Although preclinical work with rapalogs suggests potential in treatment of multiple myeloma (MM), they have been less successful clinically. These drugs allostearically inhibit the mammalian target of rapamycin kinase primarily curtailing activity of the target of rapamycin complex (TORC)1. To assess if the mammalian target of rapamycin within the TORC2 complex could be a better target in MM, we tested a new agent, pp242, which prevents activation of TORC2 as well as TORC1. Although comparable to rapamycin against phosphorylation of the TORC1 substrates p70S6kinase and 4E-BP-1, pp242 could also inhibit phosphorylation of AKT on serine 473, a TORC2 substrate, while rapamycin was ineffective. pp242 was also more effective than rapamycin in achieving cytoreduction and apoptosis in MM cells. In addition, pp242 was an effective agent against primary MM cells in vitro and growth of 8226 cells in mice. Knockdown of the TORC2 complex protein, rictor, was deleterious to MM cells further supporting TORC2 as the critical target for pp242. TORC2 activation was frequently identified in primary specimens by immunostaining for AKT phosphorylation on serine 473. Potential mechanisms of up-regulated TORC2 activity in MM were stimulation with interleukin-6 or insulin-like growth factor 1, and phosphatase and tensin homolog or RAS alterations. Combining pp242 with bortezomib led to synergistic anti-MM effects. These results support TORC2 as a therapeutic target in MM.
Introduction
Preclinical data with mammalian target of rapamycin (mTOR) inhibitors such as rapamycin, temsirolimus, and everolimus suggest these drugs may have therapeutic potential in multiple myeloma (MM).1,–3 These mTOR inhibitors associate with the FKBP12 protein and together they bind to mTOR adjacent to its kinase domain. At this site, rapamycin allostearically inhibits the kinase, primarily that which is functional within the multiprotein complex kinase called target of rapamycin complex (TORC)1.4 The TORC1 complex consists of mTOR associated with mLST8 and Raptor.4 TORC1 phoshorylates the p70S6kinase (p70) and factor 4E binding protein 1 (4E-BP1) translational repressor and both these events stimulate translation of cell cycle proteins, thus promoting cell cycle transit.5,–7 By inactivating TORC1, these first generation mTOR inhibitors prevent cell cycle protein translation and induce G1 arrest.8
Although some early results of phase I/II trials that use these mTOR inhibitors in combination with other anti-MM agents suggest modest efficacy,9,10 use of tensilorimus as a single agent was relatively ineffective in MM patients.11 Some possible reasons for these disappointing results are suggested by previous mechanistic studies. In particular, treatment of MM cells with rapamycin or temsilorimus only induces cell cycle arrest without induction of apoptosis.1 Thus, as treated MM cells maintain viability, they may resume tumor growth during the time intervals between drug administration. One potential reason for lack of apoptosis is that there is a feedback activation of AKT when MM cells are treated with mTOR inhibitors.12 Activated AKT could serve as an anti-apoptotic protein.
In addition to the multifunctional TORC1 complex, mTOR participates in a second kinase complex called TORC2. TORC2 consists of mTOR complexed with mLST8, Sin 1, Protor and Rictor.4 The major TORC2 substrates are AKT and SGK with TORC2-induced AKT phosphorylation occurring on serine 473 (S473).13,14 As AKT S473 phosphorylation is required for full activation of the antiapoptosis kinase, newer second generation mTOR inhibitors have been developed that can inhibit TORC2 as well as TORC1, with the aim of preventing AKT activation. Although TORC2 has not previously been tested as a potential therapeutic target in MM, a small immunohistochemical study15 suggests the existence of in situ TORC2 activity in patient bone marrow myeloma cells as shown by heightened AKT S473 phosphorylation. Furthermore, immunodetection of AKT S473 phosphorylation in myeloma tumor cells was present while there was no staining of nonmalignant hematopoietic tissue, suggesting a therapeutic window existed for targeting TORC2.15 For these reasons, we initiated this study testing potential efficacy of an inhibitor, pp242, which specifically inhibits the mTOR kinase domain and significantly suppresses TORC2 as well as TORC1 activity.16
Methods
Cell lines, reagents, plasmids, and transfections
The ANBL-6 wild-type (WT), N-RAS and K-RAS–transfected cell lines were gifts from Dr Brian Van Ness (University of Minnesota, Minneapolis, MN). All other MM lines were obtained from ATCC. pp242 was purchased from JiHe and Chemdea Pharmaceuticals. For in vitro experiments, pp242 was dissolved in dimethyl sulfoxide (DMSO), and for in vivo experiments in 20% DMSO, 40% polyethylene glycol-400, and 40% phosphate-buffered saline. Rapamycin and bortezomib were purchased from Calbiochem and Millenium, respectively. All antibodies were purchased from Cell Signaling Technology except for anti-actin (Santa Cruz Biotechnology) and caspase 3-phycoerythrin (BD Pharmingen) for flow analysis of apoptosis. The adenovirus used to re-express phosphatase and tensin homolog (PTEN), or its empty vector control, in OPM-2 cells was previously described.17 Briefly, OPM-2 cells were transduced with adenovirus for 2 hours with a multiplicity of infection (MOI) of 100. Virus-containing solutions were removed and the cells were incubated in complete media for designated intervals. The shRNA/pLKO.1 targeting rictor (plasmid #1854) and the control scrambled sequence (plasmid #1864) were obtained from Addgene and previously described.13 Lentiviral shRNA production and infection was performed as previously described.18 After infection at different MOIs, clones were selected in puromycin.
Primary myeloma specimens
Primary MM cells were purified from bone marrow of patients by negative selection as described19 using the RosettesSep antibody cocktail method (Stem Cell Technologies). The purity by microscopy and CD138 flow cytometric analysis was > 99% plasma cells. The project was approved by the institutional review board of the Veterans Administration, and all participants gave written informed consent in accordance with the Declaration of Helsinki.
Animal model
As previously described,20 Nonobese severe combined immunodeficiency (NOD/SCID) mice were subcutaneously injected with 7.5 × 106 8226 cells/mouse into the left flank (in 200 μL of media). Mice were randomized to receive pp242 drug or vehicle control when the tumor volume reached approximately 200 mm3. pp242 was injected intraperitoneally daily for 5 days, followed by 2 days of no drug and then 3 additional daily injections. pp242 was given at a dose of 20 mg/kg, diluted in 20% DMSO, 40% polyethylene glycol-400, and 40% phosphate-buffered saline. Tumors were measured daily at their greatest length and width and the volume was calculated as previously described.20
Cell growth and apoptosis assays
Cell survival assays were performed as previously described.1,15,17 Surviving cells were enumerated by trypan blue exclusion. In individual experiments, groups were run in triplicate and the means of the replicates were used to calculate the percentage inhibition of viable recovery compared with untreated groups. The data are presented as percent of control, means of 3 or more individual experiments. Apoptosis was identified by flow cytometric staining for expression of activated caspase 3 (BD Biosciences) or membrane annexin V as previously described.12
IHC for phospho-AKT expression
Staining of MM bone marrow sections for phosphorylated AKT was performed with an antibody specific for AKT when it is phosphorylated on S473 (Cell Signaling; clone 736E11 used at a 1:40 dilution). Briefly, endogenous peroxidase activity was blocked with hydrogen peroxide and diaminobenzadine was used as chromagen to demonstrate immunodetection. Antigen retrieval was achieved by microwaving (0.1M citrate buffer, pH 6.0). The specificity of phospho-AKT staining was validated in serial negative control sections by omitting the primary antibody for each case. Percent of myeloma cells staining for expression of phosphorylated AKT was determined in blinded fashion by enumerating positive versus negative plasma cells in at least 5 randomly selected separated fields of vision (at 400× magnification).
Statistical analysis
The effect of combining pp242 with bortezomib on induction of apoptosis was assessed by the median effect method using Calcusyn software Version 1.1.1 (Biosoft). Combination indices (CI) values were calculated using the most conservative assumption of mutually nonexclusive drug interactions. CI values were calculated from median results of apoptosis assays. Student t test was used to determine significance of differences between groups
Results
pp242 inhibits TORC1 and TORC2 function
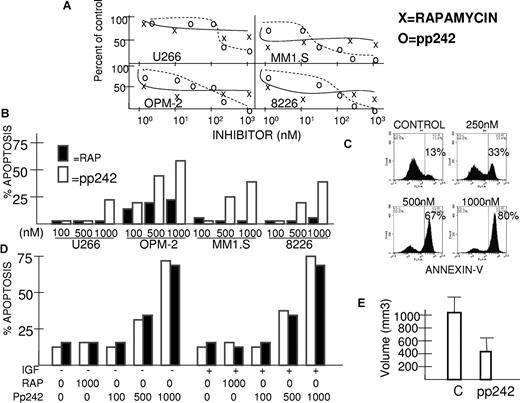
Initial studies were performed with 4 MM cell lines, U266, OPM-2, MM1.S, and 8226. U266, MM1.S, and 8226 cells were stimulated with or without insulin-like growth factor-1 (IGF-1; 250 ng/mL) for 30 minutes in the presence or absence of increasing concentrations of rapamycin versus pp242. pp242 is an ATP-competitive inhibitor that binds the mTOR catalytic site and, thus, theoretically curtails both TORC1 and TORC2 activity. In contrast, rapamycin is primarily only a TORC1 inhibitor. pp242 or rapamycin were added to cells 30 minutes before stimulation with IGF-1. Immunoblot assays were then performed to assess effects on TORC1 and TORC2 activity. For OPM-2 cells, IGF-1 treatment was not used since basal TORC1/TORC2 activity is quite high probably due to the fact this cell line is PTEN-null with heightened constitutive signaling downstream of PI3-kinase through AKT to mTOR. P70 is a direct substrate of TORC1 and phosphorylation of p70 on threonine 389 serves as a read-out for TORC1 activity. P70 was constitutively phosphorylated in all the MM cell lines but most remarkably in the PTEN-null OPM-2 cell line (Figure 1A). Phosphorylation of p70 was further induced by IGF-1 in MM1.S, 8226, and U266 cell lines. Both rapamycin and pp242 significantly inhibit p70 phosphorylation in all 4 cell lines. In contrast, the constitutive or IGF-1-induced phosphorylation of AKT on S473, used as a read-out for TORC2 activity, was only inhibited by pp242 with rapamycin having either no significant effect (in MM1.S and U266 cells) or, in fact, causing mild (OPM-2) or considerable (8226) enhancement of AKT phosphorylation. The ability of pp242 to curtail S473 AKT phosphorylation varied between the cell lines with an order of sensitivity from 8226 (most sensitive) > U266 > MM1.S > OPM-2.
Inhibition of TORC2 activity by pp242. (A-C) MM cell lines were pre-treated for 30 minutes with increasing concentrations of rapamycin (Rap) or pp242 (pp) and IGF-1 (250 ng/mL) was then added to all cell lines except OPM-2. After 30 minutes additional incubation, protein lysate was immunoblotted for ex-pression of total p70S6kinase (t-P70), phosphorylated P70 (p-P70(T389)), total AKT (t-akt), phosphorylated AKT [p-akt(S473)], total mTOR (t-mtor), phosphorylated mTOR [p-mTOR(S2481)], total NDRG1, phosphorylated NDRG1 (in C), and total ERK or phosphorylated ERK. Arrows in (C) point to the phosphorylated NDRG1 dimer present in control and rapamycin-treated cells. (D) 8226 cells treated with increasing concentrations of pp242 for 1 hour followed by immunoblot for phosphorylated ERK and total ERK. (E) MM1.S and OPM2 cells treated with 100nM of rapamycin for 0, 1, or 24 hours, followed by immunoblot assay for total AKT and S473 phosphorylated AKT.
Inhibition of TORC2 activity by pp242. (A-C) MM cell lines were pre-treated for 30 minutes with increasing concentrations of rapamycin (Rap) or pp242 (pp) and IGF-1 (250 ng/mL) was then added to all cell lines except OPM-2. After 30 minutes additional incubation, protein lysate was immunoblotted for ex-pression of total p70S6kinase (t-P70), phosphorylated P70 (p-P70(T389)), total AKT (t-akt), phosphorylated AKT [p-akt(S473)], total mTOR (t-mtor), phosphorylated mTOR [p-mTOR(S2481)], total NDRG1, phosphorylated NDRG1 (in C), and total ERK or phosphorylated ERK. Arrows in (C) point to the phosphorylated NDRG1 dimer present in control and rapamycin-treated cells. (D) 8226 cells treated with increasing concentrations of pp242 for 1 hour followed by immunoblot for phosphorylated ERK and total ERK. (E) MM1.S and OPM2 cells treated with 100nM of rapamycin for 0, 1, or 24 hours, followed by immunoblot assay for total AKT and S473 phosphorylated AKT.
