Abstract
Recombinant granulocyte colony-stimulating factor (G-CSF) is used to accelerate recovery from chemotherapy-induced myelosuppression. G-CSF has been recently shown to stimulate angiogenesis mediated by several types of bone marrow-derived cell populations. To investigate whether G-CSF may alter tumor response to therapy, we studied Lewis lung and EMT/6 breast carcinomas in mice treated with paclitaxel (PTX) chemotherapy in combination with G-CSF. We compared the results obtained to mice treated with PTX and AMD3100, a small-molecule drug antagonist of CXCR4 which, like G-CSF, can be used to mobilize hematopoietic cells. We show that PTX combined with G-CSF treatment facilitates revascularization, leading to an improvement in blood perfusion in LLC tumors, and a decrease in hypoxia in EMT/6 tumors, thus enhancing tumor growth in comparison to PTX or PTX and AMD3100 therapies. We found that hemangiocytes but not Gr-1+ CD11b+ cells colonize EMT/6 tumors after treatment with PTX and G-CSF, but not PTX and AMD3100, and therefore may contribute to angiogenesis. However, increases in hemangiocyte colonization were not observed in LLC PTX and G-CSF–treated tumors, suggesting distinct mechanisms of tumor revascularization after G-CSF. Overall, our observations suggest that despite its known considerable clinical benefits, G-CSF might contribute to tumor revascularization by various mechanisms, and diminish the antitumor activity of chemotherapy, an effect that can be prevented by AMD3100.
Introduction
Systemic chemotherapy using various types of drug, including alkylating agents, microtubule inhibitors, antimetabolites, and antibiotics, among others, continues to be the dominant therapeutic modality for the majority of cancers. Chemotherapy is increasingly being used in combination with molecularly targeted biologic agents such as VEGF-pathway targeting antibodies (eg, bevacizumab),1 EGFR family inhibitors including Her-2 targeting agents,2 among many others. In addition, it remains standard practice to administer many, if not most, chemotherapy drugs at bolus maximum tolerated doses (MTD), generally separated by long break periods between successive courses of therapy.3 Such breaks are required to allow full or nearly full recovery from the host adverse effects caused by chemotherapy administered at MTDs.3 One of the most common adverse effects is myelosuppression including a significant drop in circulating neutrophil numbers (neutropenia).4,5 Myelosuppression is associated with potentially dangerous outcomes such as severe infections that can increase the duration of hospitalization. It also can result in temporary cessation or dose reductions of chemotherapy, both of which can ultimately reduce the overall treatment efficacy.6
A major advance in dealing with neutropenia is the routine use of hemopoietic growth factor support using recombinant forms of granulocyte colony-stimulating factor (G-CSF).5 Administration of G-CSF can increase neutrophils after chemotherapy by promoting mobilization of bone marrow–derived cells (BMDCs), thus reducing both the severity and duration of neutropenia.4 This in turn is associated with fewer episodes of dose reductions or temporary suspensions of the chemotherapy treatment. In addition, the accelerated recovery from neutropenia, which takes approximately 3 weeks (hence the traditional 3-week separation between successive cycles of MTD chemotherapy associated with many regimens), means that chemotherapy can sometimes be given in a “dose-dense” fashion, that is, every 2 weeks, with the aim of increasing the cumulative dose per unit time (“dose intensity”).7,8 Such dose-dense regimens have been shown to improve overall antitumor efficacy in phase 3 trials in some situations, such as when used as postoperative adjuvant therapy of low volume residual disease in early stage breast cancer patients.8,9 However, the increased dose intensity is achieved at the expense of greater toxicity and generally with only modest gains in survival benefits in certain cancers, such as breast carcinoma.10 Thus, although the clinical benefits of using recombinant G-CSF can be significant, improvements are needed.
One aspect of G-CSF biology that might be considered for achieving such improvement concerns the possible effect of G-CSF on stimulating certain mechanisms of tumor growth. Specifically, G-CSF has been reported as mobilizing several BMDC populations that can stimulate either vasculogenesis or angiogenesis.11 These include circulating endothelial progenitor cells (CEPs),12,13 Gr1+ and CD11b+ myeloid-derived suppressor cells (MDSCs),14 and VEGFR-1+ hemangiocytes.15 In addition to G-CSF, new agents have been clinically evaluated for harvesting hematopoietic stem cells for bone marrow transplantation. AMD3100 (Mozobil) is one such agent. It is a small-molecule CXCR4 antagonist that has been found to acutely mobilize hematopoietic stem cells similar to G-CSF.16 By disrupting the SDF-1α–CXCR4 axis, AMD3100 promotes the release of BMDCs from the bone marrow compartment.
Our interest in the effects of G-CSF on tumor biology stems from our several previously reported experimental observations, as follows: First, administration of cytotoxic-like microtubule-inhibiting “vascular disrupting agents” (VDAs) known to cause acute disruptions in tumor blood flow is invariably followed by rapid tumor regrowth from the remaining rim of viable tumor tissue. This is mediated, at least in part, by a rapid mobilization of CEPs that subsequently colonize the VDA-treated tumors,17 by which they contribute to angiogenesis and tumor growth. This host response appears to be dependent on systemic VDA-induced G-CSF levels from various host cells and tissues, the source of which is currently unknown.13 As such, the VDA-induced repopulating effect was found to be largely lost in G-CSF–null mice.13 Rapid increases of circulating G-CSF were also observed in VDA-treated cancer patients.13 Second, we have found that MTD chemotherapy using certain types of drugs such as paclitaxel (PTX) can also induce increases in plasma G-CSF, along with a number of other cytokines and chemokines, such as SDF-1α,18 as can administration of certain antiangiogenic drugs such as sunitinib, again in a mainly host-dependent fashion.19 Inductions of such biologically active growth factors, including G-CSF, could contribute to drug resistance, tumor (re)growth, and even alterations in malignant aggressiveness, any of which could negate or minimize clinical benefits.
With this information in mind, we decided to further investigate the possible effect of G-CSF on tumor growth, especially after chemotherapy drug treatment. We used PTX for our studies because it is used commonly in breast cancer patients, including in dose-dense regimens along with G-CSF support for adjuvant treatment of early stage disease. Here we report that this combination treatment, when used in short-term protocols, diminishes the antitumor activity of the chemotherapy by improving tumor vessel perfusion and augmenting overall tumor cell proliferation. In contrast, another agent known to mobilize BMDCs, and which can be used as an antimyelosuppressive strategy when administered in combination with chemotherapy (such as the CXCR4 antagonist AMD3100), can increase the efficacy of chemotherapy and improve treatment outcomes. Taken together, our results highlight the need to further evaluate the impact of G-CSF supplementation, especially when administered with chemotherapy, and indicate some possible strategies to improve outcomes when using this type of combination treatment.
Methods
Tumor cell lines and animal models
LLC cells (5 × 105 cells) obtained from ATCC were subcutaneously injected in the hind flank of 8-week-old immunocompetent C57Bl/6 or 129Sv/C57Bl/6 G-CSF (G-CSF+/+) wild-type mice (The Jackson Laboratory or Harlan Israel) or 129Sv/C57Bl/6 G-CSF deficient (G-CSF−/−) mice. EMT/6 murine breast carcinoma cells (5 × 105 cells; ATCC) were injected subcutaneously in the hind flank of syngeneic BALB/c mice (Harlan Israel). Tumor size was assessed regularly with Vernier calipers using the formula width2 × length ×0.5. When tumors reached 200 or 500 mm3, treatment was initiated. Mice were randomly grouped (n = 4-5 per group) before treatment with PTX, G-CSF, AMD3100, PTX and G-CSF, or PTX and AMD3100. In some in vitro experiments, the MDA–MB-231 human breast carcinoma cell line (ATCC) was used. All animal studies were approved by the institutional committee of both the Sunnybrook Health Sciences Center (Toronto), and the Technion (Haifa).
Drug concentrations
PTX chemotherapy (25 mg/kg in BALB/c, 25 mg/kg in G-CSF−/− mice and their wild-type counterparts, or 50 mg/kg in C57Bl/6 mice) was administered intraperitoneally as a single bolus injection. The doses were chosen based on a 15% reduction in body weight as described elsewhere.18 Human recombinant G-CSF (F. Hoffmann, La Roche Ltd; 25 μg/kg) was injected intraperitoneally on the day chemotherapy was administered, and AMD3100, a small-molecule CXCR4 antagonist drug (Sigma-Aldrich; 5 mg/kg each day) was injected subcutaneously for 3 sequential days starting on the day of chemotherapy administration.13,20 Control mice received the relevant vehicles.
Flow cytometry
Blood was obtained from anesthetized mice by retro-orbital sinus bleeding. Viable CEPs, hemangiocytes, and MDSCs were quantitated using flow cytometry, as described previously.15,18,21 Briefly, blood was collected in EDTA tubes or tumors were prepared as single-cell suspensions as previously described.22 Monoclonal antibodies were used to detect viable the CEPs VEGFR2+, 7AAD−, CD117+, CD45−; the hemangiocytes CXCR4+, VEGFR1+, CD45+; and the MDSCs Gr-1+ and CD11b+. Flow cytometry studies were performed on Cyan-ADP flow cytometer (Beckman Coulter) and analyzed with Summit Version 4.3 (Beckman Coulter) or FlowJo Version 8 (Ashland) flow cytometric analysis software. All monoclonal antibodies were purchased from BD Biosciences. Representative flow cytometry plots of the various cell types are presented in supplemental Figure 1 (available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Note that a subpopulation of hemangiocytes may share markers with MDSCs (data not shown).
Quantitation and visualization of tissue hypoxia, vessel perfusion, microvessel density, tumor cell proliferation, and apoptosis
Tissue processing and immunohistochemistry were performed as described previously.17 Briefly, tumor cryosections (4 to 6 μm) were used to analyze blood vessel perfusion bythe DNA-binding dye Hoechst 33342 (40 mg/kg; Sigma-Aldrich), and hypoxia was assessed by pimonidazole hydrochloride (60 mg/kg; Natural Pharmacia International Inc) as per manufacturer's instructions. Vessels were immunostained with endothelial cell specific antibody (anti-CD31, 1:200 ratio, BD Biosciences), and the number of vessels structures per field were counted and plotted (∼ 5 fields per tumor, n > 20 fields per group). Proliferating cells were immunostained with a rabbit polyclonal Ki-67 antibody (Cell Margue). Apoptotic cells were detected by the terminal deoxynucleotidyl transferase–mediated dUTP nick end labeling (TUNEL) staining (Roche Diagnostics). SDF-1α immunostaining was performed using a mouse anti–SDF-1α antibody (1:100 R&D systems). The quantification of hypoxia, perfusion, proliferation, apoptosis and SDF-1α expression was performed by analyzing the number of positive pixels from the total pixels in a micrograph using Photoshop CS2 Version 2 software (Adobe Systems). Tumor sections were visualized under a Leica CTR 6000 microscope (Leica Microsystems) using Leica Application Suite Version 3.
alamarBlue assay for cell viability
Cell viability was evaluated quantitatively with the metabolic indicator dye alamarBlue (Serotec Ltd). Cells were obtained from subconfluent cultures and replated (500 cells per well in a 96-well plate) in complete DMEM and alamarBlue (AB; 10%) solution, in the presence of 100 ng of G-CSF or 10 μg of AMD3100 after a series of dose escalations. In some experiments 250mM cobalt chloride (CoCl2) was added to the medium to mimic hypoxic conditions. Results were presented as the percentage of AB reduction and were corrected to background values of negative controls.
Gene expression analysis by RT-PCR
RNA was extracted from tumors using the RNeasy Mini Kit (QIAGEN), and subsequently converted into cDNA by reverse transcriptase (Promega). A total volume of 20 μL contained 300 ng of total RNA template and 10 μL of Power CYBR Green Master Mix (Applied Biosystems) was prepared. Reactions were run on a 7000 Real-Time PCR system (Applied Biosystems). The amounts of target genes were determined from the comparative threshold cycle (CT) method. Expression level of each gene was further quantified against the housekeeping GAPDH gene using the same treatment and expressed as 2−ΔCT(ΔCT of target gene–CT of GAPDH). The primer sequences were as follows: for mouse Bv8 forward, GCATGACAGGAGTCATCATTTT; reverse, AAATGGCAGGATATCAGGAAA; for mouse GAPDH forward, ATGTTCCAGTATGACTCCACTCACG; reverse, GAAGACACCAGTAGACTCCACGACA.
Evaluation of VEGF-A protein levels
A total of 200-300 mm3 of tumor tissue (n = 3 samples/group) were collected and homogenized in PBS containing 20mM HEPES, 100mM NaCl, 1mM EDTA, 1% Triton, and a protease inhibitor mixture (Roche Diagnostics). After lysates centrifugation, supernatant was collected. Equal amounts of protein (30 μg) were applied on a mouse VEGF-A ELISA (R&D Systems) following the manufacturer's instructions.
Evaluation of MMP2 expression using gelatin zymography
Tumor lysates (30 μg) were loaded on 10% acrylamide Ready Gel Zymogram Gels (Bio-Rad) under nonreducing conditions, followed by 30-minute incubation in 2.5% Triton X-100. The gels were then incubated for 16 hours at 37°C in 50mM Tris, 0.2M NaCl, and 5mM CaCl2 at a pH of 7.6. Gels were then stained with 0.5% Coomassie Blue for 1 hour and the stain removed with methanol, acetic acid, and water (10:10:80 ratio). The assay was repeated 3 times.
Statistical analysis
Data are expressed as mean ± SE, and the statistical significance of differences was assessed by 1-way ANOVA, followed by Newman-Keuls ad hoc statistical test using GraphPad Prism 4 software. Differences between all groups were compared with each other, and were considered significant at values of *P < .05, **P < .01, and ***P < .001.
Results
G-CSF is associated with enhanced tumor growth
The stimulatory effect of G-CSF on hematopoiesis is well documented, but its overall impact on tumor growth remains unclear. Therefore, we first investigated whether endogenous G-CSF is associated with altered tumor growth. To assess this, 5 × 105 LLC cells were implanted in mice deficient in G-CSF (G-CSF−/− mice) and their wild-type (wt) counterparts. Tumor growth was monitored by caliper measurements until they reached a predetermined size end point (∼ 1500 mm3). The results in Figure 1A show that LLC tumors grown in G-CSF−/− mice exhibited a modest tumor growth delay compared with tumors grown in wt mice. These initial results indicate that endogenous G-CSF may support tumor growth.
LLC tumor growth in G-CSF−/− or G-CSF wt mice treated with PTX, PTX and G-CSF, or PTX and AMD3100 and the relative microvessel density, hypoxia, and perfusion. LLC cells in the quantity of 5 × 105 cells were implanted in the flanks of (A) 8- to 10-week-old G-CSF+/+ or G-CSF−/− 129Sv/C57Bl/6 mice (n = 4-5 mice per group) or (B) 8- to 10-week-old C57Bl/6 which were treated with PTX, PTX and G-CSF, PTX and AMD3100. When tumors reached 200 mm3, treatment was initiated. Tumors were measured regularly using Vernier calipers, and tumor growth (volume) was plotted against number of days after tumor cell implantation. In a parallel experiment, C57Bl/6 mice bearing 500 mm3 LLC tumors (n = 4-5 mice per group) were treated with PTX or PTX in combination with either G-CSF or AMD3100. Tumors were removed 3 days later and evaluated for (C) microvessel density using CD31 immunostaining as a marker for endothelial cells (red; scale bar = 100 μm, 20×/0.50 NA); and hypoxia (green) and vessel perfusion (blue) using hypoxic probe and Hoechst as described in “Quantitation and visualization of tissue hypoxia, vessel perfusion, microcessel density, tumor cell proliferation, and apoptosis” (scale bars = 200 μm; 10×/0.30 NA). Quantification of (D) microvessel density, and (E) perfusion and hypoxia is provided.
LLC tumor growth in G-CSF−/− or G-CSF wt mice treated with PTX, PTX and G-CSF, or PTX and AMD3100 and the relative microvessel density, hypoxia, and perfusion. LLC cells in the quantity of 5 × 105 cells were implanted in the flanks of (A) 8- to 10-week-old G-CSF+/+ or G-CSF−/− 129Sv/C57Bl/6 mice (n = 4-5 mice per group) or (B) 8- to 10-week-old C57Bl/6 which were treated with PTX, PTX and G-CSF, PTX and AMD3100. When tumors reached 200 mm3, treatment was initiated. Tumors were measured regularly using Vernier calipers, and tumor growth (volume) was plotted against number of days after tumor cell implantation. In a parallel experiment, C57Bl/6 mice bearing 500 mm3 LLC tumors (n = 4-5 mice per group) were treated with PTX or PTX in combination with either G-CSF or AMD3100. Tumors were removed 3 days later and evaluated for (C) microvessel density using CD31 immunostaining as a marker for endothelial cells (red; scale bar = 100 μm, 20×/0.50 NA); and hypoxia (green) and vessel perfusion (blue) using hypoxic probe and Hoechst as described in “Quantitation and visualization of tissue hypoxia, vessel perfusion, microcessel density, tumor cell proliferation, and apoptosis” (scale bars = 200 μm; 10×/0.30 NA). Quantification of (D) microvessel density, and (E) perfusion and hypoxia is provided.
Because G-CSF is sometimes administered in combination with chemotherapy, we investigated whether the addition of exogenous G-CSF to PTX chemotherapy can affect treatment outcomes. To this end, 8-week-old C57Bl/6 mice were implanted with 5 × 105 LLC cells. When tumors reached a size of 200 mm3, treatment with MTD PTX given in combination with G-CSF was initiated. The results in Figure 1B show that tumors exposed to PTX monotherapy were smaller than tumors treated with PTX and G-CSF. Comparable results were also observed when both LLC and EMT/6 tumors were measured only 3 days after treatment with PTX, or PTX and G-CSF (supplemental Figure 2). Overall, these results suggest that both exogenous and endogenous G-CSF may blunt the tumor growth delay caused by chemotherapy.
G-CSF supplementation promotes angiogenesis, and can be replaced by AMD3100
Given the use of G-CSF in the clinic after treatment with myelosuppressive chemotherapy, we investigated other agents known to mobilize BMDCs, and which might be used as candidates for replacing G-CSF. In other words, is there a molecule or drug that can accelerate recovery from myelosuppression, but which does not promote tumor growth? A possible agent is AMD3100, a small-molecule antagonist of CXCR4,23 which has been clinically approved for use with G-CSF for mobilizing hematopoietic stem cells.24 AMD3100 was also shown to mobilize BMDCs preclinically and clinically.25,26 In addition, it has been demonstrated that in combination with other anticancer therapeutic modalities, it may have antitumor effects.20,27 We therefore assessed the effect of AMD3100 on tumor growth when combined with PTX chemotherapy. The results in Figure 1B and supplemental Figure 2 show that tumors treated with PTX and AMD3100, are smaller than tumors treated with PTX and G-CSF, and their growth pattern is similar to PTX monotherapy. Furthermore, we observed a reduction in the degree of myelosuppression represented by WBC counts after 24 hours and 5 days in non–tumor-bearing C57Bl/6 mice treated with PTX and AMD3100 compared with PTX monotherapy (supplemental Figure 3). Taken together, our initial results suggest that AMD3100 in combination with chemotherapy is not associated with enhanced tumor growth, and can also reduce the myelosuppressive effect of chemotherapy.
Next, we investigated possible mechanisms involved in tumor growth after exogenous G-CSF supplementation. To do so, LLC tumors of 500 mm3 in size grown in 8-week-old C57Bl/6 mice were treated with PTX, PTX and G-CSF, or PTX and AMD3100. Mice were killed 3 days later and tumors removed for the evaluation of hypoxia, perfusion, and microvessel density (MVD) as previously described.17 The results shown in Figure 1C through E indicate that tumors from mice treated with the combination of PTX and G-CSF have higher MVD values, higher percentages of perfused tumor areas, and slightly lower percentages of hypoxia than tumors from the PTX and AMD3100–treated group. Some of the parameters tested reached statistical significance. Comparable results were obtained when we used the EMT/6 tumor model (supplemental Figure 4). Overall, the results indicate that exogenous G-CSF can blunt the antitumor effects of PTX by inducing or promoting angiogenesis observed as increased in MVD, whereas AMD3100 appears to avoid these proangiogenic effects.
Induction in the mobilization and tumor homing of hemangiocytes but not MDSCs after treatment with PTX and G-CSF
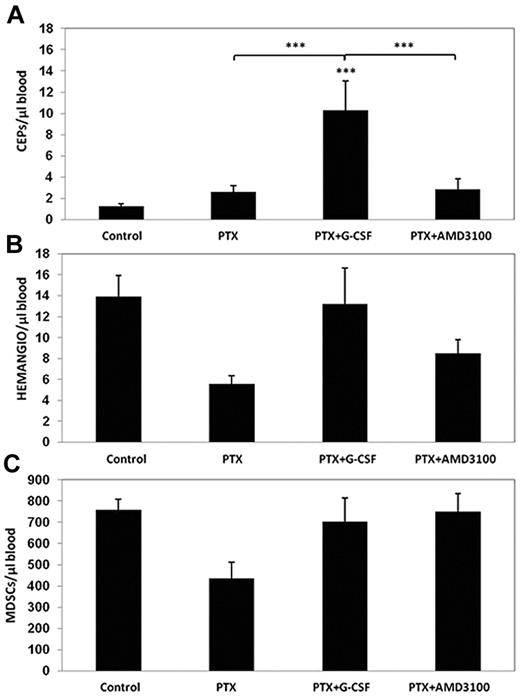
The increases in blood vessel perfusion and MVD after treatment with PTX and G-CSF, in contrast to PTX and AMD3100 treatment, led to the investigation of whether angiogenesis may play a role in mice treated with PTX and G-CSF. Because CEPs are rapidly mobilized from the bone marrow after PTX treatment, and are subsequently found in large numbers in treated tumors,18 we asked whether combined treatment of PTX with either G-CSF or AMD3100 could promote the mobilization of CEPs along with other BMDC populations known to promote tumor angiogenesis. We drew blood from the retro-orbital sinuses of mice 24 hours after they were treated with PTX, PTX and G-CSF, or PTX and AMD3100, and evaluated levels of CEPs, MDSCs, and hemangiocytes using the flow cytometry technique. We chose to focus on these particular BMDC populations because they have previously been shown to mediate G-CSF–stimulated tumor angiogenesis and growth.12,14,15 The results in Figure 2 indicate that treatment with PTX induced the mobilization of CEPs within 24 hours, consistent with our previous study.18 However, in mice treated with PTX and G-CSF, levels of CEPs significantly increased compared with PTX or PTX and AMD3100–treated groups. Furthermore, we did not observe significant changes in the levels of hemangiocytes and MDSCs in any of the treated groups, although a trend toward a reduction in levels of both cell types was observed after PTX monotherapy.
Evaluation of BMDC circulating populations after treatment with PTX or PTX in combination with either G-CSF or AMD3100. Non–tumor-bearing C57Bl/6 mice (n = 4 to 5 mice per group) were treated with PTX, PTX and G-CSF, or PTX and AMD3100. Mice were bled via retro-orbital sinus for the evaluation of (A) viable CEPs; (B) hemangiocytes; and (C) Gr-1+/CD11b+ cells (MDSCs), using flow cytometry. ***P < .001 from control group, unless indicated otherwise.
Evaluation of BMDC circulating populations after treatment with PTX or PTX in combination with either G-CSF or AMD3100. Non–tumor-bearing C57Bl/6 mice (n = 4 to 5 mice per group) were treated with PTX, PTX and G-CSF, or PTX and AMD3100. Mice were bled via retro-orbital sinus for the evaluation of (A) viable CEPs; (B) hemangiocytes; and (C) Gr-1+/CD11b+ cells (MDSCs), using flow cytometry. ***P < .001 from control group, unless indicated otherwise.
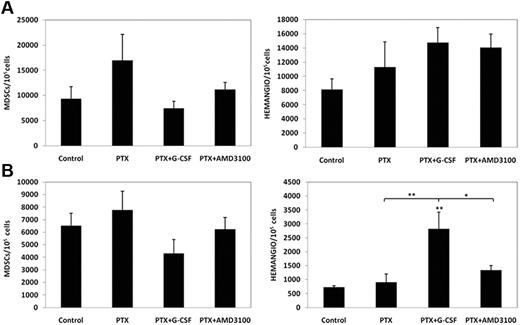
We next evaluated whether these BMDCs can colonize treated tumors. Our previous studies show that tumors in mice undergoing cytotoxic therapy are rapidly invaded by CEPs which may contribute to tumor cell repopulation commonly observed during the drug-free break periods.28 To this end, mice bearing either 500 mm3 LLC or 500 mm3 EMT/6 tumors were treated with PTX, PTX and G-CSF, or PTX and AMD3100. Mice were killed 3 days later, and tumors were removed and prepared as single cell suspensions. Subsequently, cells were analyzed by flow cytometry for the evaluation of the number of hemangiocytes and MDSCs colonizing the tumors. The results in Figure 3 indicate that the number of hemangiocytes invading EMT/6 but not LLC tumors is significantly increased after PTX and G-CSF therapy compared with PTX and PTX and AMD3100. Furthermore, we did not observe significant changes in the levels of MDSCs in any of the treated groups in either tumor model. Taken together, hemangiocytes but not MDSCs may contribute to the induction in angiogenesis in EMT/6 tumors, but not LLC tumors treated with PTX and G-CSF.
Homing and colonization of BMDCs in LLC and EMT/6 tumors after treatment with PTX or PTX in combination with either G-CSF or AMD3100. (A) C57Bl/6 mice bearing 500 mm3 LLC tumors (n = 4-5 mice per group) or (B) BALB/c mice bearing 500 mm3 EMT/6 murine breast carcinoma (n = 4-5 mice per group) were treated with PTX or PTX in combination with either G-CSF or AMD3100. Tumors were removed 3 days later and prepared as single cell suspensions for the evaluation of hemangiocytes (HEMANGIO) and MDSCs colonization of tumors using flow cytometry. *.05 > P > .01; **.01 > P > .001 from control group, unless indicated otherwise.
Homing and colonization of BMDCs in LLC and EMT/6 tumors after treatment with PTX or PTX in combination with either G-CSF or AMD3100. (A) C57Bl/6 mice bearing 500 mm3 LLC tumors (n = 4-5 mice per group) or (B) BALB/c mice bearing 500 mm3 EMT/6 murine breast carcinoma (n = 4-5 mice per group) were treated with PTX or PTX in combination with either G-CSF or AMD3100. Tumors were removed 3 days later and prepared as single cell suspensions for the evaluation of hemangiocytes (HEMANGIO) and MDSCs colonization of tumors using flow cytometry. *.05 > P > .01; **.01 > P > .001 from control group, unless indicated otherwise.
Bv8 and SDF-1α expression are down-regulated in tumors treated with PTX and G-CSF
To further assess the differences between PTX and G-CSF and PTX and AMD3100 treatment in terms of angiogenesis mediated by BMDCs, we tested the expression levels of both Bv8, a cytokine known to mobilize MDSCs,29 and SDF-1α, a chemokine attractant known to promote hemangiocyte retention in tumors,15,30 in 500 mm3 LLC tumors from mice treated with either PTX, PTX and G-CSF, or PTX and AMD3100. Tumors were removed 3 days after treatment, and either the tumors were sectioned for SDF-1α (protein) immunostaining, or mRNA was extracted for the evaluation of Bv8 mRNA levels. The results in Figure 4A and B show that SDF-1α expression at the tumor site was significantly lower in tumors treated with PTX and G-CSF compared with PTX monotherapy. Levels of SDF-1α expression in PTX and AMD3100-treated tumors were significantly higher than all other groups. The results in Figure 4C show that the mRNA levels of Bv8 were dramatically reduced in tumors treated with PTX and G-CSF or PTX and AMD3100. Comparable results of Bv8 and SDF-1α levels were observed in EMT/6 tumors after the various therapies (supplemental Figure 5). Overall, the lack of Bv8 expression in both PTX and AMD3100 and PTX and G-CSF–treated mice is in correlation with the reduced, albeit nonsignificant, number of MDSCs colonizing such tumors, and therefore, suggest that MDSCs may not play an important role in tumors treated with PTX and G-CSF. Furthermore, the increased expression of SDF-1α levels observed in PTX and AMD3100 tumors, in clear contrast to PTX and G-CSF–treated tumors, may be attributed to the increased levels of hypoxia-induced SDF-1α expression. Although the levels of SDF-1α were significantly higher in PTX and AMD3100 compared with PTX and G-CSF or PTX monotherapy, the number of hemangiocyte-colonizing PTX and AMD3100 tumors were significantly lower than PTX and G-CSF–treated tumors in the EMT/6 tumor model, probably because of the disruption of SDF-1α/CXCR4 axis by AMD3100, as was also shown by others.20,31
Relative SDF-1α protein expression and Bv8 mRNA expression in LLC tumors after treatment with PTX or PTX in combination with either G-CSF or AMD3100. (A) C57Bl/6 mice bearing 500 mm3 LLC tumors (n = 4-5 mice per group) were treated with PTX or PTX in combination with either G-CSF or AMD3100. Tumors were removed 3 days later and sections evaluated for SDF-1α expression (green; scale bar = 500 μm). (B) Quantification of relative SDF-1α expression is provided. (C) In parallel, mRNA was extracted from the same tumors, and Bv8 mRNA levels were measured by qRT-PCR. ***P < .001 from control group, unless indicated otherwise.
Relative SDF-1α protein expression and Bv8 mRNA expression in LLC tumors after treatment with PTX or PTX in combination with either G-CSF or AMD3100. (A) C57Bl/6 mice bearing 500 mm3 LLC tumors (n = 4-5 mice per group) were treated with PTX or PTX in combination with either G-CSF or AMD3100. Tumors were removed 3 days later and sections evaluated for SDF-1α expression (green; scale bar = 500 μm). (B) Quantification of relative SDF-1α expression is provided. (C) In parallel, mRNA was extracted from the same tumors, and Bv8 mRNA levels were measured by qRT-PCR. ***P < .001 from control group, unless indicated otherwise.
Increased tumor cell proliferation but not apoptosis observed after treatment with PTX and G-CSF
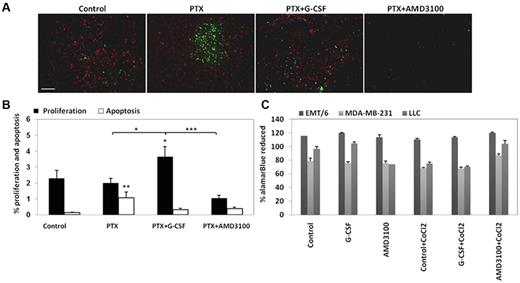
We next characterized the effect of PTX and G-CSF on tumor cell proliferation and apoptosis, and investigated whether both G-CSF and AMD3100 may contribute to tumor cell viability or apoptosis. To do so, 500 mm3 LLC tumors grown in C57Bl/6 mice were treated with PTX, PTX and G-CSF, or PTX and AMD3100. Tumors were removed 3 days later for the evaluation of tumor cell proliferation and apoptosis. The results in Figure 5A and B show that LLC tumors from mice treated with PTX and G-CSF exhibited significant increases in tumor cell proliferation compared with tumors from the untreated control group, and in clear contrast to PTX and AMD3100-treated tumors, which exhibited a significant reduction in cell proliferation. Furthermore, apoptosis measured by TUNEL staining was significantly lower in PTX and G-CSF EMT/6-treated tumors compared with PTX or PTX and AMD3100-treated tumors (supplemental Figure 6). These results indicate that the increases in MVD and perfusion probably resulted in enhanced tumor cell proliferation in LLC tumors or in reduced tumor cell apoptosis in EMT/6 tumors, both of which represent possible distinct mechanisms to explain tumor regrowth after G-CSF supplementation. To further evaluate whether G-CSF or AMD3100 have any direct effect on tumor cell viability, EMT/6, MDA-MB-231 (human breast carcinoma), and LLC tumors were cultured in the presence of either AMD3100 or G-CSF in both normoxic and hypoxic conditions. Tumor cell viability was measured by alamarBlue. The results in Figure 5C show that G-CSF and AMD3100 have minor direct effects on tumor cell viability. Of note, neither LLC nor EMT/6 tumor expressed CXCR4 (supplemental Figure 7). Taken together, our results suggest that the major effect of AMD3100 is by inhibiting tumor angiogenesis, and not by a direct effect on tumor cell apoptosis.
Evaluation of tumor cell proliferation and apoptosis in LLC tumors after treatment with PTX or PTX in combination with either G-CSF or AMD3100. (A) C57Bl/6 mice bearing 500 mm3 LLC tumors (n = 4-5 mice per group) were treated with PTX or PTX in combination with either G-CSF or AMD3100. Tumors were removed 3 days later and evaluated for proliferation (red) and apoptosis (green; scale bar = 200 μm). (B) Quantification of perfusion and hypoxia is expressed as percentage. (C) EMT/6, MDA-MB-231, and LLC cells were cultured in the presence of G-CSF or AMD3100 with or without CoCl2. Cell proliferation was evaluated quantitatively with the metabolic indicator dye alamarBlue. Results are presented as the percentage of alamarBlue reduction and were corrected to background values of negative controls.
Evaluation of tumor cell proliferation and apoptosis in LLC tumors after treatment with PTX or PTX in combination with either G-CSF or AMD3100. (A) C57Bl/6 mice bearing 500 mm3 LLC tumors (n = 4-5 mice per group) were treated with PTX or PTX in combination with either G-CSF or AMD3100. Tumors were removed 3 days later and evaluated for proliferation (red) and apoptosis (green; scale bar = 200 μm). (B) Quantification of perfusion and hypoxia is expressed as percentage. (C) EMT/6, MDA-MB-231, and LLC cells were cultured in the presence of G-CSF or AMD3100 with or without CoCl2. Cell proliferation was evaluated quantitatively with the metabolic indicator dye alamarBlue. Results are presented as the percentage of alamarBlue reduction and were corrected to background values of negative controls.
Reduced VEGF-A and MMP-2 expression in tumors treated with PTX and AMD3100
To further characterize the angiogenic properties of tumors treated with PTX and G-CSF in comparison to PTX and AMD3100, LLC and EMT/6 tumors of 200-300 mm3 in size grown in C57Bl/6 and BALB/c mice, respectively, were treated with PTX, PTX and G-CSF, or PTX and AMD3100. Tumors were removed 3 days later, and lysates were prepared. Because VEGF-A can stimulate MMP-2 activation in endothelial cells, thus promoting angiogenesis,32,33 and thereby increasing MVD in tumors,34 we considered these 2 candidates to help explain the differential effect of PTX and G-CSF compared with PTX and AMD3100 treatment on tumor growth and angiogenesis. We found that VEGF-A levels were significantly lower in LLC tumors treated with PTX and AMD3100 than in the other treated groups (Figure 6A). A trend toward reduced levels of VEGF-A was also seen in EMT/6 tumors after treatment with PTX and AMD3100 compared with all other groups (Figure 6B). We also observed a reduction in levels of MMP-2 in both LLC and EMT/6 tumors treated with PTX and AMD3100 compared with PTX and G-CSF (Figure 6C-D). Overall, these results might suggest a mechanism to explain, at least in part, why there is a reduction in angiogenesis in tumors treated with PTX and AMD3100 compared with PTX and G-CSF or PTX monotherapy, but do not explain the increase in MVD in PTX and G-CSF–treated tumors.
Analysis of VEGF-A, and MMP-2 levels in tumors after treatment with PTX, PTX and G-CSF or PTX and AMD3100. (A,C) C57Bl/6 mice bearing 500 mm3 LLC tumors (n = 3 mice per group) or (B,D) BALB/c mice bearing 500 mm3 EMT/6 tumors (n = 3 mice per group) were treated with PTX or PTX in combination with either G-CSF or AMD3100. Tumors were removed 3 days later and tumor lysates were evaluated for (A-B) MMP-2 expression by zymography, and (C-D) VEGF-A expression by ELISA. Summary of data are presented in graphs.
Analysis of VEGF-A, and MMP-2 levels in tumors after treatment with PTX, PTX and G-CSF or PTX and AMD3100. (A,C) C57Bl/6 mice bearing 500 mm3 LLC tumors (n = 3 mice per group) or (B,D) BALB/c mice bearing 500 mm3 EMT/6 tumors (n = 3 mice per group) were treated with PTX or PTX in combination with either G-CSF or AMD3100. Tumors were removed 3 days later and tumor lysates were evaluated for (A-B) MMP-2 expression by zymography, and (C-D) VEGF-A expression by ELISA. Summary of data are presented in graphs.
Discussion
Our results extend a small but growing body of literature showing circumstances where G-CSF may partially blunt the antitumor activity of chemotherapy, in part by angiogenesis-dependent mechanisms. This body of work now includes several preclinical studies showing that G-CSF can induce or promote the mobilization of several different types of proangiogenic BMDC populations, including CEPs12,35,36 and MDSCs.14,21,35 Furthermore, infiltration of MDSCs in tumors has been reported to confer “refractoriness” (resistance) to anti-VEGF therapy,14,21 or even to stimulate lung metastasis after G-CSF therapy.37 Is it possible that the G-CSF therapy in such circumstances may potentially reduce some of the beneficial antitumor effects of the chemotherapy?
These aforementioned studies raise an important question with respect to the use of recombinant G-CSF as a supportive care measure in neutropenic cancer patients who receive cytotoxic chemotherapy regimens. G-CSF support was critical in implementing the clinical concept of “dose-dense” chemotherapy, as in adjuvant therapy of women with postoperative early stage microscopic breast cancer.9,38 The use of G-CSF makes it possible to administer cytotoxic and myelosuppressive doses of chemotherapy more frequently, for example, every 2 weeks rather than the traditional once-every-3-weeks regimen.9,38 By doing so, the shorter time intervals between successive chemotherapy treatments means that there is less opportunity for surviving tumor cells to divide and repopulate, and thus increased tumor cell kill is achieved.38 However, the question arises as to whether the limited or lack of survival benefits observed in a number of dose-dense chemotherapy clinical trials or in patient subgroups39 might have been caused by the use of G-CSF supplementation. For example, this could help explain the negative outcomes of clinical trials involving dose-dense scheduling of patients with minimal residual and thus potentially “curable” disease, such as the dose dense adjuvant chemotherapy phase 3 trial in early breast cancer patients testing 5-FU, in which epirubicin and cyclophosphamide were administered every 2 weeks (FEC14) rather than a conventional once-every-3-weeks (FEC21) regimen.40 An important consideration with dose-dense chemotherapy trials is that the cumulative (“intensified”) dose over time may be greater than the standard once-every-3-weeks arm. However, this increased dose still did not translate into an improvement or benefit in survival.41 In retrospect, this last point might also be relevant to the failure of multiple randomized phase 3 trials of high-dose chemotherapy with an autologous stem cell transplant, such as in a more advanced stage (metastatic) breast cancer patients.42
Based on these studies, we decided to undertake preclinical studies of cytotoxic chemotherapy, in this case using PTX, to determine specifically whether G-CSF support might diminish some of the antitumor effects of the chemotherapy. This decision was also influenced by our own preclinical studies implicating drug-induced endogenous G-CSF as a factor that compromises the efficacy of VDAs.13
Our results support the possibility that the antitumor effects of chemotherapy using drugs such as PTX may be transiently diminished by coadministration of G-CSF. Thus, not only did we observe that the growth of LLCs was delayed in G-CSF−/− mice, but also that when exogenous G-CSF was administered in combination with PTX chemotherapy, tumor growth suppression was pronounced compared with mice which were treated with PTX alone (that is, without G-CSF).Together, these results suggest that both exogenous and endogenous G-CSF may contribute to tumor regrowth after treatment with chemotherapy.
To further show that tumor regrowth after PTX and G-CSF is due, in part, to augmented angiogenesis, we tested the direct effects of G-CSF on tumor cell viability. We found that G-CSF has only a minor direct impact on tumor cell viability with no antiproliferative effects in both normoxic and hypoxic conditions, whereas in vivo, increased numbers of proliferating LLC tumor cells (positive for Ki67) or decreased numbers of apoptotic EMT/6 tumor cells were observed. Thus, enhanced angiogenesis may be the major contributing factor to repopulation of tumor cells after PTX and G-CSF treatment, albeit via different mechanisms within the 2 tumor models tested.
Our results also suggest the consideration of a possible different or amended supportive care strategy. In previous studies we demonstrated that SDF-1α plasma levels are acutely and markedly elevated in mice treated with PTX, and the addition of an anti–SDF-1α–neutralizing antibody could block the induction of CEP mobilization, which resulted in enhanced antitumor activity of chemotherapy.18 Because the CXCR4 antagonist AMD3100 (plerexifor [Mobozil]) has been shown to cause an acute mobilization of functional BMDCs43 but can nevertheless block the retention and hence colonization of CXCR4+BMDCs in tumors (including drug-treated tumors) by disrupting the SDF-1α/CXCR4 axis,15,30 we assessed whether supplementation with AMD3100 might increase the efficacy of PTX chemotherapy treatment outcomes. Indeed, this drug combination enhanced the antitumor activity of chemotherapy, at least in part, by reducing tumor perfusion and angiogenesis. Although tumors treated with PTX or PTX and AMD3100 exhibited elevated expression of SDF-1α, the disruption of the SDF-1α /CXCR4 interaction by AMD3100 inhibited retention of hemangiocytes in the EMT/6 but not in the LLC drug-treated tumors. Our results are consistent with those published by Jin et al, who showed that CXCR4+ cells improve perfusion of hypoxic tissue via the SDF-1α.15 It should also be noted that AMD3100 has been found to have differential cell mobilization effects, which is dependent on drug treatment schedule.44 The administration of AMD3100 for 3 consecutive days, in our study, enhanced chemotherapy efficacy. Furthermore, Brown and colleagues have recently demonstrated that after localized radiation therapy of gliomas growing in the brains of mice, AMD3100 treatment blocked the colonization of the tumors by growth promoting bone marrow-derived monocytic populations, which significantly enhanced radiation treatment efficacy.20 In addition, we observed a substantial reduction in the expression levels of Bv8 in PTX and G-CSF and PTX and AMD3100 in comparison to PTX monotherapy, which was in correlation with reduced number of MDSCs colonizing treated tumors, suggesting that MDSCs may not play a significant role in PTX and G-CSF therapy. Aghi et al showed that AMD3100 can reduce angiogenesis mediated by CEPs in tumors as well as increase progenitor cell mobilization from the bone marrow.31 The fact that CEPs were found to be substantially induced in the circulation after PTX and G-CSF treatment compared with PTX or PTX and AMD3100 further suggests that these cells may contribute to angiogenesis in these treated tumors.13 Whether AMD3100 as a monotherapy can reduce tumor growth by inhibiting the interaction between CXCR4 and its ligand SDF-1α at the tumor microenvironment or not requires further investigation.
We also sought to determine whether PTX and G-CSF-treated tumors promote local angiogenesis by inducing the expression levels of proangiogenic factors within the tumor microenvironment, for example, VEGF. Ohki et al, demonstrated that G-CSF induced angiogenesis by the secretion of VEGF from neutrophils.36 Lysates of LLC tumors treated with PTX and AMD3100 revealed significantly reduced levels of VEGF-A expression compared with PTX and G-CSF treated tumors. These results may explain, at least in part, the reason for the alterations in angiogenesis observed in tumors treated with PTX and G-CSF compared with PTX and AMD3100-treated tumors. These findings are consistent with a previous study demonstrating that treatment with AMD3100 markedly reduced the expression of VEGF-A and MMPs in tumor cells.45 Furthermore, it has been demonstrated that increases in MMPs can elevate the bioavailability of VEGF-A from the extracellular matrix thus promoting the colonization of BMDCs enriched with MMPs.46 VEGF-A was found to stimulate MMP-2 activation in endothelial cells,32,33 explaining why PTX and AMD3100 therapy avoids the inductions in tumor angiogenesis that were found after PTX and G-CSF or PTX monotherapy; but this fact cannot explain why angiogenesis is increased after PTX and G-CSF treatment compared with PTX monotherapy.
In summary, our preclinical results, when considered along with some clinical outcomes, raise the possibility that tumor repopulation may be facilitated when G-CSF is administered as a supportive care measure with chemotherapy. Although there is no randomized clinical trial to indicate that survival outcomes are worsened when cancer patients receive G-CSF support along with myelosuppressive chemotherapy, it remains possible that improvements in treatment outcomes could be obtained by blocking the potential of tumor growth promoting effects of G-CSF. In this regard, our results demonstrate that mice treated with MTD PTX exhibited at least 50% reduction in WBC count, and that both G-CSF and AMD3100 treatment support reduced the myelosuppressive effects of chemotherapy. Strategies designed to maintain the anti-myelosuppressive supportive effects of G-CSF therapy while blocking the potential tumor growth promoting effects mediated by enhanced tumor colonization of certain BMDC populations should be given careful consideration. One possibility, in this regard, is the combined use of AMD3100 and G-CSF. Such a combination is used to enhance the mobilization of hematopoietic stem cell progenitors that can be obtained by G-CSF alone.47,48 In the context of cancer therapy, this combined strategy results in an enhanced mobilization of BMDCs, at the same time blocking the retention of pro-angiogenic BMDCs in tumors through the disruption of CXCR4–SDF-1α mediated by AMD3100.47,48
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by grants from the Israel Science Foundation (656/09, Y.S.), Israel Cancer Association, FP7 European Commission (Marie Curie) grant program (239212, Y.S.), and Mallat family foundation (Y.S.), and National Institutes of Health grant (R01-CA-41233, R.S.K.). S.G.-V. is supported by the Brain Power for Israel post doctoral fellowship. C.M. is the recipient of a postdoctoral fellowship from the Ontario Ministry of Research and Innovation.
National Institutes of Health
Authorship
Contribution: T.V. designed and performed the overall research, analyzed data, and wrote the paper; S.G.-V. performed research and analyzed data; R.B., L.B., and M.M. analyzed data; C.M. and S.M. performed experiments conducted on G-CSF knockout mice and analyzed data; R.S.K. analyzed data and wrote the paper; and Y.S. supervised and supported the overall study, designed research, performed experiments, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Yuval Shaked, Department of Molecular Pharmacology, Rappaport Faculty of Medicine, Technion–Israel Institute of Technology, 1 Efron St Bat Galim, Haifa, Israel; e-mail: yshaked@tx.technion.ac.il.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal