Abstract
Diarrhea-associated hemolytic uremic syndrome (D+HUS) is caused by the ingestion of Escherichia coli that produce Shiga toxin (Stx), which is composed of a cytotoxic A subunit and pentameric B subunits that bind globotriaosylceramide on susceptible cells. Stx occurs in 2 types, Stx1 and Stx2. B subunits of either type stimulate von Willebrand factor (VWF) secretion from human umbilical vein endothelial cells (HUVECs), and Stx2B can cause thrombotic microangiopathy in Adamts13−/− mice. We have now determined that Stx1B and Stx2B activate different signaling pathways in HUVECs. VWF secretion induced by Stx1B is associated with a transient rise in intracellular Ca2+ level that is blocked by chelation with 1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid-acetoxymethyl ester, removal of extracellular Ca2+, the phospholipase C inhibitor U73122, the protein kinase inhibitor staurosporine, or small interfering RNA knockdown of protein kinase Cα. In contrast, Stx2B-induced VWF secretion is associated with activation of protein kinase A (PKA) and is blocked by the PKA inhibitor H89 or small interfering RNA knockdown of PKA. Stx2B does not increase cAMP levels and may activate PKA by a cAMP-independent mechanism. The activation of distinct signaling pathways may be relevant to understanding why E coli that express Stx2 are more likely to cause D+HUS than are E coli expressing only Stx1.
Introduction
Ingestion of food contaminated with Shiga toxin (Stx)–producing Escherichia coli, such as strain O157:H7, can cause bloody diarrhea that develops into diarrhea-associated hemolytic uremic syndrome (D+HUS) that is characterized by acute renal failure, thrombocytopenia, and microangiopathic hemolytic anemia. D+HUS is the most common cause of acute renal failure in children and is fatal in ∼ 3%-5% of cases.1
Several mechanisms seem to influence the course of tissue injury by Stx. Stx-producing E coli make 2 major types of Stx, each of which is composed of 1 catalytically active A subunit (∼ 33 kDa) and 5 B subunits (∼ 7.7 kDa) that form a homopentameric ring. Stx1 is identical to the major toxin produced by Shigella dysenteriae serotype 1. Stx2 is ∼ 50%-60% identical in sequence to Stx1 and occurs in several variants. Stx1 binds to globotriaosylceramide (Gb3) more tightly than Stx2, but Stx2 is more toxic in animal models and is more often associated with D+HUS in humans than Stx1.2-4 The Stx B subunits bind to Gb3 on cell surfaces and facilitate internalization of the Stx holotoxin that undergoes retrograde transport to the endoplasmic reticulum. The Stx A subunit is then translocated into the cytoplasm where it cleaves the adenine base at position 4324 of 28S ribosomal RNA, thereby blocking protein synthesis and initiating a series of responses that culminate in cell death.5
In addition, treatment of human umbilical vein endothelial cells (HUVECs) or glomerular microvascular endothelial cells (HGMECs) with nanomolar concentrations of Stx1 or Stx2 induces rapid VWF secretion and the formation of long cell-associated VWF strings.6 Administration of Stx2 to Adamts13−/− mice also causes fatal microvascular thrombosis that resembles thrombotic thrombocytopenic purpura, and Adamts13−/− mice are protected from the lethal effects of Stx if they also lack VWF.7 We recently found that the B subunits from Stx1 or Stx2, in the absence of the A subunits, are sufficient to stimulate VWF secretion by HUVECs or HGMECs and that Stx2B subunits can induce thrombotic microangiopathy in Adamts13−/− mice.8 These responses indicate the existence of Stx B-induced signaling pathways that may be responsible for some of the biologic effects attributed previously to the cytotoxic Stx A subunit.
At least 3 signaling pathways can contribute to agonist-induced secretion of VWF. Histamine and thrombin activate phospholipase C (PLC)–β that generates inositol 1,4,5-triphosphate (IP3) and diacylglycerol. IP3 increases the level of intracellular Ca2+ that is required for VWF secretion induced by these agonists. In contrast, epinephrine and vasopressin induce VWF secretion by increasing intracellular cAMP and activating protein kinase A (PKA).9 Alternatively, vascular endothelial growth factor (VEGF) stimulates VWF secretion through a pathway that depends on protein kinase C (PKC)–δ but not on Ca2+ or cAMP.10 Whether any of these pathways contribute to Stx B-induced VWF secretion has not been determined previously.
We have now shown that Stx1B and Stx2B activate distinct signaling pathways after binding to Gb3 on the cell surface. Stx1B increases intracellular Ca2+ and in this respect resembles histamine or thrombin. In contrast, VWF secretion induced by Stx2B does not depend on intracellular Ca2+, but it involves the cAMP-independent activation of protein kinase A. The differences between signaling pathways activated by Stx1B and Stx2B may be relevant to understanding why Stx2 is more commonly associated with D+HUS.
Methods
Stx preparations
Stx1 and Stx2 holotoxins (List Biological Laboratories) and Stx1B and Stx2B (BEI Resources) were treated with Detoxi-Gel endotoxin removal columns (Pierce Chemical). Residual endotoxin was assayed with Limulus amoebocyte lysate (PYROGENT Plus test kit; Cambrex) or QCL-1000 chromogenic LAL end point assay kit (Lonza Walkersville) and was < 1 ng/mg of protein.