Immunoblot assay for phospho-mTOR gave comparable results. Phosphorylation of mTOR on serine 2481 is a marker for TORC2 activity21 and this event was constitutively present in all the MM cell lines with further IGF-1-induced increase in 8226 cells and a very modest increase in MM1.S cells. Serine 2481 mTOR phosphorylation was significantly inhibited by pp242 in MM1.S, 8226, and U266 MM cell lines while rapamycin had little effect. The order of sensitivity was similar to the aforementioned sensitivities to AKT dephosphorylation with 8226 the most sensitive (significant inhibition of mTOR phosphorylation even at 1nM) and OPM-2 the most resistant (no significant inhibition observed). The experiment shown in Figure 1A was repeated with identical results.
An additional experiment (Figure 1B) was designed at additional concentrations of rapamycin and pp242 to semiquantitatively (by densitometry) assess the ability of rapamycin versus pp242 to inhibit TORC1 activity in 8226 and U266 cell lines. This experiment tested 0.1, 1, and 10nM of rapamycin versus 10, 50, and 100nM of pp242. When using p70 phosphorylation as a readout for TORC1 activity, it is apparent that, on a molar basis, rapamycin is more effective than pp242 (Figure 1B). The effective dose of rapamycin that inhibited p70 phosphorylation by 50% (ED50) in 8226 and U266 cells was approximately 1nM while the ED50 values for pp242 in these 2 cell lines were estimated to be 75 and 30nM, respectively.
Further evidence for TORC2 inhibition achieved by pp242 was demonstrated by immunoblot assay for phosphorylation of N-myc downstream regulated gene 1 (NDRG1) on serine 330. Although antibodies are not yet available for accurately testing TORC2-mediated phosphorylation of SGK1, we were able to detect phosphorylation of NDRG1, a known SGK1 substrate.22 Phosphorylated NDRG1 runs as a dimer in a 4%-15% gradient and a clear ablation of the larger phosphorylated species was seen in MM1.S cells treated with pp242 but not with rapamycin (Figure 1C). In addition, Figure 1C shows that the pp242-induced inhibition of AKT phosphorylation is specific for the S473 residue, the TORC2 substrate, with phosphorylation of T308, the PDK-1 substrate being little affected by pp242. These data further attest to the TORC2 specificity of pp242 inhibiton.
By inhibiting TORC2-induced phosphorylation and activation of AKT, pp242 could neutralize the feedback activation through insulin receptor substrate-1 (IRS-1) of the PI3K/AKT cascade resulting from TORC1 inhibition. However, a feedback activation of the extracellular signal-regulated protein kinase/mito-gen-activated protein kinase (ERK/MAPK) pathway has also been identified.23 Figure 1C (in MM1.S cells) and Figure 1D (in 8226 cells) demonstrate that feedback ERK activation still occurs in pp242-treated cells and may provide some protection against cytotoxicity.
Sarbassov et al24 showed that TORC2 activity can be inhibited in selected tumor cell lines by rapamycin used in prolonged incubations up to 24 hours. Figure 1E demonstrates that this does not occur in MM cell lines MM1.S and OPM-2.
In a previous study,16 pp242 was more effective than rapamycin as an inhibitor of release of eIF-4E from 4E-BP1. This was thought to be due to a greater dephosphorylation of 4E-BP1 induced by pp242. To test this in our MM cell lines, we immunoblotted extracts for 4E-BP1 specifically phosphorylated at T36/45, T70, and S65 (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). It is believed that 4E-BP1 phosphorylation is hierarchical with initial phosphorylation at T36/45 in the N-terminus preceding and being required for subsequent C-terminal phosphorylation at S65 and T70.25,–27 Phosphorylation of S65 is thought to be the most important for 4E-BP1/eIF-4E association.28 As shown in supplemental Figure 1, there were some differential dose-dependent effects on phospho-P70 versus 4E-BP1 phosphorylation. For example, 10nM rapamycin abrogates P70 phosphorylation in all cell lines, indicating complete TORC1 inhibition, but only clear 4E-BP1 dephosphorylation is seen at T70 residues in 8226 and MM1.S cells and at S65 in MM1.S. Clearly, there are some TORC 1 phosphorylation events on 4E-BP1 that resist rapamycin. However, it is noteworthy that these events are not more sensitive to pp242 (supplemental Figure 1). In fact, in these MM cell lines, rapamycin is more effective than pp242 on a molar basis for inhibiting serine 4E-BP1 phosphorylation (especially S65 phosphorylation). A prior study28 also suggested that pp242 was a more effective inducer of autophagy than rapamycin. However, immunoblot assay for LC3-II accumulation demonstrated comparable degrees of autophagy induced by the 2 drugs (supplemental Figure 1).
Antimyeloma effects of pp242
We tested cytoreductive effects of the mTOR inhibitors against the above MM cell lines in 72-hour survival assays comparing pp242 to rapamycin (Figure 2). In OPM-2, MM1.S, and 8226 cell lines, rapamycin had cytotoxic effects with 50% growth inhibitory concentrations of 1nM in MM1.S cells and 1-10nM in OPM-2 and 8226 cells. In U266 cells, rapamycin achieved 40% growth inhibition at approximately 50nM. However, the rapamycin cytotoxicity dose response was rather flat, reaching an early plateau in efficacy at 40%-60% inhibition of all cell lines (Figure 2A). In contrast, although the 50% growth inhibitory concentration for pp242 was higher (20-200nM), this drug suppressed cell growth by > 90% in 8226, OPM-2, and MM1.S cell lines at higher concentrations and by 70% in U266 cells. The different shapes of these dose response curves of rapamycin versus pp242 are very similar to those previously obtained with Ph+ murine and human leukemic cells.28
pp242 is more effective than rapamycin against MM cell lines. (A) Four cell lines were treated with increasing concentrations of rapamycin (x) or pp242 (o) for 72 hours and number of recovered viable cells was assessed. Data presented as percent of control (no inhibitor treatment), mean of triplicate samples. The standard deviation (SD) in all groups was < 5% of the mean. (B) Similarly treated MM cell lines were assessed for induction of apoptosis by flow cytometric analysis for expression of activated caspase 3. Data represent percent apoptosis over control (no inhibitor treatment), mean of triplicate samples. The SD was < 5% of the mean in all groups. Rapamycin treatment represented by dark bars and pp242 represented by white bars. (C) MM1.S cells treated with 0, 250, 500, or 1000nM pp242 and apoptosis assayed by annexin V staining. Percents shown are percent annexin V-positive cells. (D) 8226 MM cells treated for 48 hours ± IGF-1 (250 ng/mL) with increasing concentrations of either rapamycin or pp242 and apoptosis assayed by activated caspase 3 staining (open bars) and annexin V staining (black bars). (E) NOD/SCID mice were challenged with SC 8226 MM cells and treated with daily intraperitoneal injections of pp242 at 20 mg/kg for 8 treatments or vehicle alone (control). Treatment started when tumors were 200 mm3. The data represent the mean ± SD volume of pp242-treated tumors versus control (N = 6). The pp242-treated tumor volumes are significantly (P < .05) lower than that of control mice.
pp242 is more effective than rapamycin against MM cell lines. (A) Four cell lines were treated with increasing concentrations of rapamycin (x) or pp242 (o) for 72 hours and number of recovered viable cells was assessed. Data presented as percent of control (no inhibitor treatment), mean of triplicate samples. The standard deviation (SD) in all groups was < 5% of the mean. (B) Similarly treated MM cell lines were assessed for induction of apoptosis by flow cytometric analysis for expression of activated caspase 3. Data represent percent apoptosis over control (no inhibitor treatment), mean of triplicate samples. The SD was < 5% of the mean in all groups. Rapamycin treatment represented by dark bars and pp242 represented by white bars. (C) MM1.S cells treated with 0, 250, 500, or 1000nM pp242 and apoptosis assayed by annexin V staining. Percents shown are percent annexin V-positive cells. (D) 8226 MM cells treated for 48 hours ± IGF-1 (250 ng/mL) with increasing concentrations of either rapamycin or pp242 and apoptosis assayed by activated caspase 3 staining (open bars) and annexin V staining (black bars). (E) NOD/SCID mice were challenged with SC 8226 MM cells and treated with daily intraperitoneal injections of pp242 at 20 mg/kg for 8 treatments or vehicle alone (control). Treatment started when tumors were 200 mm3. The data represent the mean ± SD volume of pp242-treated tumors versus control (N = 6). The pp242-treated tumor volumes are significantly (P < .05) lower than that of control mice.
To test for induction of apoptosis, the MM cell lines were treated with rapamycin or pp242 for 72 hours followed by flow cytometric analysis of activated caspase 3 expression (Figure 2B). Rapamycin had no significant apoptotic effect except for a modest induction of apoptosis in OPM-2 cells. In contrast, pp242 induced apoptosis in all 4 MM cell lines in a dose-dependent fashion. Apoptosis could also be demonstrated by annexin-V staining which increased in pp242-treated MM1.S cells in a dose depen-dent fashion (Figure 2C). The annexin V-positive cells consistedof propidium iodide–negative (early apoptotic) and propidium iodide–positive (late apoptotic) cells (propidium iodide staining not shown). Figure 2D demonstrates that annexin-V and caspase 3 staining gave very comparable apoptosis results in pp242-treated MM cells. Furthermore, the presence of IGF-1 (250 ng/mL) had no protective effect against pp242 apoptosis.
To test if pp242 could inhibit tumor growth in vivo, NOD/SCID mice were challenged subcutaneously with 7.5 × 106 8226 MM cells and, when tumor growth reached 200 mm3, mice were randomized to receive daily intraperitoneal injections of pp242 given as 20 mg/kg or, in the control group, vehicle alone. pp242-treated mice remained healthy throughout the course of the experiment without any significant weight loss. They received 5 daily injections, followed by 2 days of no treatment and then 3 additional daily injections. The experiment was then terminated because tumor growth in control mice had become distressful to mice. In the pp242-treated cohort, progression of tumor growth was significantly slowed. As shown in Figure 2E, after 8 injections of pp242, there was a 50% decrease in tumor volume compared with control mice.
Antitumor effects of pp242 against primary MM cells
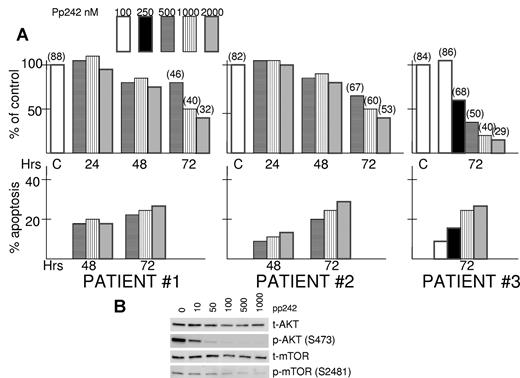
Isolated bone marrow myeloma cells from aspirates of 3 separate patients with newly diagnosed disease were exposed to increasing concentrations of pp242 to test the drug's cytotoxic effects against primary cells. As shown in Figure 3A, there was a time-dependent and dose-dependent cytotoxic response in these primary MM specimens. The viable recovery of pp242-treated cells was minimally affected at 48-hour time points but significantly inhibited by 72 hours and this was associated with increased MM cell death (parentheses above bars at 72-hour time points represent the percentage viability by trypan blue staining). In addition, these patient specimens demonstrated significantly induced apoptosis after exposure to pp242 (Figure 3A).
pp242 is effective against primary MM samples. (A) Isolated primary MM cells from 3 bone marrow aspirates were exposed to increased concentrations of pp242 for 24, 48, or 72 hours. Number of viable cells was then recorded. Data presented as percent of control (no inhibitor) where the control counts at 24, 48, or 72 hours were arbitrarily made 100%. At top of some bars is the percentage viability determined by trypan blue staining. For each primary specimen at several time points, percent apoptosis was also determined and the data represent percent apoptosis above control (no inhibitor). The latter control groups showed a mean percent apoptosis at 48 hours incubation of 28% and mean percent apoptosis at 72 hours of 39%. (B) One of the patient's cells were treated with increasing concentrations of pp242 for 2 hours after which immunoblot was performed for the designated proteins.
pp242 is effective against primary MM samples. (A) Isolated primary MM cells from 3 bone marrow aspirates were exposed to increased concentrations of pp242 for 24, 48, or 72 hours. Number of viable cells was then recorded. Data presented as percent of control (no inhibitor) where the control counts at 24, 48, or 72 hours were arbitrarily made 100%. At top of some bars is the percentage viability determined by trypan blue staining. For each primary specimen at several time points, percent apoptosis was also determined and the data represent percent apoptosis above control (no inhibitor). The latter control groups showed a mean percent apoptosis at 48 hours incubation of 28% and mean percent apoptosis at 72 hours of 39%. (B) One of the patient's cells were treated with increasing concentrations of pp242 for 2 hours after which immunoblot was performed for the designated proteins.
We were fortunate to obtain a sufficient number of MM cells from one of the above patients to perform immunoblot assays after a 2-hour exposure to pp242. As shown in Figure 3B, there was a dose-dependent inhibition of AKT 473 phosphorylation and mTOR 2481 phosphorylation in this patient's cells confirming the inhibition of TORC2 activity by pp242 in primary cells.
Effects of rictor knockdown
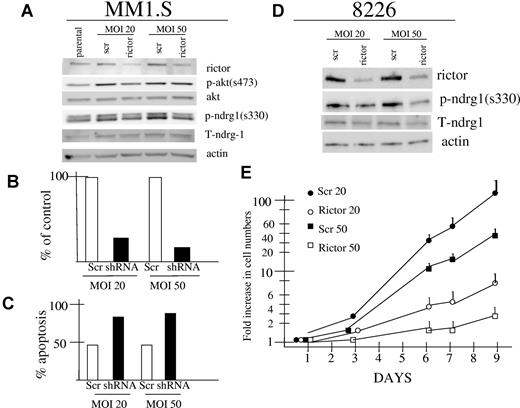
Because rapamycin was more effective than pp242 in terms of inhibiting p70S6K phosphorylation and equally effective in terms of inhibiting 4E-BP1 phosphorylation and autophagy, the above data suggested that the superior antitumor effect of pp242 (versus rapamycin) was due to its additional inhibitory effects on TORC2. To provide further support for TORC2 as a target in MM cells, we knocked down expression of rictor in MM1.S and 8226 cell lines by lentiviral infection with shRNA. As shown in Figure 4A and 4D, successful knockdown was achieved in both MM cell lines using MOIs of 20:1 or 50:1. In MM1.S cells, rictor knockdown was associated with a decrease in AKT phosphorylation on S473 best seen in the 20:1 MOI group. We also attempted to assess effects on the TORC2 substrate SGK but, as described previously, our phosphospecific SGK antibody was not successful. Thus, we immunoblotted for phosphorylated NDRG1, the SGK substrate. A decrease in phosphorylation of both NDRG bands was detected in rictor-knocked down MM1.S cells (Figure 4A). For shRNA transduced 8226 cells, it was also difficult to identify inhibitory effects on phosphorylation of AKT because the constitutive levels of phospho-AKT expression in control cells (and parental cells; see Figure 1A) is so low. However, immunoblotting for phospho-NDRG1 demonstrated clear inhibition of NDRG1 phosphorylation. In 8226 cells, the larger sized band of the NDRG dimer is very poorly expressed so that only the lower phosphorylated NDRG1 band is visible.
Effect of rictor knockdown in MM1.S and 8226 MM cell lines. (A) and (D) MM1.S and 8226 cells were infected with lentivirus expressing shRNA targeting rictor (rictor) or control shRNA containing a nontargeting sequence (scr). Cells were infected at MOIs of 20:1 or 50:1. Infected MM1.S cells were assayed by immunoblot 3 days after infection for expression of rictor, actin, phospho-AKT, total AKT, phosphorylated NDRG1 (on serine 330), or total NDRG1. Infected 8226 cells were selected in puromycin and stably expressing lines were similarly assayed by immunoblot for rictor, actin, phospho-NDRG1 and total NDRG1. (B-C) MM1.S cells infected with lentivirus expressing rictor-targeted shRNA (shRNA) or control shRNA (scr) at 20:1 or 50:1 MOIs were assayed 5 days after infection for number of viable cell recovery (B) or for percent apoptosis (C) where apoptosis was assayed by flow cytometric analysis of activated caspase 3. The experiment in (B) and (C) was repeated once with identical results. (E) Stable 8226 cell lines expressing rictor shRNA after 20 or 50:1 MOI infections and in vitro selection (rictor 20, rictor 50) or control (scr 20, scr 50) sequence and in vitro selection, were seeded at 105 cells/mL and assayed for cell growth in vitro over 9 days. Data are presented as fold increase in viable cell numbers on a log scale, mean ± SD (n = 4).
Effect of rictor knockdown in MM1.S and 8226 MM cell lines. (A) and (D) MM1.S and 8226 cells were infected with lentivirus expressing shRNA targeting rictor (rictor) or control shRNA containing a nontargeting sequence (scr). Cells were infected at MOIs of 20:1 or 50:1. Infected MM1.S cells were assayed by immunoblot 3 days after infection for expression of rictor, actin, phospho-AKT, total AKT, phosphorylated NDRG1 (on serine 330), or total NDRG1. Infected 8226 cells were selected in puromycin and stably expressing lines were similarly assayed by immunoblot for rictor, actin, phospho-NDRG1 and total NDRG1. (B-C) MM1.S cells infected with lentivirus expressing rictor-targeted shRNA (shRNA) or control shRNA (scr) at 20:1 or 50:1 MOIs were assayed 5 days after infection for number of viable cell recovery (B) or for percent apoptosis (C) where apoptosis was assayed by flow cytometric analysis of activated caspase 3. The experiment in (B) and (C) was repeated once with identical results. (E) Stable 8226 cell lines expressing rictor shRNA after 20 or 50:1 MOI infections and in vitro selection (rictor 20, rictor 50) or control (scr 20, scr 50) sequence and in vitro selection, were seeded at 105 cells/mL and assayed for cell growth in vitro over 9 days. Data are presented as fold increase in viable cell numbers on a log scale, mean ± SD (n = 4).
As a result of rictor knockdown, MM1.S cells demonstrated a significantly reduced ability to grow in vitro and an increased percent of cells spontaneously undergoing apoptosis (Figure 4B-C). The rictor-silenced MM1.S cells could not grow sufficiently to be selected by antibiotic and the data shown in Figure 4B and C are for nonselected knocked-down versus control (scrambled sequence) cells at day +5 after viral infection. In contrast, silencing rictor expression in 8226 cells was not as lethal and they, as well as scrambled sequence-control cells, were successfully selected in puromycin. After selection, these 8226 cell lines were compared for in vitro growth and Figure 4E shows a marked inhibition of growth after rictor knockdown. Stable 8226 cell lines infected with lentivirus expressing shRNA targeting rictor used at a MOI of 50:1 was more effective than that used at 20:1. These results with genetic inhibition of TORC2 confirm TORC2 as a therapeutic target in MM and strongly support the notion that pp242 cytotoxic effect in myeloma is specifically due to TORC2 inhibition.
Activation of TORC2 in MM
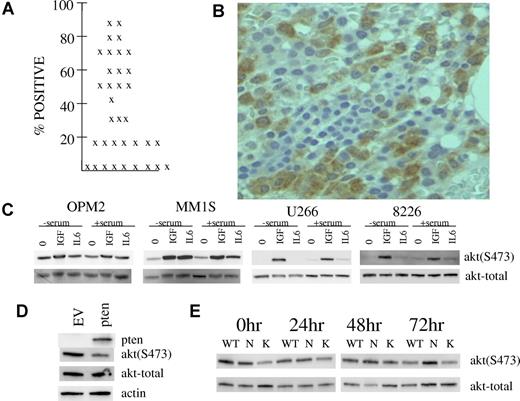
A small prior15 study of 15 patients suggested in situ TORC2 activation in primary myeloma bone marrow biopsies by immunohistochemical detection of AKT 473 phosphorylation. In an attempt to strengthen the notion that TORC2 activity is up-regulated in MM patient tumor cells, we immunostained marrow biopsies from a larger cohort of stage III patients. Of 35 patients, easily detectable phospho-AKT immunostaining for phosphorylation on S473 was present in myeloma tumor cells of 26 patients. As shown in Figure 5A, the percentage of plasma cells positively immunostained in these marrow biopsies was at least 15% and, in many patients, > 50%. An example of such immunostaining is shown in Figure 5B.
Evidence of in situ TORC2 activation in marrow myeloma cells and possible mechanisms. (A) Percent of myeloma cells positive for phospho-AKT staining in 35 patients. (B) Example of phosphorylated AKT expression in myeloma cells within a bone marrow section. Microscopic visualization was with a Nikon eclipse E400 microscope at 400× magnification and image was captured with a Microfire camera by Optometrics using the Picture Frame program Version 6.1 (Universal Imaging). (C) MM cell lines were stimulated with IGF-1 (250 ng/mL) or IL-6 (1000 U/mL) for 30 minutes after serum depletion overnight or no serum depletion. Immunoblot assay was then performed for phospho-AKT or total AKT. (D) OPM-2 MM cells were infected with adenovirus expressing PTEN or empty vector and, 48 hours later, immunoblot assay was performed for expression of PTEN, phospho-AKT, total AKT or actin. (E) ANBL-6 MM cells stably transfected with mutant N-RAS (N), K-RAS (K) or empty vector (WT) were maintained in continuous culture in 100 U/mL of IL-6. At time 0 hour, all 3 cell lines were depleted of IL-6 for 24, 48, or 72 hours. At each time point, immunoblot assay was performed for expression of phospho-AKT or total AKT. At all time points, there was no significant difference between cell lines in percent viability or viable cell recovery.
Evidence of in situ TORC2 activation in marrow myeloma cells and possible mechanisms. (A) Percent of myeloma cells positive for phospho-AKT staining in 35 patients. (B) Example of phosphorylated AKT expression in myeloma cells within a bone marrow section. Microscopic visualization was with a Nikon eclipse E400 microscope at 400× magnification and image was captured with a Microfire camera by Optometrics using the Picture Frame program Version 6.1 (Universal Imaging). (C) MM cell lines were stimulated with IGF-1 (250 ng/mL) or IL-6 (1000 U/mL) for 30 minutes after serum depletion overnight or no serum depletion. Immunoblot assay was then performed for phospho-AKT or total AKT. (D) OPM-2 MM cells were infected with adenovirus expressing PTEN or empty vector and, 48 hours later, immunoblot assay was performed for expression of PTEN, phospho-AKT, total AKT or actin. (E) ANBL-6 MM cells stably transfected with mutant N-RAS (N), K-RAS (K) or empty vector (WT) were maintained in continuous culture in 100 U/mL of IL-6. At time 0 hour, all 3 cell lines were depleted of IL-6 for 24, 48, or 72 hours. At each time point, immunoblot assay was performed for expression of phospho-AKT or total AKT. At all time points, there was no significant difference between cell lines in percent viability or viable cell recovery.
We have addressed several possible molecular reasons for such in situ TORC2 activation in MM cell line experiments. As shown above in Figure 1, IGF-1 is capable of activating TORC2 in MM cells. As IGF-1 is considered an important MM tumor growth factor in patients, this stimulation may play a role in the identified TORC2 activation in patient material. In fact, the modest constitutive TORC2 activation in our cell lines may be due to presence of IGF-1 in serum as serum depletion in all cell lines except OPM-2 prevented activation (Figure 5C). Figure 5C also demonstrates that the MM tumor growth factor interleukin-6 (IL-6) may also contribute to the observed activation in patient tumor cells. This is best shown in OPM-2 and MM1.S cell lines. In general, at the concentrations used, IGF-1 (250 ng/mL) was more effective than IL-6 (1000 U/mL) in activation of TORC2. An additional possible role of PTEN loss was addressed in OPM-2 cells. As mentioned previously, the OPM-2 line is PTEN-null.1 To address whether PTEN status may affect TORC2 activation, OPM-2 cells were infected with adenovirus expressing WT PTEN or empty vector. As shown in Figure 5D, re-expression of PTEN markedly inhibited constitutive TORC2 activation shown by a reduction in S473 AKT phosphorylation. These data suggest that loss of PTEN in MM may play a role although, as mentioned in “Discussion,” PTEN deletion or mutation in myeloma is not very frequent.29 Additional experiments in isogenic ANBL-6 MM cell lines stably transfected with mutant N-RAS, K-RAS, or control empty vectors, indicated no enhanced activation of TORC2 (473 AKT phosphorylation) when all cell lines were continuously cultured in IL-6 (the empty vector-transfected WT line is IL-6 dependent and displays slowed growth upon IL-6 depletion while mutant RAS transfection results in IL-6-independence). However, while 48 and 72 hours of IL-6 depletion results in diminished AKT 473 phosphorylation in WT and mutant K-RAS cells, phosphorylation is maintained in mutant N-RAS cells (Figure 5E). These data suggest that oncogenic N-RAS may promote constitutive TORC2 activation. As N-RAS mutations are relatively common in myeloma,30 especially in advanced disease,30 this gain-of-function mutation may also contribute to the high frequency of TORC2 activation in myeloma patients.
Synergistic interaction between pp242 and bortezomib
A previous study12 demonstrated antagonism when rapamycin was used in combination with bortezomib against OPM-2 and 8226 MM cell lines. Rapamycin inhibited bortezomib-induced apoptosis and this was possibly due to the feedback AKT activation induced by rapamycin. In contrast, as pp242 potently inhibits TORC2 activation of AKT, we hypothesized that combining it with bortezomib would not result in antagonism. Indeed, as shown in Figure 6A, there is an additive effect in some dose combinations and a synergistic effect in others when 8226 cells are studied. pp242 at 10nM and bortezomib at 1nM have no significant inhibitory effects when used alone, but in combination decrease viable recovery to 70% of control. Likewise, adding the ineffective pp242 dose of 10nM to 10nM bortezomib enhances the cyto-reductive effect, decreasing recovery from 61% down to 47% of control. Finally, adding the ineffective bortezomib dose of 1nM to 100nM pp242 also enhances cytoreduction, decreasing recovery from 58% of control to 43% of control.
Bortezomib synergizes with pp242 for enhanced antimyeloma effects. 8226 cells were incubated with increasing concentrations (shown in nM) of bortezomib ± pp242 for 48 or 72 hours. (A) Viable cell recovery was assessed after 72 hours and data are presented as percent of control where cultures without any drugs were arbitrarily made 100%. (B) Percent apoptosis (flow cytometry for activated caspase 3) is shown after 48 hours of incubation. Data are mean percent apoptosis ± SD (n = 3). (C) median effect/CI analysis is shown for 8226 cells treated for 48 hours with combinations of pp242 and bortezomib where apoptosis was assayed by annexin V staining. The ratio of concentrations of pp242:bortezomib was held constant at 10:1 for the purpose of median effect/CI method analysis with the pp242 concentration given under the horizontal axis. CI values < 1 indicate synergy. Vertical axis on the left represents fraction of cells affected (FA) and vertical axis on the right represents the CI values.
Bortezomib synergizes with pp242 for enhanced antimyeloma effects. 8226 cells were incubated with increasing concentrations (shown in nM) of bortezomib ± pp242 for 48 or 72 hours. (A) Viable cell recovery was assessed after 72 hours and data are presented as percent of control where cultures without any drugs were arbitrarily made 100%. (B) Percent apoptosis (flow cytometry for activated caspase 3) is shown after 48 hours of incubation. Data are mean percent apoptosis ± SD (n = 3). (C) median effect/CI analysis is shown for 8226 cells treated for 48 hours with combinations of pp242 and bortezomib where apoptosis was assayed by annexin V staining. The ratio of concentrations of pp242:bortezomib was held constant at 10:1 for the purpose of median effect/CI method analysis with the pp242 concentration given under the horizontal axis. CI values < 1 indicate synergy. Vertical axis on the left represents fraction of cells affected (FA) and vertical axis on the right represents the CI values.
When assessing 8226 cells for apoptosis, a synergistic interaction is also evident (Figure 6B). We used pp242 at 10 or 100nM, concentrations unable to achieve apoptosis (see Figure 2). After 48 hours incubation both concentrations of pp242 can significantly enhance bortezomib-induced apoptosis when the latter is used at 10nM. After 72 hours incubation (data not shown), both these nonapoptotic concentrations of pp242 significantly enhanced bortezomib-induced apoptosis when the latter is used at 10nM, increasing apoptosis from 27% (in absence of pp242) to 38% (with pp242 at 10nM) and to 65% (with pp242 at 100nM). To further test an interaction between pp242 and bortezomib, we assessed apoptosis by the annexin V assay after 48 hours exposure using median effect/CI analysis as shown in Figure 6C. Coexposure to both drugs resulted in synergistic drug interactions with CI values less than1 across several concentrations tested. When we tested different ratios of concentrations of pp242 to bortezomib other than the ratio used in Figure 6C (pp242:bortezomib = 10:1), the overall results of median effect/CI analysis remained the same (data not shown).
Discussion
The results of this study support the concept that TORC2 is a potential therapeutic target in multiple myeloma. Immunohistochemical staining indicated a high frequency of in situ TORC2 activation in patient myeloma cells as shown by immunodetection of AKT 473 phosphorylation. Furthermore, a newly developed inhibitor, pp242, which prevents TORC2 activity as well as TORC1 activity, was more effective than rapamycin (solely a TORC1 inhibitor) against MM cell lines. pp242's greater efficacy was demonstrated by a more complete decrease in MM cell viable recovery compared with equimolar concentrations of rapamycin and a significant induction of apoptosis wheras rapamycin was generally ineffective as a MM cell apoptosis inducer. Although direct mTOR kinase inhibitors may be superior to rapamycin in some models in terms of more complete TORC1 inhibition and, especially, greater inhibition of 4E-BP1 phosphorylation, in our MM cells, this was not the case. The greater cytoreduction of pp242 over rapamycin was not due to a greater inhibitory effect on TORC1 substrates p70S6K or 4E-BP1 (in fact, rapamycin was more effective as an mTOR inhibitor of p70 phosphorylation on a molar basis) nor a greater induction of autophagy. These data suggest pp242 toxicity to MM cells, especially its induction of apoptosis, is primarily mediated by its targeting of TORC2. Finally, knockdown of rictor with prevention of TORC2 assembly, was also lethal to MM cells confirming the promise of TORC2 as a target. One inconsistent piece of data for pp242 mediating its effect solely via TORC2 inhibition is that of the relative sensitivity among MM cell lines. OPM-2 cells are the most resistant to pp242-mediated inhibition of TORC2 activity (Figure 1) while OPM-2 cells are significantly more sensitive than 8226 and U266 cells to apoptosis (Figure 2). Thus, additional factors other than simple inhibition of TORC2 activity are important in determining responses to pp242.
It is interesting that 3 of the MM cell lines tested, 8226, OPM-2, and MM1.S, express high levels of c-maf and, as a consequence, high levels of DEPTOR, an mTOR-binding protein.31 DEPTOR inhibits TORC1 activity but, via inhibition of negative feedback effects on PI3-kinase, tends to maintain high levels of TORC2 activation.31 It is, thus, possible that high DEPTOR-expressing cell lines are extremely dependent on TORC2 activity for their survival. Although a more comprehensive series of MM cell lines needs to be tested, it was interesting that U266 cells, the sole line tested with relatively low levels of c-maf and DEPTOR,31 was the least sensitive to pp242-induced apoptosis (Figure 2) although de-monstrating comparable decreases in pp242-induced AKT dephosphorylation.
Part of the rationale for developing TORC2-targeting agents is the feedback activation of AKT that occurs when TORC1 is inhibited by rapamycin and other first generation mTOR inhibitors. A previous study12 identified such feedback activation of AKT in rapamycin and CCI-779–treated MM cells due to enhanced IGF-R/IRS-1 signaling. AKT activation could protect against apoptosis and may have been the explanation for antagonism when rapamycin was combined with bortezomib.12 The ability of rapamycin to activate AKT is again seen in this study in IGF-treated 8226 and OPM-2 cell lines (Figure 1A). As the pp242 TORC2 inhibitor prevents AKT activation by inhibiting AKT 473 phosphorylation, we tested whether it could be more successfully combined with bortezomib. As shown in Figure 6, pp242 synergized with bortezomib for enhanced MM cell cytoreduction and apoptosis. Although pp242 prevents the feedback S473 AKT phosphorylation/activation that occurs when TORC1 is inhibited, a feedback activation of ERK phosphorylation was still evident. This was expected because of the strong TORC1 inhibitory properties of pp242. Whether this ERK activation provides protection to pp242-treated MM cells remains to be tested but these data suggest that combining TORC2 inhibitors with mitogen-activated protein kinase/extracellular signal-regulated kinase kinase/ERK inhibitors may be additive or synergistic.
Immunohistochemical analysis of AKT phosphorylation on S473 in a small previous study15 as well as this one with a greater number of patient samples confirms that TORC2 is activated in bone marrow-based myeloma cells of patients. In the previous study,15 TORC2 activation was related to disease activity as AKT phosphorylation was not identified in plasma cells of monoclonal gammopathy of undetermined significance patients and was very minimal in patients with smoldering/indolent or Durie-Salmon stage I myeloma. An additional study32 showed a similar percent of primary samples (58%) expressed phosphorylation of AKT on S473 in plasma cells. Cell line experiments (Figure 5) suggest some possible mechanisms of this TORC2 activation, including IGF-1 and IL-6 stimulation as well as PTEN loss and N-RAS mutation. Although genetic alterations of PTEN have only been described in relatively few patients,29 N-RAS mutation is quite a bit more common.30 These genetic abnormalities are also more common in advanced disease29,30 and may be contributing to TORC2 activation. Additional mechanisms of TORC2 activation in MM cells may exist that we did not address such as stimulation from the microenvironment.
As a TORC2 inhibitor, pp242's adverse effects on myeloma cells may be due to one or more negative influences on TORC2 substrates. Certainly the down-regulation of AKT activity may be important but additional substrates could also be relevant. For example, the SGK kinase can regulate cancer cell growth and survival in some models33,34 and, although we could not accurately assay SGK phosphorylation, the inhibited phosphorylation of its substrate, NDRG1, in rictor-knocked-down myeloma cells (Figure 4), indicates that SGK is a TORC2 substrate in myeloma. Of note, Feldman16 reported that pp242 was a more complete inhibitor of TORC2-mediated SGK activation compared with AKT activation. Another potentially important TORC2 substrate is protein kinase C-α (PKC-α).35 Previous work36 indicates an interaction between PKCα and beta 1 integrins resulting in up-regulation of beta 1 integrin surface expression. Thus, the previously identified37 ability of IGF-1 to induce adhesion and migration in MM cells which is dependent on PI3-kinase signaling and beta 1 integrin expression, could be explained via IGF-induced TORC2 activation with downstream PKCα phosphorylation and beta 1 integrin up-regulation.
In summary, we found that pp242, a novel second generation mTOR inhibitor with negative effects on TORC2 as well as TORC1, was more effective as an antimyeloma agent than rapamycin, which solely inhibited TORC1. Additional data de-monstrating frequent TORC2 activation in myeloma bone marrow-based tumor cells and a synergistic antimyeloma effect of pp242 combined with bortezomib strongly support TORC2 as a therapeutic target in MM.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by National Institutes of Health grants RO1 CA109312 and RO1 CA111448 and research funds of the Department of Defense and Veterans Administration.
National Institutes of Health
Authorship
Contribution: B.H. designed and performed experiments; P.F., Y.S., A.B., E.B., and J.G. assisted in performance of experiments; S.C. and T.G. sectioned bone marrow biopsies, stained them for phospho-AKT expression, and analyzed staining data; G.P. analyzed bone marrow biopsy staining for phospho-AKT expression; and A.L. designed experiments and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Alan Lichtenstein, Hematology-Oncology, W111H, West Los Angeles Veterans Administration Hospital, 11301 Wilshire Blvd, Los Angeles, CA 90073; e-mail: alan.lichtenstein@med.va.gov.

![Figure 1. Inhibition of TORC2 activity by pp242. (A-C) MM cell lines were pre-treated for 30 minutes with increasing concentrations of rapamycin (Rap) or pp242 (pp) and IGF-1 (250 ng/mL) was then added to all cell lines except OPM-2. After 30 minutes additional incubation, protein lysate was immunoblotted for ex-pression of total p70S6kinase (t-P70), phosphorylated P70 (p-P70(T389)), total AKT (t-akt), phosphorylated AKT [p-akt(S473)], total mTOR (t-mtor), phosphorylated mTOR [p-mTOR(S2481)], total NDRG1, phosphorylated NDRG1 (in C), and total ERK or phosphorylated ERK. Arrows in (C) point to the phosphorylated NDRG1 dimer present in control and rapamycin-treated cells. (D) 8226 cells treated with increasing concentrations of pp242 for 1 hour followed by immunoblot for phosphorylated ERK and total ERK. (E) MM1.S and OPM2 cells treated with 100nM of rapamycin for 0, 1, or 24 hours, followed by immunoblot assay for total AKT and S473 phosphorylated AKT.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/116/22/10.1182_blood-2010-05-285726/6/m_zh89991060070001.jpeg?Expires=1767726835&Signature=PbwpdrYuJ0I7YZQAXcqcFY-SIYfhATN8HevZyk2t6wdW-x9lF6rJ0fsooHXzmVUPmvnmgcl1VLo7~YlfZoGKBFWGA7lDMMW34FReTP4ZoRZB-BbAoLGiOwJEMKqDiV7Gv~7eBLyJObKFREUpiMU1ITg6OcgWzmOeS-uxK6ZER0uiADaL4sTAkpqkR~zdPtpJ49cEiORCLdDo18oMkIbA~nDWrLRIzn7IO-brRIhLsY1mmLQih3OELngdTkGYL37YuHOby6WixtHtv4hLAw67jCtFIIWEjWYyAQHp~d-OurMItJbSoCn7f9t8qhHL7bpCBkXe0AZGl0m2OxWgNcyqtw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal