examination during their hospital stay and weekly after discharge. Laboratory work-up was performed before transplantation, daily during the hospital stay, and weekly after discharge. Cotrimoxazole and acyclovir were administered to the patients for 3 months after ASCT to prevent Pneumocystis jirovecii and viral infections, respectively.
Response criteria
Responses were evaluated according to those reported elsewhere by Cheson et al.27 OS was measured from the diagnosis to last contact. All toxicities were defined by use of the National Cancer Institute's Common Terminology Criteria for Adverse Events Version 3.0 (2003). All patients were evaluated with both computed tomography (CT) and positron emission tomography (PET) scan before entering the study and before and after ASCT.
Statistical analysis
The primary end point of the study was to assess the 3-year event-free survival (EFS) of lymphoma patients submitted to BeEAM chemotherapy followed by the reinfusion of autologous hematopoietic stem cells. Secondary objectives were to assess the safety of BeEAM chemotherapy followed by the reinfusion of autologous hematopoietic stem cells, considered as the incidence and grade of adverse event (according to World Health Organization) and clinically significant abnormal laboratory values after reinfusion; to assess the percentage of patients entering complete remission (CR); and to assess OS of resistant/relapsed lymphoma patients submitted to BeEAM chemotherapy followed by the reinfusion of autologous hematopoietic stem cells as salvage treatment.
The phase 1 section of the study was designed according to the Fibonacci increment rule. The traditional escalation rule was applied. According to the traditional escalation rule, patients are treated in cohorts of 3 each receiving the same dose x(i). If none of the 3 patients shows a dose-limiting toxicity (DLT) at level x(i), the next cohort of 3 patients receives the next greater dose x(i + 1). Otherwise, a second cohort of 3 is treated at the same level x(i) again. If exactly 1 of the 6 patients treated at the x(i) exhibits a DLT, the trial continues at the next greater level x(i+1). If 2 or more patients of the 6 exhibits DLT at level x(i), the escalation stops at that level. When the escalation has stopped, another cohort of 3 patients is treated at the next lower level x(i − 1), if 6 patients had not already been treated.
The phase 2 of the study was designed according to the Fleming method. The primary outcome was the 3-year EFS rate. Fixing the lowest acceptable rate as 40% and the successful rate as 60%, with a significance level α = 0.05 and a power 1 − β = 0.80, the sample size was estimated to be 34 patients.
Statistical analysis was performed according to a per-protocol approach. OS and EFS were estimated according to Kaplan-Meier method. The log-rank test was used to assess the significance of differences for each prognostic factor in the univariate analysis. The Cox proportional hazards regression model and the logistic regression models were used to assess how patients' characteristics predict EFS and OS. Data were analyzed with the Statistical Package for Social Science (SPSS 13.0). Two-sided test was used in all calculations. Significance level was fixed at α = 0.05 for all the statistical analyses.
Results
In vitro experiments on cell lines
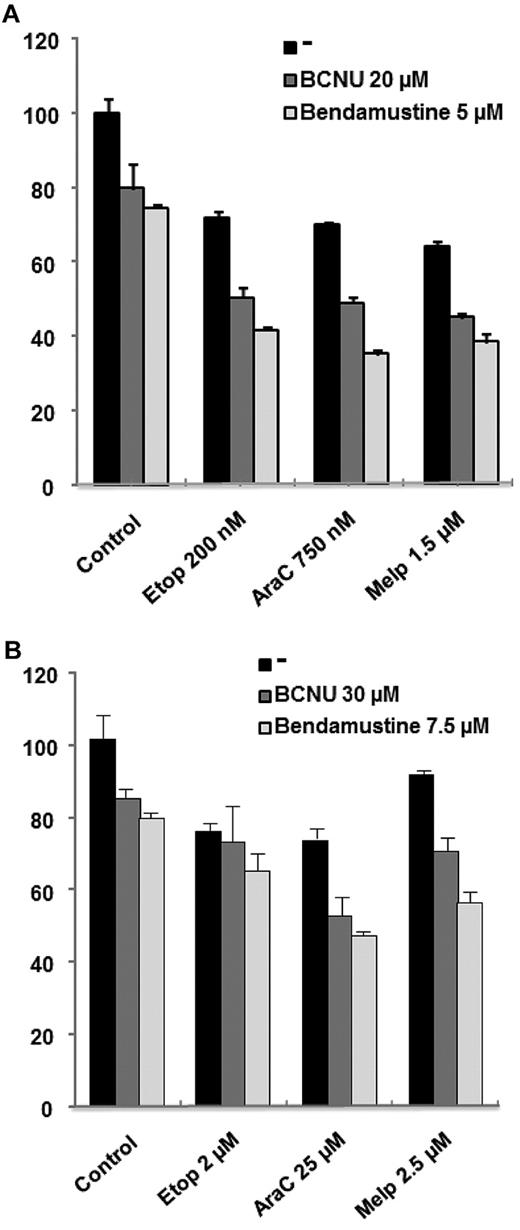
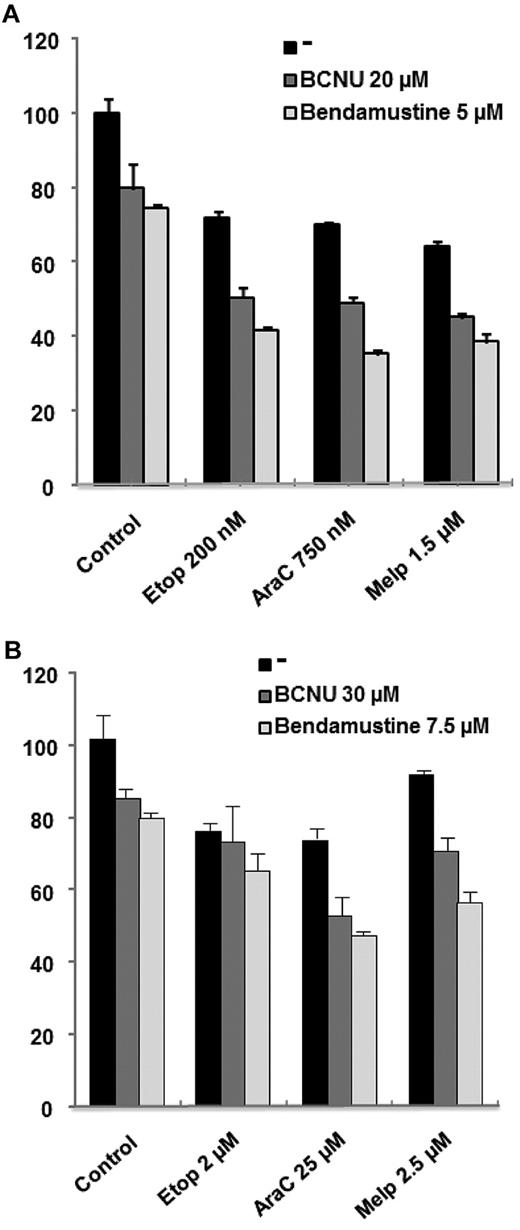
We first analyzed the ability of bendamustine to potentiate the in vitro activity of melphalan, etoposide, and cytarabine (ie, ARAC) in cell lines corresponding to a transformed CLL (MEC-1) and to a mantle cell lymphoma (JEKO1) cell line, and then compared it with that of BCNU. Viability was analyzed by MTT assay after 48 hours of culture with the drugs at the indicated doses. As can be observed in Figure 1, both alkylating agents (BCNU and bendamustine) were able to potentiate the activity of cytarabine and melphalan in both cell lines. Nevertheless, when evaluated with Calcusyn Version 2.0 software, this effect was more potent for the combinations, including bendamustine (CI in the synergistic range) compared with BCNU (CI in the additive range for melphalan and in the moderately synergistic range for cytarabine). Regarding etoposide, there was a clear potentiation for both alkylators in the cell line derived from a mantle cell lymphoma, whereas this potentiation was milder in MEC-1. This finding indicates that both alkylators potentiate the activity of these drugs in both cell lines but bendamustine would seem to be a better partner for these drugs.
Efficacy of bendamustine compared to carmustine (BCNV) on lymphoma cell lines when coupled with etoposide, cytarabine or melphalan. MTT assay showing the synergistic effect of bendamustine or BCNU with etoposide (Etop), cytarabine (AraC), and melphalan (Melp) in JEKO-1 (A) and MEC-1 (B) cells after 48 hours of culture.
Efficacy of bendamustine compared to carmustine (BCNV) on lymphoma cell lines when coupled with etoposide, cytarabine or melphalan. MTT assay showing the synergistic effect of bendamustine or BCNU with etoposide (Etop), cytarabine (AraC), and melphalan (Melp) in JEKO-1 (A) and MEC-1 (B) cells after 48 hours of culture.
Engraftment
The characteristics of the engraftment are listed on Table 2. In brief, 2 patients received BM as the source of stem cells, whereas 41 patients received peripheral blood stem cells. All patients, either undergoing transplantation with BM or with peripheral blood stem cells, fully engrafted after a median time of 10 days (range, 8-12 days). Median times to platelet count (PLT) > 20 × 109/L and PLT > 50 × 109/L were 13 days (range, 8-39 days) and 16 days (range, 11-51 days), respectively. The median number of days on G-CSF was 8 days (range, 8-13 days). These data are superimposable to those previously reported with the BEAM regimen.9,14,17
Nonhematologic toxicity
Nonhematologic toxicity, including cardiac toxicity, was not dose-limiting at any bendamustine dose with this schedule. Nineteen patients experienced a mild increase in aspartate aminotransferase and alanine aminotransferase and/or bilirubinemia. Eleven of 43 (26%) patients experienced a grade III-IV oral mucositis, whereas 15 of 43 (35%) experienced a grade II-III gastroenteritis. Nausea and vomiting were mild in all patients. All toxicities were easily managed with supportive care and adequate anti-infective therapy with intravenous antibiotics. No grade III-IV nephrotoxicity was observed. No grade III-IV cardiotoxicity was observed. No episode of veno-occlusive disease was reported. No grade III-IV interstitial pneumonitis or idiopathic pneumonia was reported. The 100-day TRM was 0%.
Infectious complications
Twenty-two of 43 patients (51%) experienced a grade I-II fever of unknown origin. The median number of days with fever was 2 days (range, 0-7 days), with a median number of 9 days of intravenous antibiotics (range, 3-22 days). Median time to hospital discharge was 24 days (range, 20-57 days). Three of 43 (7%) patients experienced a documented infection, which was easily managed with specific intravenous antibiotics. Only one documented fungal infection from Aspergillus flavus was observed, with a complete resolution of the infection after therapy with liposomal amphotericin B. Preemptive therapy with antifungal agents was required in only 3 of 43 patients (7%). Two patients (5%) developed a viral infection (1 with HSV-6, 1 with CMV) early after transplantation.
Outcome
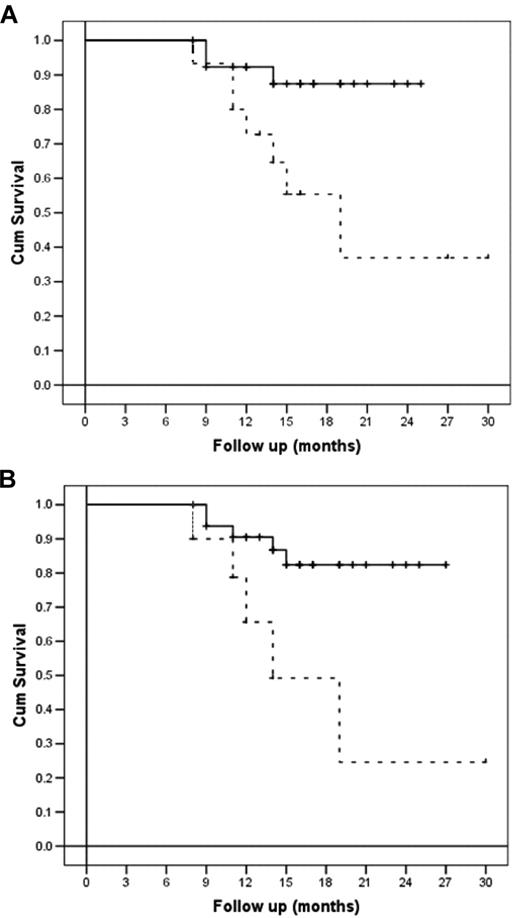
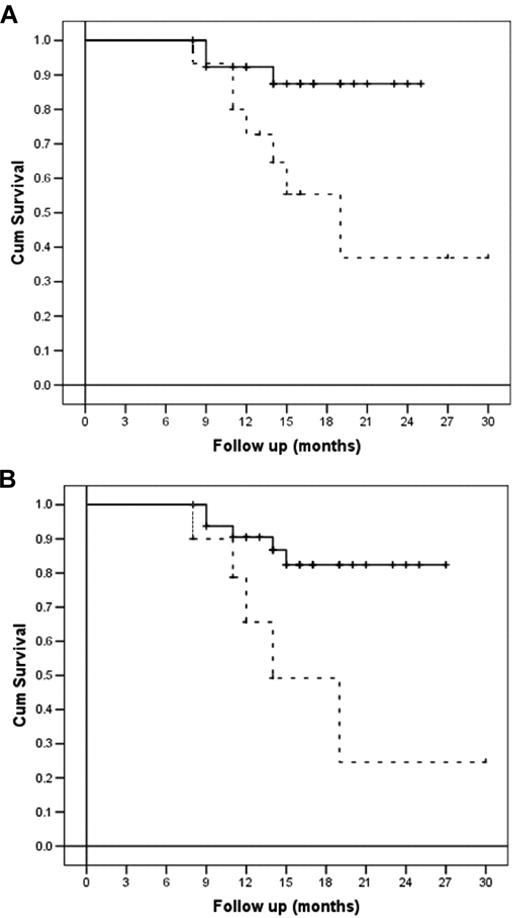
All patients enrolled in the study were refractory to at least one line of therapy or had relapsed. After receiving a salvage therapy, all of them were restaged with CT and PET scanning before receiving BeEAM and ASCT. In brief, 14 of 43 (33%) were in second or further complete remission, whereas 22 of 43 (51%) were in partial response and 7 of 43 (16%) did not respond. Log-rank comparison of survival showed a statistically greater probability of being disease-free after receiving HDT with bendamustine and ASCT for NHL compared with HD patients (P = .01) and for chemosensitive status compared with chemoresistant status (P = .007). These data were also confirmed by the Cox proportional hazards regression model, which confirmed disease type (NHL vs HD) and disease status (chemosensitive vs chemoresistant) being the only variables affecting survival in the multivariate analysis (P = .01 and P = .02, respectively).
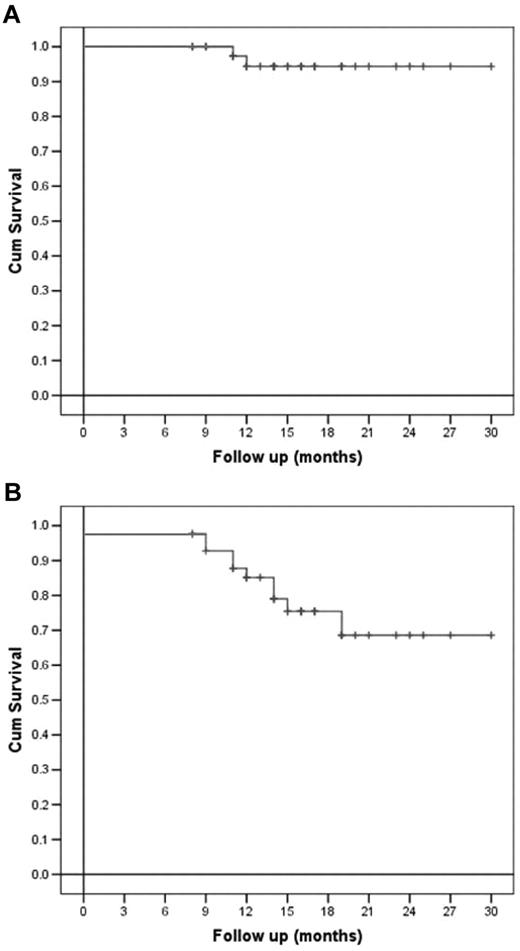
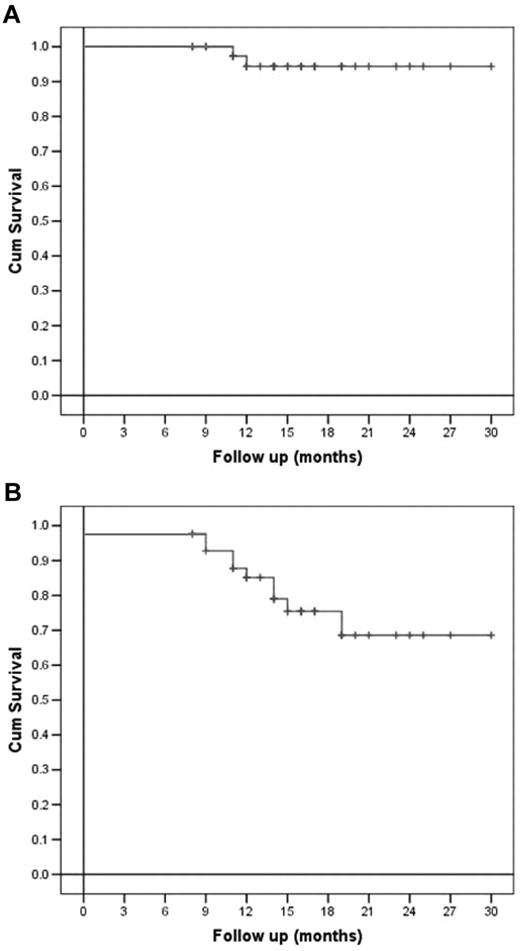
Regarding OS, a statistically greater probability of being alive after receiving HDT with bendamustine and ASCT was demonstrated for chemosensitive patients compared with chemoresistant ones (P = .004), whereas only a trend was shown for NHL patients compared with HD patients (P = .05). All the other variables analyzed, comprising sex, age, previous number of therapies, disease stage, IPI score for NHL patients, disease status before salvage treatment, and number of CD34+ cells infused did not statistically influence DFS or OS (Figures 2 and 3).
Overall and disease-free survival (per-protocol analysis). (A) OS and (B) DFS of all lymphoma patients.
Overall and disease-free survival (per-protocol analysis). (A) OS and (B) DFS of all lymphoma patients.
Disease-free and overall survival (per-protocol analysis) according to disease type at relapse and disease status at transplantation. (A) DFS of patients according to disease type (NHL, continuous line, versus HD, dashed line; P = .01). (B) DFS of patients according to disease status at transplantation (chemosensitive, continuous line, vs chemoresistant, dashed line; P = .007).
Disease-free and overall survival (per-protocol analysis) according to disease type at relapse and disease status at transplantation. (A) DFS of patients according to disease type (NHL, continuous line, versus HD, dashed line; P = .01). (B) DFS of patients according to disease status at transplantation (chemosensitive, continuous line, vs chemoresistant, dashed line; P = .007).
After a median follow-up of 18 months from transplantation, 35 of 43 patients (81%) are alive and disease-free (CT and PET). Median DFS for HD patients was 19 months (95% confidence interval 11.4-26.5), whereas it has not been reached for NHL patients. Six of 43 (14%) patients relapsed after a median time of 3 months from ASCT, and only 2 of 43 (5%) did not respond at all to therapy. Notably, 4 of 43 (9%) patients achieved their first complete remission after HDT and ASCT. At the time of this report, only 2 patients with HD have died, at 3 and 4 months after ASCT, respectively.
Regarding the outcome after ASCT on the basis of pre-ASCT PET, it is not possible, at present, to draw any sure conclusion. In brief, 14 patients had a negative PET scan at ASCT, and all of them are still in PET-negative CR. The remaining 29 patients were PET positive at transplantation, with 22 patients showing a reduction in the number of the sites involved or in the Δstandard uptake value and 7 patients with a progression of disease documented by PET. Notably, only 4 of 43 patients (9%) were still PET positive 3 months after transplantation, whereas additional 4 of 43 (9%) became PET positive between 6 and 12 months after ASCT. In other words, 21 of 29 patients who were still PET positive at ASCT became PET negative after ASCT and, with a median follow up of 18 months, are still in PET-negative CR. However, because of the small size of our study population and also because of the heterogeneity of histologic subtypes of lymphoma (HD, diffuse large B-cell [DLBC], mantle cell, grade IIIb follicular, T-cell–rich) we were not able to identify a significant difference between the various subgroup of patients. However, it is of note that almost 50% of the patients being PET positive at ASCT became PET negative after BeEAM and ASCT.
Discussion
On the basis of the results of the PARMA study group trial, high-dose chemotherapy followed by ASCT has become the standard of care for patients with relapsed, chemosensitive aggressive NHL.7-12 Recently, the randomized Collaborative Trial in Relapsed Agressive Lymphoma (CORAL) study,28 performed in the rituximab era, confirmed the results of the PARMA study, reporting a 3-year progression-free survival of 53% in patients receiving high-dose BEAM therapy after having obtained a response with R-ICE (ifosfamide, carboplatin, etoposide, and rituximab) or R-DHAP (rituscinab, dexamethasone, high-dose cytarabine, cisplatin). Nonetheless, after the pioneering work by Philip et al in 1986, a significant proportion of HD patients with resistant or relapsed disease may benefit from HDT and ASCT.7-12
However, several clinical needs still remain unmet in this field. The identification of effective salvage regimens to maximize disease response before ASCT is one. In this regard, the CORAL study showed comparable results between rituximab-containing regimens in relapsed DLBC patients (R-ICE vs R-DHAP, CR rate 63.5% and 62.8%, respectively).28 However, only 50% of patients were able to proceed with ASCT.28 Moreover, in patients relapsing > 12 months after ASCT, previous rituximab treatment did not affect progression-free survival.28
Because of the results reported from the randomized CORAL study, which are less favorable than those reported in nonrandomized study,29 testing effective HDT schedules to increase responses and to reduce both TRM and the incidence of secondary diseases becomes an important area of research in relapsed/refractory lymphomas.30 Furthermore, there is no evidence, to date, for a superior HDT regimen in the treatment of refractory or relapsed disease, in NHL or in HD. Carmustine (BCNU), etoposide, cytarabine, and melphalan (BEAM regimen) is one of the most used schedules to proceed to ASCT in either NHL and HD patients since its introduction in the 1980s.9 However, major pitfalls still affect the BEAM regimen regarding safety and efficacy. Toxicities of BCNU-containing regimens, such as BEAM or BEAC, are concerning because of a high incidence of interstitial pneumonitis.9,11 Patients who received carmustine as part of their preparative regimen for stem cell transplantation offer a longer window in which the development of pulmonary complications can occur. In this setting the incidence of interstitial pneumonitis or idiopathic pneumonia have been reported to be 2%-23%.31-35
Furthermore, results from the Bone Marrow Transplant Survivor Study showed a relative risk of death not related to relapse of 2.27 (95% confidence interval 1.42-3.64) for patients receiving ASCT for hematologic malignancies with BCNU-based regimens compared with those treated with busulfan- or total body irradiation–based regimens.36 Nonetheless, a high incidence of relapse is still an important concern of either BEAM or BEAC therapy.7-17 Therefore, new conditioning regimens are required, and few trials are currently running, all of which have BEAM as either a standard comparison with the new proposed treatment or as the link for new components, such as high-dose rituximab or radioimmunoconjugates (BMT-CTN Prot 0401, NCT00472056, NCT00591630).
In this search for the optimal conditioning, we decided to test the safety and the efficacy of a modified BEAM combination, with the substitution of carmustine by high-dose bendamustine (“Supercharge BeEAM”), a drug with proven clinical activity compared with standard therapies in resistant/relapsed B-cell malignancies, such as indolent lymphomas and CLL.19-22 The basis for this change arose from preliminary in vitro data on lymphoma cell lines that demonstrated the greater efficacy bendamustine has in combination with etoposide, cytarabine, and melphalan with respect to carmustine (Figure 1).
From bench to bedside, 9 patients were initially treated with increasing doses of bendamustine (160 mg/m2, 180 mg/m2, and 200 mg/m2). All patients fully engrafted, with a median time to achieve absolute neutrophil count > 0.5 × 109/L and PLT > 20 × 109/L superimposable to those of other conventional conditioning regimens (10 and 13 days, respectively). None of them experienced a dose-limiting toxicity, and all of them achieved a response after HDT and ASCT. As a matter of fact, we did not observe a dose-efficacy correlation among 160, 180, and 200 mg/m2. This would probably mean we are most likely in a plateau with high doses of bendamustine and no further increase in activity may be expected.
Finally, we decided to carry on the phase 2 study using a fixed dose of 200 mg/m2 because of the reported cardiotoxicity in previous series25,26 at doses of bendamustine of 280 mg/m2, keeping in mind the high dose of anthracyclines already received from most of the patients. Again, all patients fully engrafted, with manageable grade III-IV oral mucositis in a minor number of cases being the only major side effect. The cumulative TRM of the procedure was 0%, with no transplantation-related deaths occurring within 100 days of transplantation. The extremely low-toxicity profile of the “Supercharge BeEAM” needs to be adequately underlined, especially if we bear in mind that the study population was pretreated with a median number of 2 lines of therapy. Furthermore, the toxicity of regimens considered safer than BCNU-containing schedules (eg, intravenous busulfan and cyclophosphamide) are greater if compared with BeEAM, with a reported 100-day TRM between 0% and 10% among the studies37-40 and with an incidence of approximately 20% of mild-to-severe clinically relevant side effects (eg, seizures, interstitial pneumonia, veno-occlusive disease). We reported a cumulative incidence of infectious complications (bacteremia plus fever of unknown origin) of approximately 60%, which is greater than other series, but we did not observe any of the serious adverse events reported with other schedules.
Considering efficacy, BeEAM was very effective, with 81% of patients in complete response after a median observation time of 18 months. In this regard, chemosensitive patients performed better than chemoresistant patients in terms of both DFS and OS (P = .007 and P = .004, respectively), as expected, and clearly demonstrated from previous studies. Interestingly, the probability of being disease free was statistically superior for NHL patients compared with HD patients (P = .01). These data could be because of the lower number of patients enrolled in the HD group (15 patients), and further studies are warranted to confirm the greater efficacy of the bendamustine-based combination in the NHL setting than the HD one. In any case, even incorporating the probability of further relapses at later observation times, “Supercharge BeEAM” performed favorably if compared with common clinical practice in this subset of poor prognosis patients. In fact, the analyses of relapsed/refractory lymphoma patients treated with SCT are frequently hampered by an institution-to-institution, if not a case-to-case, difference in post-SCT policy. Thus, the random incorporation (often on a single case-based clinical decision) of maintenance with monoclonals for DLBCL, as well as radiotherapy use in the bulky site, in HD as well as NHL frequently occurs.41,42 In the present study, patients were not allowed to receive any therapy after ASCT. In conclusion, BeEAM appears as a promising novel HDT regimen pre-SCT for relapsed/refractory lymphomas; further observation and research is warranted and is planned to confirm the favorable safety and efficacy profile of this novel HDT regimen.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Mundipharma International Ltd for providing bendamustine for the study.
The study was supported in part by AIL Pesaro Onlus.
Authorship
Contribution: G.V. and A.I. equally proposed, designed, and supervised the study and wrote the manuscript; L.M., P.M.S., S.C., P.G., F.G., G.S., G.M., F.G., C.G., S.F., F.C., M.G., B.S., A.S., and F.F. treated the patients, collected the data, and commented on the manuscript; M.R. and A.I. were responsible for statistical analysis; and E.M.O. and M.D.C. performed in vitro experiments and commented on the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Giuseppe Visani, MD, Hematology and Hematopoietic Stem Cell Transplant Center, Marche Nord Hospital, Via Lombroso, 61100 Pesaro, Italy; e-mail: g.visani@ospedalesansalvatore.it.
References
Author notes
G.V. and A.I. contributed equally to the manuscript.