Endothelial cell culture and quantitation of VWF strings
Pooled donor HUVECs (Lonza Walkerville) between the third to fifth passages were cultured in endothelial growth medium-2 supplemented with growth factors (Lonza Walkerville) on collagen-coated glass coverslips and grown to confluence. Perfusion assays were performed in a parallel plate flow chamber (Glycotech) as described previously.11 In brief, HUVECs were perfused using a syringe pump (Harvard Apparatus) at 2.5 dyn/cm with medium 199 containing 2% BSA, polyclonal rabbit anti–human VWF antibody (Dako North America) conjugated to Alexa Fluor 594 (Invitrogen), and other reagents as indicated. Two minutes after initiating perfusion, time-lapse images were collected with an Axiovert 200M inverted microscope, a 40×/0.5 numeric aperture objective, standard filter sets, AxioCam MRm camera, and Axiovision Version 4 software (Carl Zeiss). Fluorescent VWF strings longer than 20 μm were counted in at least 10 images corresponding to 10 consecutive optical fields (×400 magnification) for each perfusion condition. To adjust for variations in responsiveness to histamine or Stx among HUVECs, responses were normalized within each experiment to the number of VWF string induced by 5nM Stx1B. Differences between mean values were assessed using the unpaired Student t test or 1-way ANOVA.
Measurement of intracellular Ca2+ and cAMP
HUVECs were plated at 5 × 105 cells/well in black 96-well clear-bottomed plates 1 day before use. Intracellular Ca2+ levels were measured using a BD calcium assay kit (BD Biosciences) and a VICTOR 2 microplate reader (PerkinElmer Life and Analytical Sciences). After injection of agonists, fluorescence was monitored with excitation at 490 nm and emission at 535 nm.
For calcium imaging in individual cells, HUVECs at 37°C were incubated for 20 minutes with 1 μg/mL Fura-2/AM (Invitrogen), washed in Ringer solution (150mM NaCl, 5mM KCl, 2mM CaCl2, 1mM MgCl2, 10mM glucose, and 5mM HEPES, pH 7.4) and incubated for another 30 minutes. Cells were examined with an Axiovert 200 microscope (Carl Zeiss), equipped with a xenon light source, X20 Fluar objective (numeric aperture 0.75), in a 37°C environmental control chamber. Cells were excited at 340 and 380 nm, and the ratio of fluorescence emission at 510 nm was determined as a function of time after exposure to agonist. Data acquisition and analysis were performed using MetaMorph 6.0 (Molecular Devices). Ca2+ concentration was estimated as described previously.12
To measure intracellular cAMP, HUVECs were pretreated for 10 minutes with 0.75mM 3-isobutyl-1-methylxanthine (IBMX) and challenged with agonists for 1-5 minutes. Levels of cAMP were determined in cell lysates with a CatchPoint cAMP fluorescent assay kit (Molecular Devices) and VICTOR 2 microplate reader.
Alternatively, HUVECs were transfected with 1 μg/mL of plasmid Epac1-camps13 using a Nucleofector and HUVEC nucleofection solution (Lonza Walkersville) and cultured on glass-bottomed dishes. Plasmid Epac1-camps encodes the cAMP binding domain of Epac1 flanked by enhanced yellow fluorescent protein (YFP) and enhanced cyan fluorescent protein (CFP). Binding of cAMP to Epac1 relieves internal fluorescence quenching of CFP by YFP.13 Cells were treated with agonist or forskolin as indicated and examined using an LSM510 meta laser confocal microscope (Carl Zeiss), with illumination at 436 nm and collection of fluorescence intensity for CFP (470 nm) and YFP (525 nm) simultaneously from single cells. Images were acquired every 50 seconds, and fluorescence intensity ratios (470 nm/525 nm) were analyzed with ImageJ (Version 1.34r; National Institutes of Health, rsbweb.nih.gov/ij).
Detection of phosphatidylinositol-4,5 bisphosphate hydrolysis
Plasmid pUG36-2XPLCδPH encodes green fluorescent protein (GFP) fused to 2 tandem copies of the pleckstrin homology (PH) domain of human PLCδ1.14 HUVECs were transfected with pUG36-2XPLCδPH and cultured on glass-bottomed dishes as described in “Measurement of intracellular Ca2+ and cAMP.” Cells were treated with agonist or control conditions and examined by confocal microscopy. Images were acquired every 50 seconds and analyzed using ImageJ to determine the relative change in fluorescence intensity of the plasma membrane compared with that of the cytosol.15
PKCα and PKA RNA interference
RNA oligonucleotides were synthesized by Sigma-Aldrich. PKCα silencing was performed with a mixture of 2 duplex oligonucleotides: 5′-CAGAAGAACUGUAUGCAAU[dTdT] and 5′-GAGUUUCGGAGCUGAUGAA[dTdT]. PKA silencing was performed with a mixture of 2 duplex oligonucleotides: 5′-GAUUGUGGAUGUAAUAGGAdTdT and 5′-CCUGCAAGCUGUCAACUUUdTdT. The siRNA Universal Negative Control 1 (Sigma-Aldrich) was used as a control in all experiments. HUVECs were transfected using a Nucleofector. After 72 hours, cell lysates were prepared and analyzed by Western blotting with anti-PKCα and anti-PKA antibodies. Blots were stripped with Restore Western blot stripping buffer (Pierce Chemical) and reprobed with anti–β-actin antibody (Sigma-Aldrich).
Phosphorylation of PKCα and 14-3-3ζ
Confluent HUVECs in 10-cm plates were treated with the indicated agonist or control conditions in M199 medium for 5 minutes. Cells were harvested with a scraper and incubated on ice for 40 minutes using a cell lysis solution (Cell Signaling Technology) containing 1mM PMSF and phosphatase inhibitor cocktail (Pierce Chemical). Lysates were centrifuged for 15 minutes at 20 000g, and the supernatants were stored at −80°C until analyzed by SDS-PAGE and Western blotting. Antibodies specific for PKCα (2056; Cell Signaling Technology), phospho-PKCα/βII Thr638/641 (9375; Cell Signaling Technology), phospho-PKC (pan) Thr514 (9379; Cell Signaling Technology; detects phospho-PKCα Thr479), 14-3-3ζ (101623; Santa Cruz Biotechnology), phosphorylated 14-3-3ζ (51109; Abcam), and GAPDH (9484; Abcam), were used at appropriate dilutions. Secondary antibodies (Invitrogen) were Alexa Fluor 633–conjugated or Alexa Fluor 594–conjugated goat anti–mouse IgG. Imaging and quantitation of Western blots was performed on a Typhoon scanner (GE Healthcare) or by exposure to film and analysis with ImageJ.
PKA activity
Confluent HUVECs in 10-cm dishes were treated with agonists for 5 minutes. Cell lysates were prepared, and PKA activity was assayed using a PKA kinase activity kit (Assay Designs) that uses a synthetic peptide PKA substrate and polyclonal antibody that recognizes the phosphorylated product by ELISA.
Results
Stx B subunits induce acute VWF secretion by human endothelial cells
Nolasco et al first demonstrated that Stx holotoxins induce the rapid secretion of VWF from HUVECs and HGMECs.6 We recently confirmed these findings and also found that Stx B subunits are sufficient to induce VWF secretion.8 The assay for these studies involves perfusion with fluorescently labeled anti-VWF antibody and allows the visualization of VWF secretion continuously in living endothelial cells, indicating that secretion occurs within 30 seconds of exposure to Stx holotoxins or B subunits.
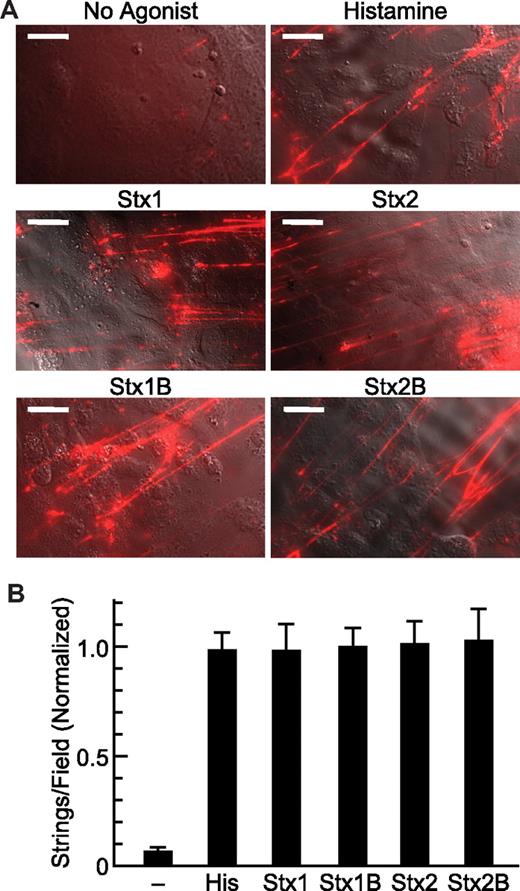
Different preparations of HUVECs differ in their sensitivity to agonists. For example, the mean number of VWF strings induced by histamine (100μM) varied from 4-10 (per high-power field) among 3 independent experiments. To facilitate comparisons among replicates, data are normalized to the response obtained for 5nM Stx1B, a concentration that induces maximal VWF secretion.8 At this concentration, Stx1, Stx1B, Stx2, and Stx2B are approximately equipotent and stimulate VWF responses similar to those elicited by 100μM histamine (Figure 1).
Stx holotoxin and StxB induced secretion of VWF. (A) HUVECs were perfused in a parallel plate flow chamber at 2.5 dyn/cm2 with medium M199 containing fluorescent anti-VWF antibody without or with 100μM histamine, 5nM Stx1, 5nM Stx2, 5nM Stx1B, or 5nM Stx2B and images were acquired at 10 minutes. Scale bars are 20 μm. Images were prepared with Photoshop CS4 (Adobe Systems). (B) VWF strings were counted in 10 fields and values shown as the mean ± SE, normalized to the mean value for Stx1B. Experiments were repeated at least 3 times with similar results.
Stx holotoxin and StxB induced secretion of VWF. (A) HUVECs were perfused in a parallel plate flow chamber at 2.5 dyn/cm2 with medium M199 containing fluorescent anti-VWF antibody without or with 100μM histamine, 5nM Stx1, 5nM Stx2, 5nM Stx1B, or 5nM Stx2B and images were acquired at 10 minutes. Scale bars are 20 μm. Images were prepared with Photoshop CS4 (Adobe Systems). (B) VWF strings were counted in 10 fields and values shown as the mean ± SE, normalized to the mean value for Stx1B. Experiments were repeated at least 3 times with similar results.
Stx1B and Stx2B have distinct effects on intracellular Ca2+
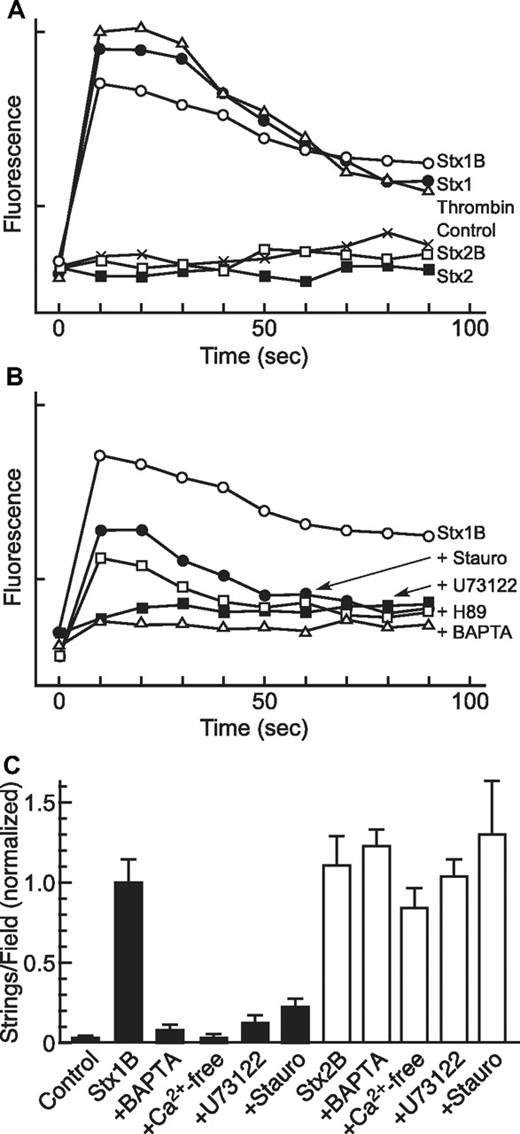
Thrombin-induced VWF secretion depends on an increase in the intracellular Ca2+ concentration16 and, as expected, HUVECs treated with thrombin (1 U/mL) exhibited a maximal increase in Ca2+ within 10 seconds that declined toward baseline over ∼ 2 minutes. Treatment with Stx1 or Stx1B (5nM) also induced rapid changes in intracellular Ca2+ of the same magnitude and time course as those induced by thrombin (Figure 2A). Retreatment with thrombin, Stx1, or Stx1B induced a similar change in intracellular Ca2+ (data not shown). A similar magnitude and time course of Ca2+ changes was observed in single HUVECs loaded with Fura-2 after treatment with thrombin or Stx variants, as determined by fluorescence videomicroscopy (data not shown). In contrast, concentrations of Stx2 or Stx2B (5nM) that stimulate maximal secretion of VWF (Figure 1B) did not alter intracellular Ca2+ (Figure 2A), indicating that Stx1B and Stx2B activate different signaling pathways.
Inhibitors of Stx-induced Ca2+ responses and VWF secretion. (A-B) Changes in intracellular Ca2+ concentration were measured in 96-well plates with a BD calcium assay kit. Fluorescence signals were recorded for 6 wells per condition at 10-second intervals, and the means are plotted; SE values are < 2% of the means. Each experiment repeated at least 3 times with similar results. (A) HUVECs were treated without (Control) or with 1 U/mL thrombin, 5nM Stx1, 5nM Stx1B, 5nM Stx2, or 5nM Stx2B. (B) HUVECs were stimulated with 5nM Stx1B after pretreatment as indicated with 50nM staurosporine (Stauro), 5μM U73122, 5μM H89, or 100μM BAPTA-AM. (C) HUVECs were stimulated without (Control) or with 5nM Stx1B (closed bars) or 5nM Stx2B (open bars) after pretreatment with 100μM BAPTA-AM, Ca2+-free medium, 5μM U73122, or 19-31 amide. All comparisons between treatment with Stx1B alone and Stx1B with any inhibitor are statistically significant (P < .01); no comparisons between any Stx2B condition are statistically significant (P > .05). Each experiment repeated at least 3 times with similar results.
Inhibitors of Stx-induced Ca2+ responses and VWF secretion. (A-B) Changes in intracellular Ca2+ concentration were measured in 96-well plates with a BD calcium assay kit. Fluorescence signals were recorded for 6 wells per condition at 10-second intervals, and the means are plotted; SE values are < 2% of the means. Each experiment repeated at least 3 times with similar results. (A) HUVECs were treated without (Control) or with 1 U/mL thrombin, 5nM Stx1, 5nM Stx1B, 5nM Stx2, or 5nM Stx2B. (B) HUVECs were stimulated with 5nM Stx1B after pretreatment as indicated with 50nM staurosporine (Stauro), 5μM U73122, 5μM H89, or 100μM BAPTA-AM. (C) HUVECs were stimulated without (Control) or with 5nM Stx1B (closed bars) or 5nM Stx2B (open bars) after pretreatment with 100μM BAPTA-AM, Ca2+-free medium, 5μM U73122, or 19-31 amide. All comparisons between treatment with Stx1B alone and Stx1B with any inhibitor are statistically significant (P < .01); no comparisons between any Stx2B condition are statistically significant (P > .05). Each experiment repeated at least 3 times with similar results.
The signaling pathways activated by Stx1B were investigated further by assessing the effects of selected inhibitors on Ca2+ flux (Figure 2B) and VWF secretion (Figure 2C). Pretreatment of HUVECs with the cell permeable chelator 1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid-acetoxymethyl ester (BAPTA-AM; 0.1mM) prevented a rise in Ca2+. The Ca2+ response to Stx1B also was blocked completely by the PLC inhibitor U73122 and was partially inhibited by the PKC inhibitor staurosporine or PKA inhibitor H89 (Figure 2B). These treatments had very similar effects on both Ca2+ levels and VWF secretion (Figure 2C). Stx1B-induced VWF secretion was largely prevented by chelation of Ca2+ with BAPTA-AM, removal of extracellular Ca2+ from the perfusate, the PLC inhibitor U73122, or the protein kinase inhibitor staurosporine. In contrast to their ability to inhibit Stx1B-induced VWF secretion, none of these treatments significant decreased VWF secretion in response to Stx2B (Figure 2C). These results indicate that Ca2+ from at least extracellular sources is required for VWF secretion induced by Stx1B, possibly mediated through activation of PLC and PKC. In contrast, VWF secretion induced by Stx2B does not involve Ca2+ and seems to be independent of PLC and PKC.
PLC activation and PIP2 hydrolysis in response to Stx1B
The role of PLC activation in Stx1B-induced VWF secretion was evaluated further by confocal microscopy of HUVECs transfected with a plasmid encoding GFP fused to the PH domain of PLCδ1, which binds membrane-associated phosphatidylinositol bisphosphate (PIP2) in the plasma membrane of living cells.14 When these HUVECs were treated with thrombin (1 U/mL), the fluorescent GFP-PH redistributed from plasma membrane to the cytoplasm within 50 seconds, indicating the activation of PLC and cleavage of PIP2 (Figure 3). A similar translocation of GFP-PH was observed after treatment with Stx1B (5nM) but not after treatment with Stx2B (5nM). Cells pretreated with Stx2B remained responsive to a subsequent treatment with thrombin. Thus, Stx1B rapidly activates PLC to cleave PIP2, and the IP3 produced presumably is required for Ca2+ mobilization and VWF secretion.
Stx1B-induced phosphatidylinositol-4,5 bisphosphate hydrolysis. (A) Confocal fluorescence images of cells expressing GFP-PH-PLCδ before (0 seconds) and after (50 and 300 seconds) treatment with 1 U/mL thrombin, 5nM Stx1B, 5nM Stx2B, or with thrombin after pretreatment with Stx2B for 300 seconds. Images were prepared with Photoshop CS4 (Adobe Systems). (B) Time course of cytoplasmic fluorescence changes relative to t = 0 (F/F0; data are means ± SE; *P < .01, +P < .05 relative to the initial condition).
Stx1B-induced phosphatidylinositol-4,5 bisphosphate hydrolysis. (A) Confocal fluorescence images of cells expressing GFP-PH-PLCδ before (0 seconds) and after (50 and 300 seconds) treatment with 1 U/mL thrombin, 5nM Stx1B, 5nM Stx2B, or with thrombin after pretreatment with Stx2B for 300 seconds. Images were prepared with Photoshop CS4 (Adobe Systems). (B) Time course of cytoplasmic fluorescence changes relative to t = 0 (F/F0; data are means ± SE; *P < .01, +P < .05 relative to the initial condition).
PKCα activation in response to Stx1B
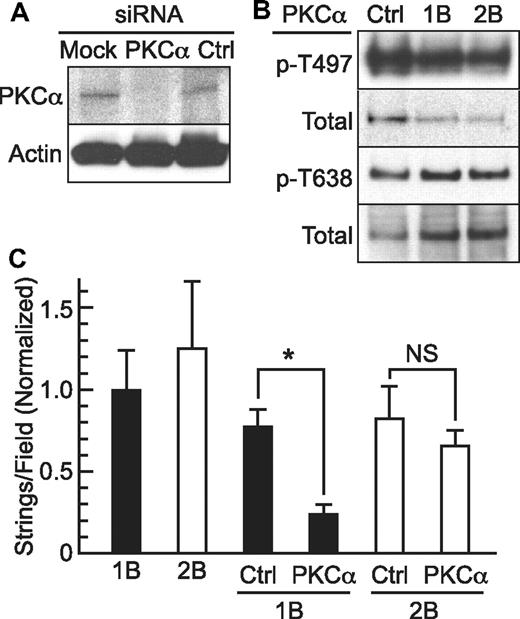
The inhibition of Stx1B-induced VWF secretion by staurosporine (Figure 2) suggests that protein kinases may participate, and PKCα has been identified as a mediator of VWF secretion in response to several agonists. We therefore assessed the role of PKCα by small interfering RNA (siRNA) knockdown. Transduction with 2 PKCα siRNA duplexes decreased PKCα expression in HUVECs, achieving ∼ 80% reduction in PKCα in 72 hours (Figure 4B) and decreasing the Stx1B-induced secretion of VWF strings by ∼ 70% compared with HUVECs transfected with control siRNA; the response to Stx2B was not decreased significantly (Figure 4B). Thus, PKCα seems to contribute to VWF secretion in response to Stx1B but not Stx2B. Treatment of HUVECs with Stx1B or Stx2B was accompanied by relatively small changes (< 2-fold) in PKCα phosphorylation at Thr497 and Thr638 (Figure 4C). These data do not exclude the possibility that the effects of Stx1B on PKCα activity are mediated in part by phosphorylation, but they suggest that allosteric activation by diacylglycerol and Ca2+after PLC activation (Figure 3) mediates the response to Stx1B.
PKCα activation and Stx1B-induced VWF secretion. (A) HUVECS were transfected with PKCα siRNA or negative control siRNA oligonucleotides. Cell lysates were prepared after 72 hours and analyzed by 4%-12% SDS-PAGE and Western blotting to detect total PKCα and β-actin. (B) Secretion of VWF strings was assessed in HUVECs treated similarly. Values are shown as mean ± SE; *P < .00005 and NS indicates P = .4, by Student t test. (C) HUVECs were treated for 5 minutes with 5nM Stx1B or Stx2B, and cell lysates were prepared for 3%-8% SDS-PAGE and Western blotting with primary antibodies specific for PKCα phosphorylated on Thr638 or Ser497 and appropriate fluorescently labeled secondary antibodies. Blots were stripped and reprobed with antibody recognizing total PKCα.
PKCα activation and Stx1B-induced VWF secretion. (A) HUVECS were transfected with PKCα siRNA or negative control siRNA oligonucleotides. Cell lysates were prepared after 72 hours and analyzed by 4%-12% SDS-PAGE and Western blotting to detect total PKCα and β-actin. (B) Secretion of VWF strings was assessed in HUVECs treated similarly. Values are shown as mean ± SE; *P < .00005 and NS indicates P = .4, by Student t test. (C) HUVECs were treated for 5 minutes with 5nM Stx1B or Stx2B, and cell lysates were prepared for 3%-8% SDS-PAGE and Western blotting with primary antibodies specific for PKCα phosphorylated on Thr638 or Ser497 and appropriate fluorescently labeled secondary antibodies. Blots were stripped and reprobed with antibody recognizing total PKCα.
PKA activation in response to Stx2B
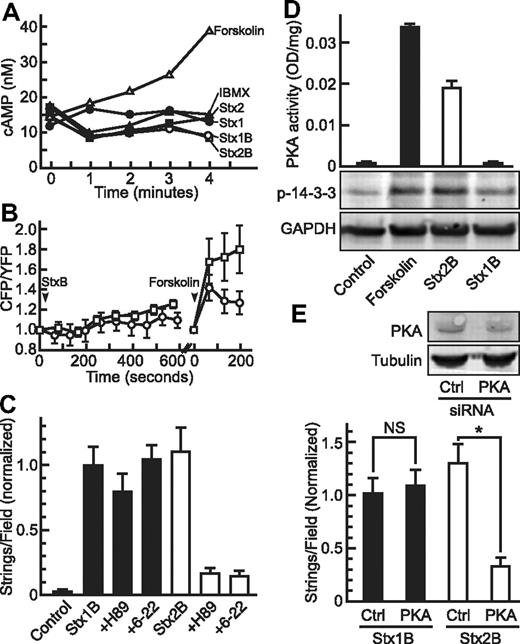
Some agonists induce VWF secretion by generating cAMP and activating PKA. Forskolin rapidly increased cAMP levels, demonstrating the presence of functional adenylyl cyclase in HUVECs, but treatment with Stx holotoxins or B subunits did not cause a detectible rise in intracellular cAMP levels (Figure 5A).
PKA activation and Stx2B-induced VWF secretion. (A) HUVECs in buffer containing 0.75mM IBMX were treated for the indicated times without (IBMX, ▴) or with 20μM forskolin (▵), 5nM Stx1 (●), 5nM Stx1B (○), 5nM Stx2 (■), or 5nM Stx2B (□), and intracellular cAMP concentration was measured using a CatchPoint cAMP fluorescent assay kit. (B) HUVECs expressing CFP-Epac-YFP were examined by fluorescence microscopy, CFP and YFP fluorescence emission was monitored, and 5nM Stx1B (○) or Stx2B (□) was added at 50 seconds. As a positive control, forskolin (20μM) was added, and cells were monitored another 200 seconds. The CFP/YFP fluorescence intensity ratio reflects the intracellular cAMP level. (C) HUVECs in parallel plate perfusion chambers were pretreated without or with the PKA inhibitors 5μM H89 or 1mM 6-22 amide, followed by 5nM Stx1B or Stx2B. Control cells were perfused with buffer only. VWF strings were counted and values normalized to the Stx1B condition. H89 and 6-22 amide inhibited the secretion of VWF induced by Stx2B (P < .0001) but not by Stx1B. (D) HUVECs were treated 5 minutes without (Control) or with 20μM forskolin, 5nM Stx1B, or 5nM Stx2B. Cell lysates were assayed for PKA activity with a synthetic peptide substrate (top) or analyzed by SDS-PAGE and Western blotting for phosphorylated 14-3-3ζ or GAPDH (bottom). (E) HUVECs were transfected with control siRNA (Ctrl) or PKA siRNA. After 72 hours, cell lysates (top) were analyzed by SDS-PAGE and Western blotting for PKA or tubulin. Parallel cultures treated similarly (bottom) were perfused with 5nM Stx1B or Stx2B. VWF strings were counted and normalized to the Stx1B control condition. *P < .0005, NS indicates P = .25 by Student t test.
PKA activation and Stx2B-induced VWF secretion. (A) HUVECs in buffer containing 0.75mM IBMX were treated for the indicated times without (IBMX, ▴) or with 20μM forskolin (▵), 5nM Stx1 (●), 5nM Stx1B (○), 5nM Stx2 (■), or 5nM Stx2B (□), and intracellular cAMP concentration was measured using a CatchPoint cAMP fluorescent assay kit. (B) HUVECs expressing CFP-Epac-YFP were examined by fluorescence microscopy, CFP and YFP fluorescence emission was monitored, and 5nM Stx1B (○) or Stx2B (□) was added at 50 seconds. As a positive control, forskolin (20μM) was added, and cells were monitored another 200 seconds. The CFP/YFP fluorescence intensity ratio reflects the intracellular cAMP level. (C) HUVECs in parallel plate perfusion chambers were pretreated without or with the PKA inhibitors 5μM H89 or 1mM 6-22 amide, followed by 5nM Stx1B or Stx2B. Control cells were perfused with buffer only. VWF strings were counted and values normalized to the Stx1B condition. H89 and 6-22 amide inhibited the secretion of VWF induced by Stx2B (P < .0001) but not by Stx1B. (D) HUVECs were treated 5 minutes without (Control) or with 20μM forskolin, 5nM Stx1B, or 5nM Stx2B. Cell lysates were assayed for PKA activity with a synthetic peptide substrate (top) or analyzed by SDS-PAGE and Western blotting for phosphorylated 14-3-3ζ or GAPDH (bottom). (E) HUVECs were transfected with control siRNA (Ctrl) or PKA siRNA. After 72 hours, cell lysates (top) were analyzed by SDS-PAGE and Western blotting for PKA or tubulin. Parallel cultures treated similarly (bottom) were perfused with 5nM Stx1B or Stx2B. VWF strings were counted and normalized to the Stx1B control condition. *P < .0005, NS indicates P = .25 by Student t test.
Similar results were obtained by monitoring intracellular cAMP levels in single cells with a chimeric CFP-Epac1-YFP construct (Figure 5B).13 Neither Stx1B nor Stx2B induced a significant rise in cAMP over the 5-minute time course of Stx-induced VWF secretion, although monitoring for longer times suggested that Stx2B may cause a small increase in cAMP. Subsequent treatment with forskolin triggered the expected increase in cAMP.
Despite the absence of detectable rapid cAMP synthesis, PKA seemed to be activated selectively by Stx2B. Pretreatment of HUVECs with PKA inhibitors (H89, 6-22 amide) reduced Stx2B-induced VWF secretion by 80% but had no significant effect on the response to Stx1B (Figure 5C). PKA activity in cell lysates increased after treatment with forskolin as expected and also increased after treatment with Stx2B but not Stx1B (Figure 5D). Increased PKA activity was accompanied by increased phosphorylation of 14-3-3ζ, a downstream target of PKA (Figure 5D).
To confirm the role of PKA in StxB-induced VWF secretion, we transfected HUVECs with siRNA to decrease PKA expression and observed an ∼ 50% reduction in PKA antigen compared with control siRNA after 72 hours (Figure 5E). Knockdown of PKA was associated with a 75% decrease in VWF string formation in response to Stx2B, but it had no effect on the response to Stx1B (Figure 5E). Therefore, PKA participates in VWF secretion induced by Stx2B but not by Stx1B.
Discussion
Stx ultimately causes D+HUS by killing cells through the action of the Stx A subunit on cytoplasmic ribosomes, and Stx variants with inactive A subunits, or isolated Stx B subunits, are not toxic to cells or healthy animals even at high concentrations.5 For example, Stx1B (10μg/mL) did not injure Ramos Burkitt lymphoma cells17,18 or Vero cells,19 and Stx2B (0.1μg/mL) was nontoxic for primary human renal tubular epithelial cells.20 The LD100 dose of Stx1 is ∼ 100 ng/mouse, but administration of 1000-fold more Stx1B (10 μg) has no adverse effects on wild-type mice.21 Nevertheless, either Stx holotoxins or their B subunits cause VWF secretion from endothelial cells, and otherwise nontoxic Stx2B subunits can induce lethal thrombotic microangiopathy in mice that have been sensitized by genetic deletion of ADAMTS13.6,8 Whether Stx-induced VWF secretion contributes to the pathogenesis of D+HUS in humans is unknown, but such a role would be consistent with a recent linkage analysis. The risk of developing D+HUS was associated (odds ratio, 3.08; P < .001) with the expression of a platelet GPIbα 145M variant that binds VWF with higher affinity than the more common GPIbα 145T variant,22 suggesting that increased Stx-induced interactions between VWF and platelets may promote renal injury in this setting.
Although Stx1 and Stx2 both bind Gb3 and injure cells by the same mechanism, E coli that express Stx2—with or without Stx1—have a higher likelihood of causing D+HUS than do E coli expressing only Stx1.23 This difference in the propensity of E coli strains to cause disease in humans may have many causes. However, differences between the Stx1 and Stx2 proteins have been identified that may be relevant: Stx1 binds Gb3 on cells 10-fold more tightly than does Stx2,2 but Stx2 is more potent for inducing VWF secretion from HUVECs8 and is ∼ 400-fold more toxic to mice.3 We now find that the B subunits of Stx1 and Stx2 rapidly induce the secretion of VWF through distinct signaling pathways. Stx1B increases the intracellular concentration of Ca2+, mediated by activation of PLC and PKC (Figures 2,Figure 3–4); in this respect, Stx1B resembles several other endothelial cell agonists, including thrombin, histamine, and sphingosine-1-phosphate. In contrast, Stx2B does not increase intracellular Ca2+ (Figure 2) and instead activates PKA (Figure 5), as do the agonists serotonin, epinephrine, and vasopressin.9 The activation of PKA by Stx2B is unusual because intracellular levels of cAMP do not increase detectably during the time course of maximal VWF secretion (Figure 5A), suggesting that Stx2B might activate PKA by a cAMP-independent mechanism, perhaps analogous to pathways described for cAMP-independent PKA activation by IL-1,24 sphingosine,25 transforming growth factor-β,26 or endothelin-1.27
The activation of distinct signaling pathways represents a previously unsuspected qualitative difference between the events initiated by Stx1 and Stx2 binding at the cell surface. Although we identified these differences while investigating VWF secretion, other consequences of Stx B signaling may contribute to the pathogenesis of D+HUS independently of VWF. How these homologous toxins engage different signaling pathways remains to be determined. The B subunits of Stx1 and Stx2 are 50%-60% identical in amino acid sequence, and comparisons of their crystal structures show that each has 3 conserved binding sites per subunit for the terminal Galα(1,4)Gal(β1,4)Glcβ trisaccharide of Gb3. Slight differences between these sites in Stx1B and Stx2B influence ligand binding affinity, and some variants of Stx2B may bind Gb4 preferentially at Site 3.28-30 However, the major differences between Stx1B and Stx2B are elsewhere, on the lateral surface of the pentamer where they could mediate differential binding or clustering of other signaling molecules.
A recent study identified TLR4 as a coreceptor that facilitates the binding of Stx1 to Gb3. Depletion with siRNA of either TLR4 or Gb3 synthase markedly inhibited the cell surface binding and endocytosis of Stx1 or Stx1B by SW480 colon carcinoma cells and primary HUVECs.31 Stx1 was not compared with Stx2 for dependence on TLR4, and the effect of TLR4 depletion on VWF secretion was not evaluated in that study.
Additional potential Stx-GB3 coreceptors have been reported in other cell types. For example, the Src family kinases Yes and Lyn are activated and recruited to Gb3-containing membrane domains of ACHN renal cells32 or Ramos Burkitt lymphoma cells,33 respectively, within 5 minutes of binding Stx1. In these cells or HeLa cells, additional responses to Stx1 or Stx1B include the activation of Syk, p38 MAPK and PKCδ, phosphorylation of clathrin heavy chain, and an increase in clathrin-coated endocytosis.33-37 Whether Stx2 or Stx2B also can elicit these responses has not been reported, and their relevance to Stx-induced VWF secretion by endothelial cells needs to be evaluated. Further study is required to understand how the distinct signaling events initiated by Stx1B and Stx2B converge on a common pathway to induce WPB exocytosis.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Kendall J. Blumer (Washington University) for plasmid pUG36-2XPLCδPH, Martin J. Lohse (University of Würzburg) for plasmid Epac1-camps, and Andrey S. Shaw (Washington University) for assistance with confocal fluorescence videomicroscopy.
This work was supported by National Institutes of Health grants HL72917 and HL89746.
National Institutes of Health
Authorship
Contribution: F.L. and J.H. designed and performed research, analyzed and interpreted data, and wrote the manuscript; and J.E.S. designed research, analyzed and interpreted data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: J. Evan Sadler, Washington University School of Medicine, 660 S Euclid Ave, Box 8125, St Louis, MO 63110; e-mail: esadler@dom.wustl.edu.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal