Abstract
Factors associated with spermatogenesis after allogeneic hematopoietic stem cell transplantation (HSCT) were assessed in this prospective, single-center, cross-sectional study. All consecutive men aged 18 years or older and in complete remission 2 years or longer after HSCT were invited to participate. Seminal fluid analysis was performed on freshly collected samples according to World Health Organization guidelines. Between April 2003 and June 2004, 39 patients were included. The median age at semen analysis was 34 years (range, 20-59 years), and the median time interval between HSCT and sperm analysis 9 years (range, 2-20 years). Thirty-two patients (82%) underwent total body irradiation (TBI; ≥ 10 Gy) as part of their conditioning regimen. Eleven of 39 (28%) patients showed some spermatogenesis. Patients with detectable spermatozoa in the ejaculate were younger at HSCT (median age, 19 versus 28 years; P = .004), had a longer interval since HSCT (median time, 12 versus 7 years; P = .01), and were more often without chronic graft-versus-host disease (GvHD; 2 of 11 patients versus 16 of 28; P = .03). Nine of 16 patients (56%) undergoing transplantation when younger than age 25 years showed some degree of spermatogenesis. In conclusion, men who are long-term survivors, who were younger than 25 years at HSCT, and who apparently do not have chronic GvHD have a reasonable likelihood of spermatogenesis even when conditioned with standard-dose TBI.
Introduction
Allogeneic hematopoietic stem cell transplantation (HSCT) is the treatment of choice for a wide variety of malignant and nonmalignant diseases and has been increasingly performed during the last 25 years. The number of patients free of the disease for which they were treated is continuously increasing. About 40% to 60% of patients receiving transplants survive 5 years after transplantation, and nearly 90% of the patients still alive 2 years after HSCT will become long-term survivors.1,2 Therefore, the aim of allogeneic HSCT now is not only to cure a patient's underlying disease but also to minimize the incidence of posttreatment complications and ensure the best possible long-term health status and quality of life.
A number of late malignant and nonmalignant complications have already been described. Although the latter usually are not life-threatening, quality of life may be considerably reduced.3 Gonadal dysfunction with absence of sperm production leading to definitive infertility, a common long-term sequela following cytotoxic treatment of malignancies, is a major concern. A recent survey revealed that 51% of men with cancer expressed their wish to preserve their capacity for procreation in the future, including 77% of men who were still childless when their cancer was diagnosed.4 Total body irradiation (TBI) used as part of the conditioning regimen plays a central role in posttransplantation infertility.5-7 In women, it provokes durable ovarian damage with both hypergonadotropic ovarian failure and lasting infertility. Pregnancies are rare, with an incidence well below 3%, and are associated with an increased risk of spontaneous abortion, preterm delivery, and low-birth-weight babies,7 but not with a higher risk for congenital malformation.8
In male recipients, endocrine dysfunction of the testes is less pronounced. Usually the serum levels of the follicle-stimulating hormone (FSH) are increased, indicative of impaired or absent spermatogenesis, but in most cases testosterone levels remain within the normal limits. The absence of spermatozoa in the semen is a common long-term sequela of both chemotherapy and irradiation given prior to HSCT and as conditioning before transplantation. However, with increasing follow-up time of the long-term survivors, the rate of patients with spermatogenesis might rise and modify our knowledge on the long-term effects of HSCT on male fertility.5
We investigated prospectively the sperm production in long-term male survivors who had undergone allogeneic HSCT and we analyzed factors that might determine spermatogenesis.
Patients and methods
Study design
This was a prospective, single-center, and cross-sectional study, conducted by the Basel Stem Cell Transplant Team of the University Hospital of Basel, Switzerland, for a defined time period. The study was performed in the context of the regular annual follow-up controls, which included an update of the patients' history, a full clinical investigation and laboratory tests such as whole blood counts, chemistry analysis, examination to assess the remission status of the primary disease, and peripheral blood chimerism using a polymerase chain reaction–based assay analyzing polymorphic short tandem repeats. The study protocol was approved by the Ethics Committee of the University Hospital Basel, and patients were included once written informed consent was obtained in accordance with the Declaration of Helsinki. The informed consent included a question concerning the patient's willingness to be informed about his results.
