Abstract
A sequential regimen of chemotherapy, reduced-intensity conditioning (RIC) for allogeneic stem cell transplantation (SCT), and prophylactic donor lymphocyte transfusion (pDLT) was studied in 103 patients with refractory acute myeloid leukemia (AML). According to published criteria, refractoriness was defined by primary induction failure (PIF; n = 37), early (n = 53), refractory (n = 8), or second (n = 5) relapse. Chemotherapy consisted of fludarabine (4 × 30 mg/m2), cytarabine (4 × 2 g/m2), and amsacrine (4 × 100 mg/m2), followed 4 days later by RIC, comprising 4 Gy total body irradiation (TBI), cyclophosphamide, and antithymocyte globulin. Patients without graft-versus-host disease (GvHD) at day +120 received pDLT in escalating doses. Patients' median age was 51.8 years. Before conditioning, 99 patients had active disease, 3 were aplastic, 1 was in second complete remission (CR2). Forty-one patients had family donors, 62 had unrelated donors. With a 25-month median follow-up, overall survival (OS) at 1, 2, and 4 years was 54%, 40%, and 32%; the respective leukemia-free survival (LFS) was 47%, 37%, and 30%. Patients with PIF showed a 2-year OS of 62.5%. OS was 87% in 17 patients receiving pDLT. One-year cumulative incidence of leukemic death and non–relapse-mortality was 28.7% and 17.2%. In a multivariate analysis, more than 2 courses of prior chemotherapy were the strongest predictor for poor outcome (P = .007; HR = 3.01 [OS]; P = .002; HR = 3.25 [LFS]). These results indicate a high activity of the regimen in refractory AML.
Introduction
Allogeneic stem cell transplantation (SCT) is the most effective treatment for a variety of hematologic malignancies.1-3 However, regimen-related toxicities of conventional conditioning regimens have limited the procedure to medically fit patients generally younger than 50 to 55 years. This limitation was partially overcome by the development of reduced-intensity conditioning (RIC),4 which is associated with a considerably lower treatment-related toxicity. RIC regimens take advantage of the graft-versus-leukemia (GvL) effect mediated by the transferred immunocompetent donor cells5 and do not rely on the eradication of the leukemic clone by high-dose radiochemotherapy. Consequently, sibling and unrelated SCTs were performed in patients at older age and with considerable comorbidities.6-14 After initial studies had shown the feasibility of allogeneic SCT following RIC regimen in a broad variety of hematologic malignancies at different clinical stages, subsequent protocols were directed more specifically to defined clinical entities, such as high-risk myeloid leukemias and myelodysplastic syndrome (MDS).15-22 These regimens were classified as “reduced, but still myeloablative” and were aimed to find a balance between the reduction of toxicity and a sufficient antileukemic efficacy.
Recently, our group has introduced a sequential conditioning regimen for patients with high-risk acute myeloid leukemia (AML) and MDS, consisting of cytoreductive chemotherapy, followed by reduced-intensity conditioning and prophylactic transfusion of donor lymphocytes.23 Preliminary subgroup analysis of this trial suggested that patients with refractory AML might particularly benefit from the procedure. Hence, the current study was initiated to assess the feasibility and efficacy of the approach in a homogenous group of patients with refractory AML as defined by established criteria24-26 in the setting of a multicenter trial, in which 8 institutions in Germany and Austria participated.
Patients and methods
Eligibility criteria
Patients were included if they fulfilled at least one of the following criteria defining refractory AML24-26 : (1) primary induction failure (PIF) after 2 or more cycles of chemotherapy, (2) first early relapse after a remission duration of fewer than 6 months, (3) relapse refractory to salvage combination chemotherapy containing high-dose AraC, and (4) second or subsequent relapse. Further inclusion criteria were age between 18 and 70 years and the availability of a family or unrelated stem cell donor with 8 of 8 or 10 of 10 HLA match or 1 major mismatch. Exclusion criteria were creatinine clearance less than 50 mL/min, bilirubin or transaminases greater than 3 times the upper limit of normal, cardiac shortening fraction less than 30%, and pregnancy.
HLA typing
Standard serologic typing was used for HLA A, B, and C, the latter only typed in 75% of the patients. High-resolution molecular typing using polymerase chain reaction in the sampled DNA with sequence-specific primers was performed for class II alleles (HLA DRB1 and DQB1).
Treatment
Low-dose AraC (10 mg/m2 subcutaneously every 12 hours or 100 mg/24 hours as continuous infusion) was given for a maximum of 14 days to control the disease in patients with rapidly progressive leukemia, if conditioning could not be started immediately (eg, if clearance for an unrelated donor was pending). Patients with central nervous system (CNS) leukemia received repeated applications of intrathecal chemotherapy before start of the preparative regimen. The FLAMSA (fludarabine, AraC, Amsa)–RIC protocol for allogeneic SCT has recently been described in detail.23 In brief, patients received fludarabine (30 mg/m2), high-dose AraC (2 g/m2), and amsacrine (100 mg/m2) from day –12 to –9 (FLAMSA regimen) for initial reduction of leukemic burden. Following 3 days of rest, RIC consisted of 4 Gy total body irradiation (TBI) on day –5, cyclophosphamide (40 mg/kg with related donors, 60 mg/kg with unrelated or mismatched donors) on days –4 and –3, and rabbit antithymocyte globulin (ATG; Fresenius, Munich, Germany; 10 mg/kg with related donors, 20 m/kg with unrelated or mismatched donors) on days –4, –3, and –2. For transplantation, G-CSF–mobilized peripheral blood stem cells (PBSCs) were preferred; bone marrow (BM) was accepted at the donor's preference. No graft manipulation was performed; 5 × 106 CD34+ cells/kg were requested. Graft-versus-host disease (GvHD) prophylaxis consisted of cyclosporine A (CyA; target levels between 250 and 500 ng/mL) from day –1 and mycophenolate mofetil (MMF; 4 × 500 mg/day) from day 0. In the absence of GvHD, MMF was discontinued by day +50; CyA was tapered from day +60 to +90. Infection prophylaxis was used according to the rules of the respective transplantation centers. From day +120 prophylactic transfusion of donor lymphocytes (pDLT) was recommended per protocol in patients in remission, without infection, and free of GvHD after 30 days off immunosuppression. It was given in a dose-escalating fashion to achieve a sustained graft-versus-leukemia effect with limited risk of GvHD. The initial cell dose for pDLT was 1 × 106 CD3+ cells/kg; it could be increased to 5 × 106 CD3+ cells/kg in patients without a history of acute GvHD. In the absence of GvHD, pDLT was repeated at 4- to 6-week intervals up to 3 times, using an escalating cell dose regimen with 5- to 10-fold increase per transfusion.
Evaluations and definitions
Concomitant diseases were classified according to the modified Charlson Comorbidity Index (CCI).27,28 During hospitalization at a laminar air flow unit, clinical status, adverse events, and hematologic and clinical chemistry parameters were monitored daily. Following discharge, patients were seen in the outpatient clinic of their respective transplantation center once to twice a week up to day +100 and at gradually increasing intervals thereafter. Toxicities were graded according to WHO criteria; acute and chronic GvHD were graded as described.29
Date of neutrophil recovery was defined as the first of 2 consecutive days with an absolute neutrophil count within the peripheral blood (PB) exceeding 500/μL. Date of platelet engraftment was defined as the first of 3 days with an absolute platelet count exceeding 20 G/L without transfusion support. At day +30, disease response and chimerism were assessed in PB and bone marrow (BM). Because thrombocyte regeneration could be postponed by factors other than leukemia and cytotoxic therapy (ie, GvHD, virus infection, drugs), complete remission (CR) was defined as less than 5% blasts without evidence of dysplasia in BM, and more than 1500 neutrophils/μL in PB. Donor chimerism among peripheral CD3+ cells and in unfractionated BM was studied at day +30 and +90, using fluorescence in situ hybridization in sex-mismatched30 and short tandem repeat analysis in sex-matched transplantations.31 Hematologic relapse was defined by reappearance of blasts in the PB, by any manifestation of leukemia outside the hematopoietic system, or by BM infiltration by greater than 5% of blasts in a representative smear. Death from leukemia was defined as death with refractory disease after transplantation, or as death from any cause following posttransplantation relapse. Nonrelapse mortality (NRM) was defined as death from any cause other than refractory disease or relapse, including death from preexisting comorbidities.
Study end points and statistical analysis
Results were analyzed as of May 4, 2005. Overall survival (OS) at 2 years from transplantation was the primary end point of the study. Secondary end points included leukemia-free survival (LFS; defined as being alive and free of disease), nonrelapse mortality, and relapse incidence, as well as incidence and severity of acute and chronic GvHD. The day of stem cell transfusion was counted as day 0, and all intervals were calculated based on this date.
Numeric variables were analyzed as categories considering their value below or above the median of the entire cohort, as indicated in “Results.” For comparison of group characteristics (eg, sex, donor type, CMV status), chi-square test, Fisher exact test, and Student t test were applied for univariate analysis; stepwise logistic regression was used for multivariate analysis. Acute and chronic GvHD were analyzed as time-dependent variables. Additionally, a landmark analysis was performed at several dates after transplantation to evaluate the role of GvHD on survival. OS and LFS were estimated using the Kaplan-Meier method. Log-rank test and a Cox proportional hazard regression model were used for analysis of risk factors for time-to-event variables. Variables showing a P value less than .1 in univariate analysis were considered for multivariate testing. Probabilities of nonrelapse mortality and death from leukemia were calculated using reciprocal cumulative incidence estimates to account for competing risks. SAS (SAS Institute, Cary, NC) and SPSS (SPSS, Chicago, IL) software packages were used for data analysis.
Study conduct
The study was performed according to the modified Helsinki declaration. The protocol was approved by the respective local ethical review boards prior to its initiation. Every patient gave written informed consent after being informed about the investigational nature of the study.
Results
Patient and donor characteristics
One hundred three patients from 8 institutions in Germany and Austria were entered into the study. Characteristics of patients, donors, and transplantations are displayed in Table 1. The median patient age at transplantation was 51.8 years; 18 patients were older than 60 years. Within the study population, 35.9% of the patients had primary induction failure (PIF), 51.5% had early relapse at fewer than 180 days from CR1 (ER), and 12.6% had second relapse or first relapse that was refractory to high-dose AraC. Forty-two percent of the patients had received 4 or more courses of chemotherapy, and 10 had undergone myeloablative therapy followed by autologous SCT at a median of 10 months (range, 3-72 months) before allografting. At time of transplantation, 99 patients had active disease, 1 patient with ER was in second remission, and 3 patients were in aplasia following intensive reinduction therapy. The median percentage of leukemic blasts in BM before start of FLAMSA chemotherapy was 30% (range, 0%-90%). Extramedullary disease, including CNS, skin, and soft tissue infiltration, was present before start of conditioning in 11 cases. The leukemic karyotype according to the SWOG/ECOG criteria32 was favorable in 5 cases, whereas 47 patients had unfavorable cytogenetics, including 22 with complex aberrations.
Patient, donor, and transplant characteristics
. | Values . |
|---|---|
| No. patients | 103 |
| Sex, no. (%) | |
| Female | 49 (47.6) |
| Male | 54 (52.4) |
| Median age, y (range) | 51.8 (18.5-68.2) |
| Diagnosis, no. (%) | |
| De novo AML | 76 (73.8) |
| AML secondary to MDS | 21 (20.4) |
| AML secondary to other malignancies | 6 (5.8) |
| Cytogenetic subgroups, no. (%)* | |
| Good | 5 (4.9) |
| Intermediate | 49 (47.6) |
| Poor | 47 (45.6) |
| Unknown | 2 (1.9) |
| Stage at transplantation, no. (%) | |
| Primary induction failure (PIF)† | 37 (35.9) |
| Relapse after CR1 < 6 mo (ER) | 53 (51.5) |
| Untreated/Ld AraC, no. | 29 |
| In aplasia, no. | 3 |
| Refractory,‡ no. | 20 |
| In CR2, no. | 1 |
| Refractory relapse after > 6 mo in CR,‡ no. (%) | 8 (7.8) |
| 2nd relapse | 5 (4.8) |
| Untreated, no. | 4 |
| Refractory, no. | 1 |
| Median marrow blasts, % (range) | 30 (0-90) |
| Extramedullary disease | |
| Total, no. (%) | 11 (10.6) |
| Present from time of diagnosis, no. | 4 |
| