Extensive evidence suggests that the malignant cells of chronic lymphocytic leukemia (CLL) patients are in close contact with activated T lymphocytes, which secrete a range of cytoprotective cytokines including interleukin-4 (IL-4). IL-4 induced the rapid phosphorylation and activation of the signal transducer and activator of transcription 6 transcription factor in CLL cells in vitro. Longer incubation with IL-4 resulted in up-regulation of the antiapoptotic proteins, Mcl-1 and Bcl-XL. All of these events were blocked by the JAK3-selective inhibitor, PF-956980. A dye reduction cytotoxicity assay showed that IL-4 induced resistance to the cytotoxic drugs fludarabine and chlorambucil and to the novel p53-elevating agent nutlin 3. IL-4–induced drug resistance was reversed by PF-956980. These conclusions were confirmed by independent assays for apoptosis induction (annexin V binding, cleavage of poly[ADP-ribose] polymerase, and morphologic analysis). Coculture with bone marrow stromal cells in the presence of supernatants derived from activated T-lymphocyte cultures also protected CLL cells from apoptosis induction by chlorambucil. Protection by these combined signals was reversed by PF-956980. The data here provide a preclinical rationale for the possible therapeutic use of PF-956980 in conjunction with conventional cytotoxic drugs to achieve more extensive killing of CLL cells by overcoming antiapoptotic signaling by the microenvironment.
Introduction
Once thought to be a benign disease, chronic lymphocytic leukemia (CLL) is now known to comprise an aggressive subset, characterized by unmutated immunoglobulin heavy-chain variable (IGHV) genes, high expression of cell-surface CD38, and of the protein tyrosine kinase, zeta-chain–associated protein kinase 70 (ZAP-70).1 The proliferative compartment in CLL is located in “pseudofollicles” within bone marrow and lymphoid tissue,2 and access of CLL cells to these compartments is dependent on migratory chemokine signals, including chemokine (C-X-C motif) ligand 12 (CXCL12),2 chemokine (CC motif) ligand 19 (CCL19), and CCL21.3 CLL is treated with chlorambucil or fludarabine, but is incurable by chemotherapy alone.4 Because drug resistance is a formidable obstacle to treatment, it is important to identify and evaluate the mechanisms that contribute to apoptosis resistance in CLL and to develop strategies to overcome them.
Conventional DNA-damaging cytotoxic agents, including fludarabine and chlorambucil, kill CLL cells via the induction of the proapoptotic p53 protein.5 Therefore, mutations or deletions affecting the p53 genes are a major cause of drug resistance.6 However, genetic changes involving p53 are selected for during treatment, and this type of resistance is acquired over time.6 In contrast, environmental factors mediate drug resistance from the initiation of treatment, resulting in the protection of tumor cells from diverse chemotherapeutic agents and in the survival of foci of residual disease.2,7 It is therefore desirable to develop strategies that can be applied during initial treatment and are designed to minimize the survival of malignant cells and the consequent emergence of acquired resistance.
CLL cells, especially those located in bone marrow or lymphoid tissue, are exposed to extracellular signals that promote their survival and proliferation through the suppression of apoptotic pathways.2 Interleukin-4 (IL-4) is a Th2 lymphokine that signals via receptor-mediated activation of the Janus protein tyrosine kinases, JAK1 and JAK3, and subsequent phosphorylation and activation of the signal transducer and activator of transcription 6 (STAT6) transcription factor.8 IL-4 prolongs the basal survival of CLL cells in vitro,9 and the IL-4 receptor is abundantly expressed by CLL cells, compared with normal B lymphocytes, correlating with the substantially greater antiapoptotic effect of IL-4 on CLL cells.10,11 Extensive evidence suggests that IL-4/CLL interactions occur in vivo as well. First, an IL-4–secreting CD3+/CD8+/CD28− T-lymphoid population is expanded in CLL.12 Second, CLL cells secrete IL-6, which switches T lymphocytes from a T-helper 1 (Th1)– to an IL-4–secreting Th2 phenotype.13 Third, 40%-65% of CD8+ T cells of CLL patients contained IL-4, while < 10% of these cells from normal subjects did so.14 These T cells were also able to secrete IL-4 without activation. Fourth, the proportion of IL-4–positive T cells was greater in patients with progressive disease, compared with stable patients, suggesting that IL-4 played pathophysiologic roles in suppressing cell death.15 Finally, CLL cells within lymph nodes and bone marrow are in intimate contact with T cells,2 which stain strongly for IL-4 and also other T cell–derived cytokines, including IL-2, IL-6, and IL-10, which may additionally contribute to antiapoptotic signaling.16 It is therefore likely that cytoprotective interactions between CLL cells, IL-4, and other cytokines, which result in drug resistance, may occur predominantly in these complex microenvironments rather than in the periphery.2
Bone marrow mesenchymal stromal cells (MSCs) represent the in vitro counterpart of the predominant cell type of the bone marrow microenvironment in CLL.17 MSCs were initially shown to protect CLL cells from basal apoptosis.18 Cytoprotection is mediated by multiple contact-dependent and -independent signaling proteins, including the chemokine, CXCL12.19 CXCL12 binding to its receptor, CXCR4 (CD184), results in the activation of JAK2 and 3 and phosphorylation of STAT1, 3, and 5.20 MSCs protect CLL cells from apoptosis induction after treatment with cytotoxic agents17,21 and contribute to the persistence of foci of residual disease.
The studies reported here were designed to identify the intracellular pathways that are activated after IL-4 treatment of CLL cells to determine whether PF-956980, a JAK3-selective inhibitor,22 would abrogate IL-4–mediated signaling and reverse the ability of IL-4 to block the apoptotic killing of CLL cells by cytotoxic agents and to determine whether PF-956980 could also limit the antiapoptotic protection provided by MSC.
Methods
Reagents
Tissue-culture materials and preformed 12% polyacrylamide gels were from Invitrogen. Fludarabine was purchased from Schering Healthcare. Chlorambucil and 3-4,5-dimethylthiazol-2,5-diphenyl tetrazolium bromide (MTT) were from Sigma-Aldrich. Nutlin-3 and PD98059 were from Merck. Carbobenzoxy-valyl-alanyl-aspartyl-[O-methyl]-fluoromethylketone (Z-VAD-FMK) was from Biomol International. PF-95698022 was a generous gift from Dr Donnie Owens from Pfizer Inc. All other reagents were of the highest grade available.
Patients and cells
CLL was diagnosed according to established clinical criteria.23 A total of 29 isolates were studied (supplemental Table 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). The median lymphocyte count was 44 × 106/mL. IGHV genes were unmutated in 8 of the samples and mutated in 17, while the remaining 3 samples were not analyzed or were not quantifiable for technical reasons. This study was approved by the Local Research Ethics Committee of the Royal Free Hospital, London, United Kingdom. Written consent was obtained from patients before the collection of heparinized peripheral blood samples in accordance with the Declaration of Helsinki. Procedures for the isolation of malignant cells and the determination of their purity have been described in detail.24 All isolates used contained > 95% CD5/CD19-positive cells. All experiments were carried out using freshly isolated cells.
Cell culture and protein extraction
CLL cells were cultured in RPMI 1640 medium supplemented with 10% fetal calf serum and antibiotics. Differential detergent fractionation was carried out by a published procedure.5
Normal MSC cultures were established using cryopreserved human mononuclear cells as previously described.25 Before each experiment, stromal cells were harvested by brief trypsinzation. One milliliter of stromal-cell suspension (105 cells/mL) was plated in each well of a 24-well culture plate. The cells appeared confluent after 3 days of culture. The medium was then removed, and 1 mL CLL cells (5 × 106/mL) were added to each well. After a further 18 hours of culture, CLL cells were recovered for analysis by gentle pipetting, leaving the MSC layer undisturbed. CLL cells recovered in this way contained less than 1% of MSCs, as judged by microscopic examination of Giemsa-stained cytospin preparations and by flow cytometric analysis.
Normal human mononuclear cells (5 × 106/mL) were cultured in serum-supplemented RPMI 1640 medium. T lymphocytes were stimulated by the addition of 10 μg/mL phytohemagglutinin (PHA-P; Sigma-Aldrich). Three days after activation, the cells were recovered by centrifugation, washed twice in medium, and resuspended in fresh medium. After 6 hours of further culture, the cells were removed by centrifugation. The T-cell supernatant was filter sterilized and stored in aliquots at −80°C.
Determination of IC50
Cytotoxicity was assessed by carrying out triplicate assays for the reduction of MTT, after a 24- to 72-hour incubation of cells with serial 2-fold dilutions of drugs.26 Half-maximal inhibitory concentration (IC50) values were computed using CalcuSyn Version 1.2 software (Biosoft).
Quantitation of apoptosis
In accordance with recently published guidelines, apoptosis was quantified by multiple methodologically unrelated criteria.27 Flow cytometric analysis of CLL cells labeled with fluorescein isothiocyanate (FITC)–annexin V and propidium iodide (PI) was performed as previously described.24 Apoptosis was also quantified by Western blot assessment of the cleavage of the caspase 3 substrate, poly(ADP ribose) polymerase (PARP).5 Late-stage apoptosis was quantified morphologically by the counting of cells showing evidence of nuclear condensation and fragmentation in Giemsa-stained cytospin preparations, following established guidelines as previously described.27 A total of at least 800 cells were counted in 3 randomly selected fields. The percentage of apoptotic cells in each field was computed. The data are presented as means of the triplicate percentage values.
Western blotting
Procedures for extraction of cellular proteins, gel electrophoresis, and Western transfer have been described elsewhere.24 The following antibodies were from the indicated sources: PARP (BD Biosciences), p53 and Mcl-1 (Santa Cruz Biotechnology), actin (Sigma-Aldrich), Bcl-2 (Dako), Hsp60 (Stressgen), and lactate dehydrogenase (Abcam). All other antibodies were from Cell Signaling Technology.
Results
IL-4 treatment of CLL cells results in the rapid tyrosine phosphorylation of STAT6, which is inhibited by PF-956980
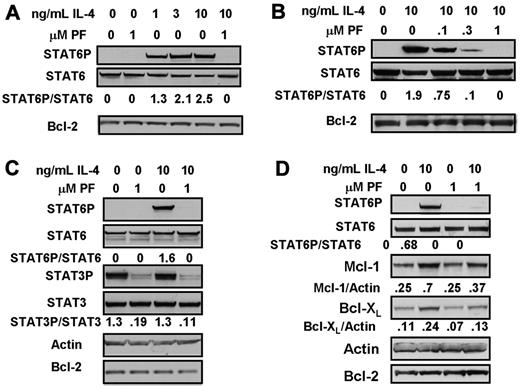
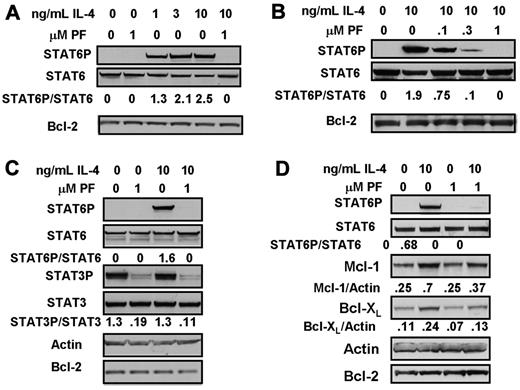
Phosphorylation of STAT6 was undetectable in unstimulated CLL cells, but was rapidly and strikingly induced by the addition of 1-10 ng/mL IL-4. IL-4–induced STAT6 phosphorylation was completely abolished by the JAK3-selective inhibitor, PF-956980 (Figure 1A). Figure 1B shows that 0.1μM of PF-956980 resulted in a 60% inhibition of STAT6 phosphorylation, and that between 0.3 and 1μM of the inhibitor was required to completely block phosphorylation induced by 10 ng/mL IL-4.
Inhibition of IL-4-induced STAT6 activation by treatment of CLL cells with PF-956980. (A-C) CLL cells were incubated for 20 minutes with the indicated concentrations of IL-4. When present, 1μM PF-956980 (abbreviated as PF) was added to cultures 20 minutes before the addition of IL-4. Protein lysates were analyzed by Western blotting using the antibodies indicated. In this and all figures, the densitometric band intensities determined using phosphorylation state-specific antibodies were normalized with respect to the intensities obtained by application of the corresponding phosphorylation state-independent antibody to a duplicate blot. The normalized band intensities are shown numerically. (D) CLL cells were incubated in the presence of the pan-caspase inhitor, Z-VAD-FMK (100μM), and with additions of IL-4 and/or PF-956980, as indicated. Lysates were prepared for Western blot analysis after 18-hour incubation. The isolates used here were from patients CLL1 (A), CLL 12 (B), CLL 19 (C), and CLL 28 (D). The data in each panel are representative of experiments carried out using isolates from 4 to 7 patients.
Inhibition of IL-4-induced STAT6 activation by treatment of CLL cells with PF-956980. (A-C) CLL cells were incubated for 20 minutes with the indicated concentrations of IL-4. When present, 1μM PF-956980 (abbreviated as PF) was added to cultures 20 minutes before the addition of IL-4. Protein lysates were analyzed by Western blotting using the antibodies indicated. In this and all figures, the densitometric band intensities determined using phosphorylation state-specific antibodies were normalized with respect to the intensities obtained by application of the corresponding phosphorylation state-independent antibody to a duplicate blot. The normalized band intensities are shown numerically. (D) CLL cells were incubated in the presence of the pan-caspase inhitor, Z-VAD-FMK (100μM), and with additions of IL-4 and/or PF-956980, as indicated. Lysates were prepared for Western blot analysis after 18-hour incubation. The isolates used here were from patients CLL1 (A), CLL 12 (B), CLL 19 (C), and CLL 28 (D). The data in each panel are representative of experiments carried out using isolates from 4 to 7 patients.
Unlike STAT6, STAT3 was constitutively phosphorylated in the cells from this patient, and this phosphorylation was not further enhanced by IL-4. PF-956980 completely abolished STAT3 phosphorylation in both untreated and IL-4–treated CLL cells (Figure 1C). Constitutive STAT3 phosphorylation was detectable in some CLL isolates (eg, Figure 1C), but not in others (eg, Figure 6A).
IL-4 treatment of CLL cells results in up-regulation of Mcl-1 and Bcl-XL and the activation of the ERK–signaling cascade
Apoptosis induction during longer incubation times may compromise the interpretation of Western blot data. Therefore, the long-term consequences of IL-4 treatment were determined by incubating CLL cells for 18 hours in the presence 100μM of the pan-caspase inhibitor, Z-VAD-FMK,28,29 a concentration shown to completely block caspase activation and apoptosis induction in CLL cells.29 Treatment of CLL cells with IL-4 for 18 hours resulted in a greater than 2-fold up-regulation of the antiapoptotic proteins, Mcl-1 and Bcl-XL. Up-regulation of both these proteins was blocked by PF-956980 (Figure 1D).
IL-4 induction of STAT 6 phosphorylation and PF-956980's ability to inhibit this event were both maintained over an 18-hour incubation (Figure 2A). However, the inhibition of STAT6 phosphorylation by 1μM PF-956980 was incomplete at this time of incubation, whereas complete inhibition was seen at 20 minutes (Figure 1A-C). It is likely that the modest decrease in inhibition seen at longer incubation times results from partial degradation of the drug.
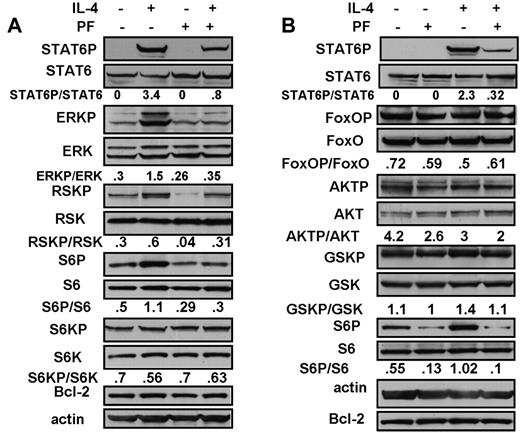
Analysis of downstream signaling events in CLL cells treated with IL-4. CLL cells were incubated for 18 hours in the presence of 100μM Z-VAD-FMK. IL-4 (10 ng/mL) or PF-956980 (1μM) were added, as indicated. Protein lysates were analyzed by Western blotting using phosphorylation state-specific antibodies, as indicated. Isolates from CLL18 and 16 were used in the experiments depicted in panels A and B, respectively. The data are representative of 9 (A) and of 5 (B) independent experiments.
Analysis of downstream signaling events in CLL cells treated with IL-4. CLL cells were incubated for 18 hours in the presence of 100μM Z-VAD-FMK. IL-4 (10 ng/mL) or PF-956980 (1μM) were added, as indicated. Protein lysates were analyzed by Western blotting using phosphorylation state-specific antibodies, as indicated. Isolates from CLL18 and 16 were used in the experiments depicted in panels A and B, respectively. The data are representative of 9 (A) and of 5 (B) independent experiments.
In addition to STAT6 activation, IL-4 can also up-regulate the extracellular signal–related kinase (ERK) pathway and the antiapoptotic pathway initiated by the sequential activation of phosphatidylinositol 3′-kinase and AKT protein kinase.8 ERK was constitutively phosphorylated and activated in CLL cells. The ERK target, ribosomal S6 kinase (RSK), as well as its substrate, the ribosomal S6 protein, were also constitutively phosphorylated (Figure 2A). IL-4 addition also resulted in a 5-fold increase in phospho-ERK, accompanied by an augmented phosphorylation of both RSK and S6 protein. These increases were reversed by PF-956980 (Figure 2A), showing that the IL-4 addition to CLL cells resulted in increased signaling through the ERK/RSK/S6 cascade, and that this was blocked by JAK3 inhibition.
The antiapoptotic actions of the AKT protein kinase pathway are mediated via the phosphorylation and inactivation of several proapoptotic downstream targets, including the transcription factor, FoxO3a, and of glycogen synthase kinase 3β (GSK-3β).30 AKT itself as well as FoxO3a and GSK-3β were constitutively phosphorylated in CLL cells (Figure 2B). Although the IL-4 receptor is known to activate this pathway in some cellular contexts,8 IL-4 did not augment the phosphorylation of any of these proteins (Figure 2B). In contrast, the cytokine triggered the increased phosphorylation of both STAT6 and S6 ribosomal protein in the same experiment. Activation of the AKT pathway was also not seen in CLL cells treated with IL-4 for shorter time intervals (20-60 minutes; data not shown).
The IL-4–induced blockade of CLL cell killing by cytotoxic agents is reversed by PF-956980
The concentrations of cytotoxic agents required to induce 50% loss of viability of CLL cells (ie, IC50 values) were determined by the incubation of cells with doubling dilutions of the drugs, followed by an estimation of the remaining viable cells by the MTT dye-reduction assay. In addition to fludarabine and chlorambucil, experiments were also done using the novel agent, nutlin-3, which results in p53 elevation and apoptosis induction by directly blocking the ability of Mdm2 to tag the p53 protein with ubiquitin residues and, consequently, target the latter protein for proteasomal degradation.31 IC50 values for each of these drugs were determined in the absence of further additions, in the presence of IL-4 or of PF-956980, or of a combination of IL-4 and PF-956980. The data are summarized in Figure 3, which shows that IL-4 decreased the sensitivity of CLL cells to each of the cytotoxic agents tested, as evidenced by an increase in the mean IC50. Coincubation with PF-956980 reversed the protective action of IL-4.
The impact of IL-4 and PF-956980 on the IC50 values of cytotoxic agents toward CLL cells. IC50 values for fludarabine, chlorambucil, and nutlin-3 were determined (“Methods”) in the presence of no further additions, 10 ng/mL IL-4, 1μM PF-956980, or IL-4 plus PF-956980, as indicated. Data for each treatment group are presented as mean values + standard errors. Statistical significance of the differences between means was assessed by computing P values, as indicated (2-tailed Student t test for paired samples). Isolates from patients 1–16 were used in panel B, with the omission of patient 8 in panel A and patients 4, 8, and 14 in panel C.
The impact of IL-4 and PF-956980 on the IC50 values of cytotoxic agents toward CLL cells. IC50 values for fludarabine, chlorambucil, and nutlin-3 were determined (“Methods”) in the presence of no further additions, 10 ng/mL IL-4, 1μM PF-956980, or IL-4 plus PF-956980, as indicated. Data for each treatment group are presented as mean values + standard errors. Statistical significance of the differences between means was assessed by computing P values, as indicated (2-tailed Student t test for paired samples). Isolates from patients 1–16 were used in panel B, with the omission of patient 8 in panel A and patients 4, 8, and 14 in panel C.
Both the IL-4–induced increase in mean IC50 values for each cytotoxic agent and its reversal by PF-956980 were statistically significant (Figure 3) and were observed in every isolate tested (not shown). PF-956980 alone (in the absence of IL-4) decreased the IC50 values less dramatically than in the presence of IL-4 and with lower levels of statistical significance in fludarabine- or chlorambucil-treated cells (Figure 3A-B). The inhibitor-induced decrease in IC50 values in the absence of IL-4 was evident, but not statistically significant, in experiments that used nutlin-3 as the cytotoxic agent (Figure 3C).
We attempted to determine whether the cytoprotective actions of IL-4 were related to the IGHV gene-mutation status or to the expression of CD38. We computed the fold increase in IC50 values for each cytotoxic agent used, in the presence of IL-4 relative to the IC50 value in the absence of IL-4 (“fold protection”). We also computed the fold decrease in IC50 value produced in IL-4–protected cultures by the addition of PF-956980 (“fold reversal”). The means of these parameters were separately evaluated for groups of isolates from patients with low vs. high IGHV mutation (supplemental Table 2A) or with low vs. high expression of CD38 (supplemental Table 2B). Using either the parametric t test or the nonparametric Mann-Whitney test, we observed no statistically significant difference (P > .05) between these parameters between isolates grouped by either the IGHV mutation or CD38 expression criteria. Although the number of isolates analyzed here is relatively small, we cautiously conclude that the protective actions of IL-4 and the ability of PF-956980 to reverse this protection are independent of these clinical prognostic criteria. We also failed to observe significant differences in fold protection among CLL isolates grouped according to the expression of ZAP-70 (data not shown).
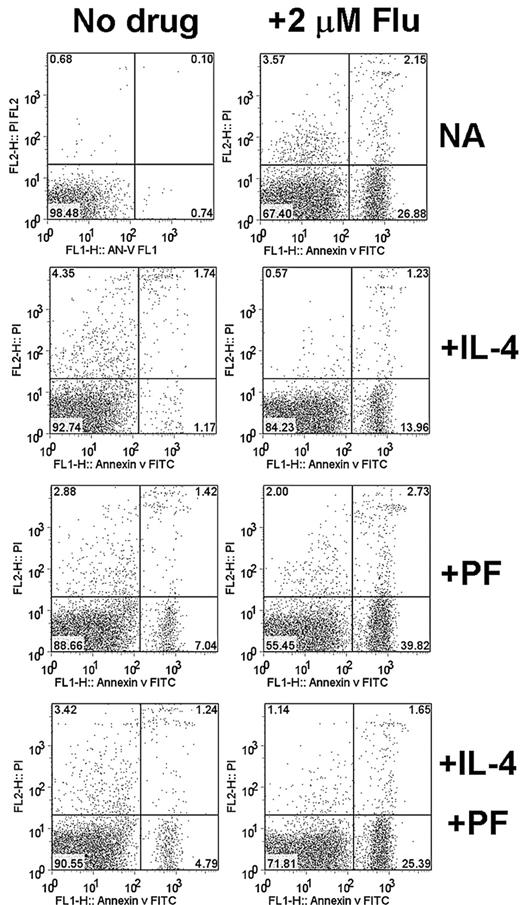
Quantitation of annexin V binding to CLL cells also showed that apoptosis induction by fludarabine was decreased in the presence of IL-4, and that this decrease could be reversed by PF-956980 (Figure 4). This conclusion was confirmed by data obtained using isolates from 3 additional patients, which are summarized in Table 1, together with the data from Figure 4. These data also showed that PF-956980 augmented apoptosis induction by fludarabine in the absence of IL-4 in 3 of the 4 isolates studied by annexin V staining (CLLs 13, 21, and 23).
Annexin V/propidium iodide (PI) analysis of CLL cells. Cells from CLL13 were incubated for 18 hours with the indicated additions. IL-4 was added at 10 ng/mL and PF-956980 at 1μM. Apoptosis was quantified by FACScan analysis of cells. Annexin V–FITC fluorescence is displayed on the vertical axis and PI fluorescence on the horizontal axis. Annexin V–positive/PI-negative cells in the lower right quadrant were considered as being apoptotic.
Annexin V/propidium iodide (PI) analysis of CLL cells. Cells from CLL13 were incubated for 18 hours with the indicated additions. IL-4 was added at 10 ng/mL and PF-956980 at 1μM. Apoptosis was quantified by FACScan analysis of cells. Annexin V–FITC fluorescence is displayed on the vertical axis and PI fluorescence on the horizontal axis. Annexin V–positive/PI-negative cells in the lower right quadrant were considered as being apoptotic.
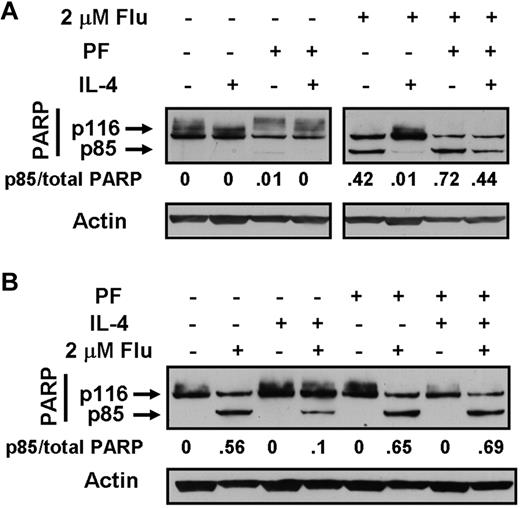
Cleavage of PARP, a caspase-3 substrate, is an established molecular criterion for apoptosis induction in CLL cells.5,24,26 PARP cleavage was quantified by Western blotting. Data obtained using isolates from 2 patients are shown in Figure 5 and confirm the antiapoptotic actions of IL-4 and the ability of PF-956980 to reverse cytoprotection by the cytokine. These conclusions were confirmed using isolates from a further 8 patients. Supplemental Figure 1 shows that the pattern of response established using fludarabine as the cytotoxic agent was also mirrored when chlorambucil or nutlin-3 were used to induce apoptosis. In all of the studies described, PF-956980 was added simultaneously with IL-4 and cytotoxic agents. However, the addition of PF-956980 3 hours before the addition of the other reagents produced the same reversal of cytoprotection (not shown) as was seen in the data presented here.
Western blot analysis of PARP cleavage in CLL cells. Cells from CLL 21 (A) or CLL 17 (B) were incubated for 18 hours with the indicated additions. IL-4 was added at 10 ng/mL and PF-956980 at 1μM. Lysates were analyzed by Western blotting. PARP cleavage was quantified by dividing the densitometric band intensity of the 85-kDa band by the total intensities of the 116- and 85-kDa bands. The data are representative of 10 experiments.
Western blot analysis of PARP cleavage in CLL cells. Cells from CLL 21 (A) or CLL 17 (B) were incubated for 18 hours with the indicated additions. IL-4 was added at 10 ng/mL and PF-956980 at 1μM. Lysates were analyzed by Western blotting. PARP cleavage was quantified by dividing the densitometric band intensity of the 85-kDa band by the total intensities of the 116- and 85-kDa bands. The data are representative of 10 experiments.
The PF-956980 dose dependence of the reversal of IL-4–induced cytoprotection was determined by PARP cleavage analysis (supplemental Figure 2A-B) and by morphologic quantitation (supplemental Figure 2C). Modest reversal was evident at 0.1μM of the inhibitor, with increasing apoptosis induction at 0.3 and 1.0μM. In an earlier study, IC50 values for the inhibition of JAK3-dependent signaling in cell-based assays were found to lie in the range 100-200 nM,22 similar to the levels of PF-956980 required to half-maximally inhibit STAT6 phosphorylation in CLL cells (Figure 1B). It is noteworthy that the PF-956980 dose response for the reversal of IL-4–mediated blockade of drug-induced apoptosis (supplemental Figure 2) was similar to the dose response for the inhibition of IL-4–triggered STAT6 phosphorylation (Figure 1B). However, slightly higher concentrations of the inhibitor were required for the reversal of drug resistance relative to the concentrations required for the inhibition of IL-4–induced STAT6 phophorylation in short-term assays. We attribute this modest discrepancy to a low level of drug inactivation in long-term culture. Therefore, our data are consistent with the interpretation that the ability of PF-956980 to reverse drug resistance was, indeed, attributable to the inhibition of JAK3.
The cytoprotective action of IL-4 intersects the apoptotic pathway at a point between the elevation of p53 and the release of mitochondrial cytochrome c
Treatment of CLL cells with DNA-damaging cytotoxic agents, including fludarabine and chlorambucil, results in the induction of the p53 protein.5 Conventional models of p53 action focus on its role as a transcription factor and its ability to transactivate proapoptotic proteins of the BH3-only subset of Bcl-2 family proteins.32 In contrast, recent studies have established that p53 can induce apoptosis via transcription-independent mechanisms involving the direct binding of p53 to antiapoptotic Bcl-2 family proteins at the mitochondrial surface, thus neutralizing their antiapoptotic function and triggering the release of cytochrome c from the mitochondria to the cytosol.33 Binding of cytochrome c to the Apaf-1 protein complex then initiates a cascade of caspase protease-activation events, resulting in the apoptotic demise of the cell.34 The major mechanism for apoptosis induction in CLL cells has been shown to be transcription independent and involves a direct biochemical interaction between p53 and Bcl-2 at the mitochondrial surface, resulting in cytochrome c release and caspase activation.5 This model was used as the basis for designing experiments aimed at locating approximately the point in the CLL apoptotic pathway at which IL-4–mediated signaling blocked cell killing
The induction of p53 by fludarabine-treated CLL cells was unaffected by concentrations of IL-4 between 2 and 10 ng/mL (supplemental Figure 3A), showing that the protective action of IL-4 was not dependent on inhibiting the complex mechanisms of p53 elevation.32 This conclusion is concordant with the observation that IL-4 blocks the cytotoxic action of nutlin-3 (Figure 3 and supplemental Figure 1), an agent that elevates p53 by direct inhibition of the p53-Mdm2 interaction31 and that bypasses the upstream pathways involved in the elevation of p53 by conventional cytotoxic agents. Furthermore, PF-956980 did not affect p53 levels in fludarabine-treated cells when added together with IL-4 (supplemental Figure 3A). Lysates for Western blot analysis were prepared from cultures incubated in the presence of Z-VAD-FMK. Parallel cell aliquots incubated in the absence of Z-VAD were used to assess apoptosis induction, confirming that fludarabine-induced apoptosis was blocked by IL-4 in this experiment, and that this blockade was reversed by PF-956980 (supplemental Figure 3A).
Differential detergent extraction experiments were then carried out to quantify the redistribution of cytochrome c between the mitochondrial-organellar fraction (fraction 2) and the cytosolic fraction (fraction 1). Fludarabine treatment resulted, predictably, in the release of cytochrome c from fraction 2 to 1 (supplemental Figure 3B). The selective nature of cytochrome c release is emphasized by the observation that the mitochondrial/organellar markers, heat-shock protein 60, Bcl-2, and cytochrome c oxidase IV, remained in fraction 2 under all incubation conditions and were undetectable in the cytosolic fraction (fraction 1). Cytochrome c release was blocked by IL-4 and was partially restored when PF-956980 was added in the presence of IL-4 and fludarabine. Supplemental Figure 3B also confirms that IL-4 did not block either the ability of fludarabine to induce p53 or the association of p53 with the mitochondrial/organellar fraction (fraction 2). The data therefore show that IL-4–mediated signaling blocks the intrinsic apoptotic pathway at a point beyond the association of up-regulated p53 with the organellar/mitochondrial fraction, but preceding the release of cytochrome c from the mitochondria to the cytosol.
PD-98 059 blocks IL-4–induced activation of ERK and RSK, but fails to reverse inhibition of apoptosis induction by IL-4
PD-98 059 is a chemical inhibitor of MEK, the protein kinase immediately upstream of ERK.35 PD-98 059 markedly reduced the constitutive phosphorylation of ERK and RSK and also eliminated the activation of these enzymes by IL-4 (supplemental Figure 4A). However, PD-98 059 had no effect on the blockade of fludarabine-induced apoptosis by IL-4, as quantified by PARP cleavage analysis or by morphologic criteria (supplemental Figure 4B-C). This conclusion was confirmed in studies using the annexin V binding assay (not shown).
PF-956980 reverses the blockade of CLL cell killing induced by T-cell culture supernatants and by MSC
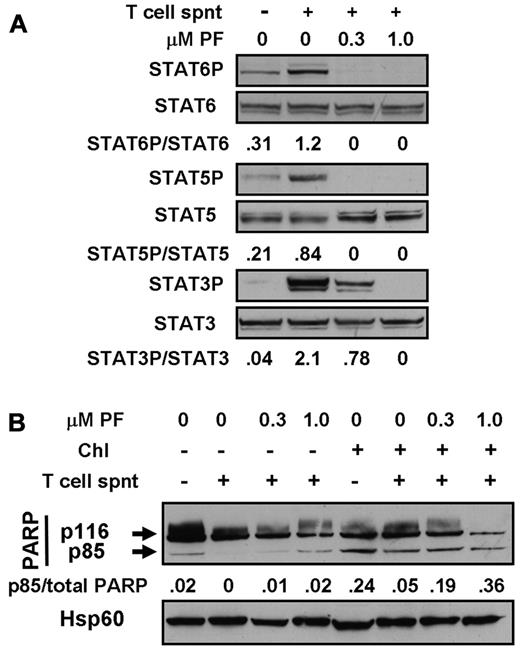
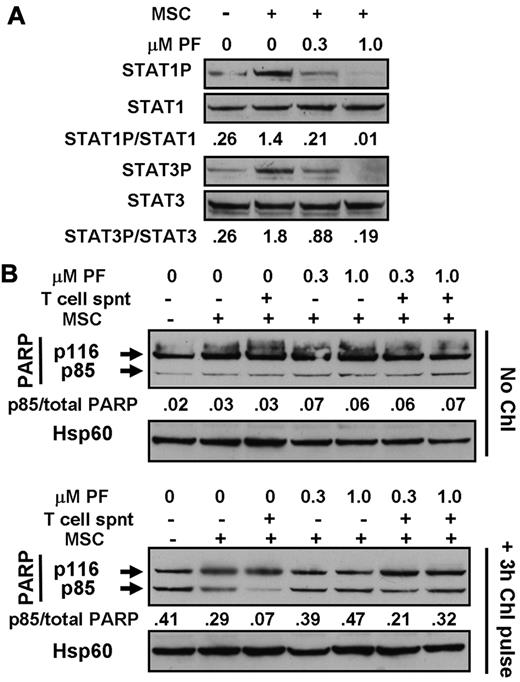
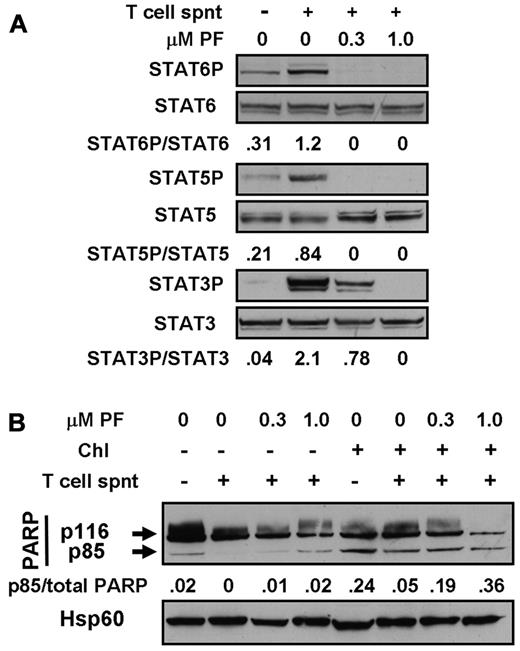
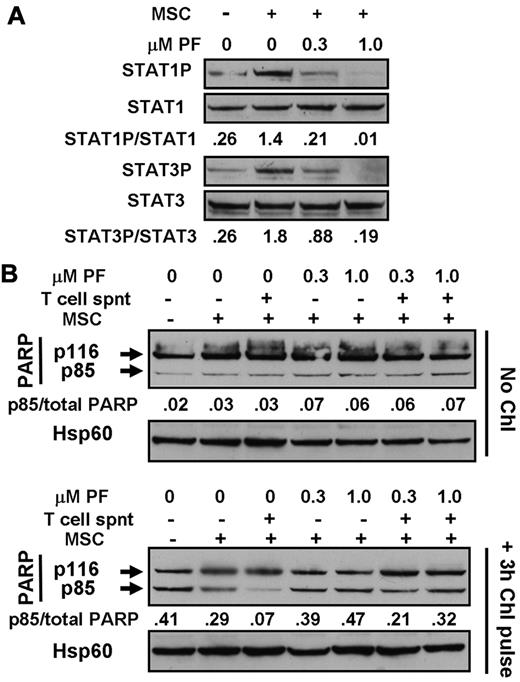
Experiments were done to mimic the cytoprotective actions of CLL cell microenvironments2 in vitro by carrying out experiments using T-cell culture supernatants and MSC cocultures. Incubation with T-cell supernatants induced the phosphorylation of STAT3, 5, and 6 (Figure 6A), consistent with the previously documented presence of IL-6, IL-2, and IL-4 in these supernatants.36,37 Culture of CLL cells on MSC layers also resulted in the up-regulation of phosphorylation of STAT1 and 3 (Figure 7A), but not of STAT6 (not shown). These observations are consistent with reports that MSCs secrete CXCL12,19 which activates the JAK-mediated phosphorylation of multiple STAT proteins via binding to its receptor, CXCR4.20 STAT phosphorylation induced by either T-cell supernatants (Figure 6A) or by coculture with MSC (Figure 7A) was abolished in a dose-dependent manner by PF-956980.
Cytoprotection by T-cell supernatants. (A) Cells from patient CLL16 were incubated for 20 minutes in the absence or presence of 20% T-cell supernatant (spnt) and/or PF-956980, as shown. Proteins were extracted and analyzed by Western blotting using the antibodies indicated. (B) Cells from patient CLL18 were left untreated or incubated with 100μM chlorambucil, as indicated, washed, and resuspended in fresh medium. PF-956980 and/or 20% T-cell supernatant were added, as indicated. After 18 hours of further incubation, proteins were extracted and PARP cleavage was quantified by Western blotting. Data are representative of 3 experiments.
Cytoprotection by T-cell supernatants. (A) Cells from patient CLL16 were incubated for 20 minutes in the absence or presence of 20% T-cell supernatant (spnt) and/or PF-956980, as shown. Proteins were extracted and analyzed by Western blotting using the antibodies indicated. (B) Cells from patient CLL18 were left untreated or incubated with 100μM chlorambucil, as indicated, washed, and resuspended in fresh medium. PF-956980 and/or 20% T-cell supernatant were added, as indicated. After 18 hours of further incubation, proteins were extracted and PARP cleavage was quantified by Western blotting. Data are representative of 3 experiments.
Cytoprotection by bone marrow stromal cells. (A) Cells from patient CLL1 were incubated for 18 hours with or without contact with marrow stromal cells (MSCs), as indicated. PF-956980 was added at the concentrations shown. Proteins were extracted and analyzed by Western blotting. (B) Cells from CLL12 were left untreated or incubated with 100μM chlorambucil for 3 hours, washed, and resuspended in fresh medium. Cells were placed in wells of a 24-well culture plate in the absence or presence of MSCs, as indicated. PF-956980 was added at the concentrations shown. After 18 hours of further incubation, proteins were extracted and PARP cleavage was quantified by Western blotting. Data are representative of 5 experiments.
Cytoprotection by bone marrow stromal cells. (A) Cells from patient CLL1 were incubated for 18 hours with or without contact with marrow stromal cells (MSCs), as indicated. PF-956980 was added at the concentrations shown. Proteins were extracted and analyzed by Western blotting. (B) Cells from CLL12 were left untreated or incubated with 100μM chlorambucil for 3 hours, washed, and resuspended in fresh medium. Cells were placed in wells of a 24-well culture plate in the absence or presence of MSCs, as indicated. PF-956980 was added at the concentrations shown. After 18 hours of further incubation, proteins were extracted and PARP cleavage was quantified by Western blotting. Data are representative of 5 experiments.
In these experiments, MSC layers were damaged by cytotoxic agents, but not by PF-956908 (not shown). Therefore, the experiments were done by exposing CLL cells to a 3-hour pulse of chlorambucil to induce DNA damage, followed by washing and resuspension of the cells and subsequent incubation for 18 hours in the presence of culture medium alone or with T-cell supernatants and/or MSCs. Pulsed exposure of CLL cells to chlorambucil followed by incubation with medium alone resulted in subsequent PARP cleavage, consistent with a previous report.38 Incubation with 20% T-cell supernatant substantially blocked PARP cleavage. This block was reversed in a dose-dependent manner by PF-956980 (Figure 6B). Quantitation of apoptosis by annexin V binding confirmed the blockade of chlorambucil-induced killing by T-cell supernatants and its reversal by PF-956980 (supplemental Figure 5A-D).
Culture of chlorambucil-pulsed CLL cells in contact with MSCs also resulted in a substantial reduction of PARP cleavage relative to that seen when the cells were cultured in medium alone (Figure 7B). The addition of 20% T-cell supernatant to the CLL/MSC cultures resulted in a further reduction in PARP cleavage. The cytoprotective actions of MSC alone or MSC plus T-cell supernatant were reversed by PF-956980 (Figure 7B). The conclusions drawn from these observations of PARP cleavage were supported by the results of experiments in which apoptosis was quantified by annexin V binding (supplemental Figure 5F-G). The annexin V experiments also show that T-cell supernatants alone (supplemental Figure 5A) or in combination with MSCs (supplemental Figure 5E) provided modest protection against spontaneous apoptosis, which was reversed by PF-956980. The negative values in these panels result from normalization to annexin V binding measured in controls with no additions.26
Discussion
Because IL-4 is likely play a major role in inducing drug resistance in vivo,10,,,,,–16 we initially used this cytokine in experiments designed to analyze the mechanisms by which cytokine signaling blocks apoptosis induction by cytotoxic agents and to establish the feasibility of using a JAK inhibitor to reverse cytoprotective signaling. These experiments have shown that IL-4 treatment of CLL cells resulted in the rapid activating phosphorylation of the STAT6 transcription factor. Longer incubation of CLL cells with the cytokine resulted in the up-regulation of the ERK/RSK pathway, culminating in phosphorylation of the ribosomal S6 protein. The antiapoptotic proteins, Mcl-1 and Bcl-XL, were also elevated in IL-4–treated cells. AKT was constitutively activated in CLL cells, in agreement with an earlier report,39 as were its substrates, FoxO3a and GSK-3β. Treatment with IL-4 did not increase the phosphorylation of these proteins.
PF-956980, an inhibitor highly selective for JAK3, blocked the ability of IL-4 to trigger STAT6 phosphorylation, to activate the ERK/RSK/S6 pathway, and to up-regulate Mcl-1 and Bcl-XL. Experiments using multiple independent criteria for the assessment of cytotoxicity and apoptosis induction showed that IL-4 blocked the killing of CLL cells by fludarabine, chlorambucil, and nutlin-3, and that PF-956980 strikingly reversed the cytoprotective action of IL-4 on CLL cells. Neither the IL-4–induced blockade of apoptosis nor its reversal by the JAK inhibitor appeared to correlate with IGHV mutation status or ZAP-70 and CD38 expression.
The ability of IL-4 to block cytotoxic drug action and of PF-956980 to reverse this blockade was seen in every CLL isolate studied. However, PF-956980 alone augmented apoptosis induction by cytotoxic agents in the absence of IL-4, and this effect was more variable among the different isolates. In addition to STAT6, STAT3 has also been shown to exert potent antiapoptotic actions and to contribute to malignant transformation in a wide range of both solid and hematopoietic human tumors.40,41 In agreement with a previous report,42 this study shows that constitutive tyrosine phosphorylation of STAT3 was detectable in some, but not all, CLL isolates, and that this phosphorylation was completely eliminated by PF-956980. This may explain the ability of PF-956980 to augment CLL cell killing even in the absence of IL-4. The mechanisms resulting in constitutive STAT3 activation and the downstream signaling events by which active STAT3 may modulate apoptosis sensitivity are unclear at present and warrant further investigation.
RSK is the principal mediator of cell-survival modulation by the ERK pathway in some cellular contexts.35 In this study, the MEK inhibitor, PD98059, at a concentration that clearly blocked the up-regulation of ERK and RSK by IL-4, had no effect on the antiapoptotic action of IL-4, although it did augment the cytotoxic actions of fludarabine in the absence of IL-4. The data show that the ERK/RSK/S6 pathway is constitutively active in CLL cells, in agreement with the data of Muzio et al,43 suggesting that that the constitutively active component of the ERK/RSK/S6 pathway impacts on apoptosis resistance and that its inhibition in the absence of IL-4 can augment the killing of CLL cells. The failure of PD-98 059 to reverse the cytoprotective actions of IL-4 suggests further that induction by the cytokine of mechanisms additional to the ERK/RSK pathway are likely to have potent antiapoptotic actions that may override the proapoptotic consequences of inhibiting the ERK pathway in the presence of IL-4.
Up-regulation of Mcl-1 and Bcl-XL by IL-4–mediated signaling may contribute to the generation of cytotoxic drug resistance, and the inhibition of these events by PF-956980 may explain the ability of this inhibitor to reverse the antiapoptotic actions of the cytokine. STAT6 has been shown to bind to the promoter of the Bcl-XL gene and to up-regulate Bcl-XL and, consequently, block apoptosis in mast cells44 and in mediastinal B-cell lymphoma cells.45 While the mechanisms by which IL-4 up-regulates Mcl-1 are unclear, the importance of this protein in apoptosis resistance is suggested by the positive correlation between constitutive Mcl-1 levels and fludarabine resistance in vitro.46 High Mcl-1 levels were also predictive of shorter time to first treatment.46 Up-regulation of Mcl-1 after engagement of the B-cell antigen receptor on the CLL cell surface also increased resistance to chemotherapy-induced apoptosis.47 The mechanistic experiments described in this study showed that the block to apoptosis induction is located at a point between up-regulation and mitochondrial association of p53 and the release of cytochrome c to the cytosol. The Mcl-1 and Bcl-XL proteins are located at the mitochondrial surface and act to block the intrinsic apoptotic pathway at a point preceding cytochrome c release.34 These data are consistent with roles for Mcl-1 and Bcl-XL in mediating the antiapoptotic actions of IL-4, but the participation of additional antiapoptotic targets of STAT6 and of other STAT6-independent signaling mechanisms triggered by IL-4 cannot be ruled out at present.
In experiments designed to replicate more closely the in vivo environment in which CLL cells interact with cytotoxic agents, CLL B cells pulsed with chlorambucil were cultured in the presence of T-cell culture supernatants alone or in tissue-culture wells containing monolayers of MSCs. Either T-cell supernatants or MSCs alone could block apoptosis induction, with evidence of enhanced protective effects when supernatants and MSCs were present in the same culture well. PF-956980 effectively reversed the apoptotic block induced by supernatants, by MSCs, or by a combination of supernatants and MSCs. These observations are consistent with the ability of both T-cell supernatants and MSCs to up-regulate STAT phosphorylation in CLL cells and with the reversal of STAT activation by the JAK inhibitor. Experiments in which CLL cells were incubated with MSC together with IL-4 (in place of T-cell supernatants) also showed that cytoprotection by this combination of signals were effectively reversed by PF-956980 (not shown). JAK3-mediated signaling is used by the receptors for IL-2, IL-4, IL-7, IL-9, IL-13 IL-15, and IL-21.37 Therefore, PF-956980 would inhibit prosurvival signaling initiated by any of these cytokines.
In conclusion, the studies here show that the JAK3-selective inhibitor, PF-956980, effectively reversed the ability of IL-4, MSCs, or T-cell supernatants to block the killing of CLL cells by cytotoxic agents. Among the 4 JAK family members, JAK3 is unique in that it associates exclusively with the γc transmembrane receptor subunit, which participates in signaling by only a limited subset of cytokines. It is therefore likely that JAK3 inhibitors may result in lower collateral cytotoxicity than other JAK inhibitors.48
PF-958960 is highly selective for JAK3, inhibiting this enzyme with an IC50 value of approximately 4nM cell-free enzyme assays. IC50 values toward a panel of 30 other protein kinases tested were in excess of 5μM.22 PF-956980 was shown to be effective in blocking delayed hypersensitivity in a murine model, demonstrating its in vivo efficacy and tolerability.22 A closely related JAK inhibitor, CP-690550, is currently being evaluated in clinical trials for rheumatoid arthritis and for the prevention of allograft rejection.22 Therefore, PF-956980 and other JAK3-selective inhibitors are candidate drugs for future use in the treatment of CLL as adjuvants that may overcome the resistance to cytotoxic drugs induced by microenvironmental signals.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This study was supported by Leukaemia and Lymphoma Research, United Kingdom. We also thank the family of Cyril Shack and the Dreyfuss Foundation for additional support.
Authorship
Contribution: A.J.S., A.G.P., M.W.L., and R.G.W. designed studies, analyzed data, and wrote the manuscript; A.J.S., S.M.H, E.R.S., and R.G.W. carried out research; and A.G.P., A.V.H., and K.C. recruited and obtained written consent from patients and collated clinical data used in this study.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: R. Gitendra Wickremasinghe, Department of Hematology, Cancer Institute, University College Medical School, Rowland Hill St, London NW3 2PF, United Kingdom; e-mail: r.wickremasinghe@medsch.ucl.ac.uk.