Target patient population
Male patients 18 years of age or older who had survived 2 years in remission of their primary disease after allogeneic HSCT were asked to participate. For organizational reasons, we mailed an invitation letter 1 month before the consultation. Those patients who scheduled the yearly control visit without having a 1-month previous arrangement were considered as lost patients and were not invited to participate.
According to our local protocols, various regimens were used as conditioning for the allogeneic HSCT, depending on the disease, the stage of the disease, the patient's age, and the risk profile of the patient at transplantation. Patients with acute leukemia (AL), chronic myeloid leukemia (CML), and multiple myeloma (MM) received either 120 mg/kg cyclophosphamide and 16 mg/kg busulfan, or 120 mg/kg cyclophosphamide and TBI with or without 30 mg/kg etoposide. Single-dose TBI with 10 Gy was applied before 1985, and fractionated TBI with 6 × 2 Gy since 1985. Elderly patients and those with reduced clinical condition received reduced-intensity conditioning with 90 mg/m2 fludarabine and a single-dose TBI with 2 Gy. Patients given transplants for severe aplastic anemia (SAA) received 200 mg/kg cyclophosphamide alone. Cyclosporine with or without methotrexate was used to prevent graft-versus-host disease (GvHD).
Semen analysis
The semen specimens were collected and processed in the Laboratory of Andrology at the Women's Hospital of the University of Basel. Information on fatherhood that had occurred before and after HSCT was obtained by questioning the patient. No paternity tests were performed. Fertility counseling was offered to all recipients participating in the study when they were informed about the results of the sperm analysis.
Semen analysis was performed on freshly collected samples. The volume of the ejaculate, the concentration of spermatozoa, motility, and morphology were assessed according to the World Health Organization (WHO) guidelines.9 Patients were considered to be normozoospermic when sperm concentration exceeded 20 × 106/mL, oligozoospermic when the sperm count was between 5 and 20 × 106/mL, severely oligozoospermic with a sperm count below 5 × 106/mL, and cryptozoospermic when spermatozoa were detected only after careful analysis of the concentrated sample. When no spermatozoa were detected in any field, both before and after centrifugation, patients were considered azoospermic. Sperm motility was assessed microscopically with a grading system according to the WHO protocol. Motility was classified as normal when at least more than 50% of spermatozoa were motile. Sperm morphology was assessed on Papanicolaou-stained preparations using a × 100 oil immersion bright-field objective and a × 10 eye piece, when at least 200 spermatozoa were available for examination. It was considered to be normal when more than 25% of the spermatozoa had a normal morphology.
Statistical analysis
Patients with proven sperm production were compared to patients without spermatogenesis, using the χ2 test for categorical data and the Mann-Whitney U test for continuous unrelated variables. Variables included age, disease, donor sex, source of the stem cells, type of conditioning regimen and in particular the use of TBI, occurrence of acute or chronic GvHD (or both), time between transplantation and last follow-up, and the time free of chronic GvHD. The time free of chronic GvHD was defined as the interval between the last date with chronic GvHD (date of cessation of therapy) and the date when the semen analysis was performed. In patients without chronic GvHD, the time free of chronic GvHD was defined as the interval between day + 100 and the date of the spermiogram. Variables were presented as median and range. To identify independent prognostic risk factors, a multivariate stepwise regression analysis was performed. In all statistical procedures, P values below .05 were considered significant. Statistical analysis was performed by using SPSS statistical software (SPSS for Windows, Release 12.0, SPSS, Chicago, IL). For cumulative risk analysis, the NCSS Trial and PASS Trial software (Number Cruncher Statistical Systems, Kaysville, UT, released March 17, 2004) was used.
Results
Feasibility of the study and characteristics of patient populations
Between April 2003 and June 2004, 62 male recipients were asked to participate in this study. Five were excluded because of a previous vasectomy, and 18 declined to take part. Thirty-nine patients (63%) agreed to participate in the study. Data characteristics from patients included in the study and patients who declined their participation are outlined in Table 1. In patients included in the study, the median time interval between HSCT and seminal fluid analysis was 9 years (range, 2-20 years) and the median age at study time was 34 years (range, 20-59 years). Thirty-three (84%) of the 39 patients were treated with a standard conditioning regimen, including TBI with a total dose of 10 Gy or more in 32 and cyclophosphamide and busulfan in one patient. Three patients received reduced-intensity conditioning (fludarabine and TBI 2 Gy); one of them with MM had previously had an autologous HSCT, a second patient with non-Hodgkin lymphoma was treated with bischloro-ethylnitrosourea (BCNU), carmustine, etoposide, cytarabine, and melphalan (BEAM) immediately prior to HSCT, and a third patient with myelodysplastic syndrome did not receive any therapy before the transplantation. Grade II to IV acute GVHD was observed in 31 patients (79%) and localized or extended chronic GVHD in 18 patients (46%). In regard to paternity before HSCT, differences were observed between patients included in the study and those who refused to take part (Table 1). Participating patients were significantly more often childless (29 of 39; 74%) and were less likely to have 2 or more children (8 of 39; 21%) compared with nonparticipating patients (childless in 6 of 18; 33%; P = .003; 2 or more children in 10 of 18; 56%; P = .008). Six of the nonparticipating patients were childless, and 3 of them wanted to cryopreserve sperm before HSCT. One patient had a successful cryo-preservation and 2 were azoospermic at that time. One childless patient refused to participate because he previously had an andrology check-up showing a normal spermiogram. Two childless patients, who were 40 and 51 years old at HSCT with a follow-up of 10 and 18 years, respectively, refused to participate.
In reference to sperm cryopreservation, 9 patients of the participating group were younger than 18 years at the time of HSCT, and 3 patients did a sperm cryopreservation before transplantation (none had children at that time).
Results of semen analysis
Of all 39 examined patients, spermatozoa were detected in the semen of 11 men (28%), whereas 28 patients (72%) were identified with complete azoospermia. No patient had an absolutely normal result of the semen analysis, because those diagnosed as normozoospermic had normal motility but all of them had abnormal spermatozoa morphology. Sperm motility was found to be normal in 7 (64%) of 11 sperm samples, but none of them fulfilled the criteria for normal sperm morphology. Details of semen analysis results are shown in Table 2.
Paternity
Three patients experienced posttransplantation paternity; one of them fathered 2 children. All 3 recipients were normozoospermic and presented with normal sperm motility. Two of them had been conditioned with cyclophosphamide alone for aplastic anemia, and one had received standard conditioning with TBI, cyclophosphamide, and etoposide for acute myeloid leukemia (AML). The time interval between transplantation and paternity was 6 and 9 years, respectively, for aplastic anemia patients, and 9 and 11 years for the patient with AML. Patients who refused to participate in the study did not father any children after transplantation.
Factors associated with spermatogenesis
The median age at transplantation of those patients who later presented with at least some sperm production was 19 years (range, 5-42 years), as compared with 28 years (range, 16-56 years) for the patients who remained azoospermic (P = .004). The median time interval between transplantation and seminal fluid analysis was 12 years (range, 3-19 years) for patients with and 7 years (range, 2-20 years) for patients without recovery of sperm production (P = .01; Table 3).
Patients presenting with signs of an ongoing sperm production were more often free of chronic GvHD than were those without detectable sperm in their ejaculate (P = .03). The time free of any chronic GvHD was longer for patients with sperm compared with those with azoospermia (P = .05). Of the 18 patients with chronic GvHD, only 2 showed some sign of an active sperm production. Both of them had a mild form of chronic GvHD. One had had skin and oral mucosa involved and was off immunosuppression therapy for more than 4 years at study time. The other patient had eye and oral mucosa involvement and was still under low-dose immunosuppression (cyclosporine 50 mg/d) when semen analysis was performed.
Regarding the primary disease, none of the recipients with detectable spermatozoa in their ejaculate had CML, but 8 (38%) of 21 patients with AL and all 3 patients with SAA showed some sperm production activity (P = .008). For CML recipients, several confounding factors could explain this difference. Compared with patients with AL, patients with CML were older (median age, 36 vs 20 years) at transplantation, presented a shorter time interval between transplantation and seminal fluid analysis (median, 5 vs 11 years), and more often had chronic GvHD (7 of 11 patients with CML vs 2 of 21 with AL). We did not find a statistical correlation effect between donor type (P = .1) or the occurrence of acute GvHD (P = .7) and the semen analyses.
The effect of the conditioning regimen on testicular sperm production was analyzed. Thirty-two (82%) of 39 patients had received 10 Gy or more TBI for conditioning, 30 of them fractionated and 2 as single-dose TBI. In univariate analysis, we observed a trend in the sperm production in patients conditioned without TBI (P = .08). A 42-year-old patient conditioned with busulfan and cyclophosphamide for AML showed spermatogenesis after a follow-up period of 5 years. Three patients received nonmyeloablative conditioning and none had sperm in the seminal fluids. The ages at transplantation of these 3 patients were 24, 48, and 56 years, respectively, and all of them had a follow-up of less than 3 years. Three patients were conditioned with cyclophosphamide for SAA; they all showed sperm in their semen analysis, and 2 of them fathered a normal child (see “Paternity”).
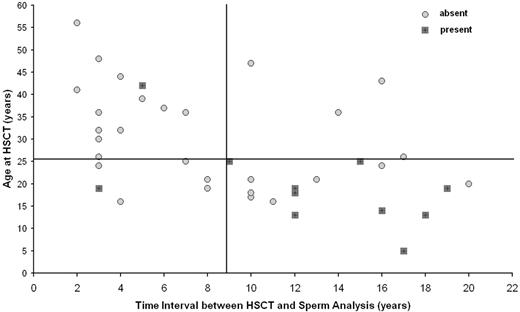
The combination of 2 risk factors, age at transplantation and follow-up time, defined patients with high probability of spermatogenesis (Figure 1). Nine (56%) of 16 patients younger than 25 years at HSCT and with an interval time between HSCT and seminal fluid analysis of 9 years or longer presented detectable spermatozoa in the semen, compared with 2 (9%) of 23 patients who fulfilled one or none of these criteria (P < .002). In addition, we observed that the 3 recipients receiving transplants before adolescence (one at 5 years old and 2 at 13 years old) and with a follow-up longer than 12 years (12, 17, and 18 years) independent of the diagnosis (1 SAA, 1 ALL, and 1 AML) and type of conditioning (2 received TBI) presented with sperm production. None of them had had chronic GvHD.
Multivariate analysis
In stepwise multivariate Cox regression analysis, age older than 25 years at transplantation and conditioning with TBI were statistically associated with low probability for spermatogenesis. The occurrence of chronic GvHD showed a trend for patients to remain azoospermic (Table 4).
Discussion
In this study on sperm production activity after HSCT, 63% of the invited men agreed to participate. Those patients who did not agree were older and had a higher frequency of paternity before HSCT than the participating group. In particular, having 2 or more children was more frequent in the nonparticipating group. Having fathered 2 or more children seems therefore to the patient as an achieved family planning. We can speculate that men's interest in knowing their sperm production and their potential fertility decreases with age and the number of children.
Presence or absence of spermatozoa after HSCT as a function of age at HSCT and time interval from HSCT. Detection of spermatozoa in the seminal fluid.
Presence or absence of spermatozoa after HSCT as a function of age at HSCT and time interval from HSCT. Detection of spermatozoa in the seminal fluid.
Twenty-eight percent of the recipients presented some sperm production during long-term follow-up after allogeneic HSCT. We were able to identify that with increasing follow-up, the impact of TBI on gonadal damage after allogeneic transplantation became more attenuated. Indeed, 7 of 11 patients with sperm production had previously been treated with TBI. In addition, we were able to demonstrate that younger age at transplantation, a longer interval between HSCT and the seminal fluid analysis, and apparently the absence of chronic GvHD were associated with a higher incidence of spermatogenesis production. Fifty-six percent of the recipients younger than 25 years of age and with an interval of more than 9 years between transplantation and semen analysis presented with some sperm production, whereas less than 10% of the older patients or patients with a shorter observation period since transplantation had sperm in their semen analysis.
In cancer survivors, the degree to which testicular function is affected depends on the dose and the type of treatment used.10,11 After allogeneic HSCT, both TBI and intensive chemotherapy, such as the combination of cyclophosphamide and busulfan, have been demonstrated to be the most detrimental factors contributing to durable infertility in male recipients. Rare cases of recovery of testicular sperm production have been described after HSCT with TBI.5,7,12 One of the reasons for the lower statistical impact of TBI on testicular dysfunction in our study might be the relatively low number of patients at risk. However, the present study is particularly noteworthy due to its long follow-up time after HSCT. This long observation period has allowed us to demonstrate the capacity of some younger patients not presenting with GvHD to produce spermatozoa after a decade or longer. Leydig cell injury following the infiltration of donor alloreactive T cells as a manifestation of GvHD has been recently demonstrated in a mouse model.13 In humans, lower sperm counts have been described after allogeneic HSCT associated with extensive chronic GvHD.14 We observe here a trend to spermatogenesis production after HSCT correlated with longer intervals free of any chronic GvHD. However, so far we do not know whether this possible difference could be the result of a general illness of the patients with chronic GvHD leading to a decreased sperm production or of the direct effect of donor alloreactive T cells on spermatogenesis.
Chemotherapy used before HSCT may play an important role in gonadal toxicity.15 Indeed, pretreatment evaluation of patients with cancer frequently demonstrates significant impairment of the quality of their semen. In men with Hodgkin lymphoma, 21% have oligozoospermia and 2% azoospermia before treatment.16 In the present study, we intended to evaluate factors that influence presence of spermatogenesis in long-term recipients, which includes inevitably the injury of the testes before transplantation. Unfortunately, in the majority of the patients we have no data on the spermatogenesis status before transplantation; only 3 participating patients cryopreserved sperm immediately before transplantation, and so far no one recovered spermatogenesis. Data from patients treated with reduced-intensity conditioning suggest that the dose and the type of agent used for conditioning cannot alone explain the azoospermia. Therefore, even in patients given transplants with reduced-intensity conditioning, the age of the patient, the treatment before transplantation, the kinetics of spermatogenesis after allogeneic HSCT, and the presence or not of chronic GvHD have to be considered. The reason for the high rate of spermatogenesis usually observed in patients with aplastic anemia conditioned with cyclophosphamide alone5,8 is likely to be related to the younger age at transplantation and the absence of chemotherapy before transplantation.
Despite the fact that we collected the information concerning paternity before and after HSCT, this study was not designed to evaluate paternity as a consequence of presence of spermatogenesis. We do not have information concerning the intention of male recipients to become fathers after HSCT, nor did we perform paternity tests in case of a child's birth occurring after transplantation. Nevertheless, 3 of the 11 patients with sperm production reported fathering a child after HSCT. In the present series, only recipients with normal sperm cell counts fathered a child. Therefore, the question is whether incomplete sperm production with oligozoospermic or even cryptozoospermic seminal fluid is of clinical relevance for the recipient after transplantation. Fathering of a healthy child from a severely oligozoospermic patient after autologous HSCT using an assisted reproductive technique has been reported.17 But more importantly, reduced fertility due to low sperm count and poor sperm motility can be circumvented by assisted reproduction, in particular with intracytoplasmic sperm injection.18
At present, cryopreservation of spermatozoa is an established option for sustaining the viability of sperm over a long period of time, allowing the sperm to be used for insemination at a later date.19-21 Cryopreservation should be offered to all men at risk of infertility and should be done prior to treatment, and it must be emphasized and encouraged. According to our findings, young patients undergoing transplantation before adolescence and without cryopreserved sperm have a reasonable chance of spermatogenesis after HSCT.
In conclusion, the results of our prospective cohort study suggest that long-term survivors after stem cell transplantation have a substantial likelihood of recovering some degree of testicular sperm production even when conditioned with TBI, provided they are younger than 25 years at HSCT and apparently when they remain free of chronic GvHD.
Prepublished online as Blood First Edition Paper, March 16, 2006; DOI 10.1182/blood-2006-01-0176.
Supported in part by a grant of the Swiss National Research Foundation and by grant NFP46 No. 404640-101297 from the Swiss National Research Project.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Alexander Genitsch who helped with the database production; our colleagues and nurses at Zellersatzambulatorium, the Nurses Data Team of the Hematology Department of University Hospital Basel, and the Laboratory Team of Andrology at the Women's Hospital of the University of Basel for all their contributions to this work.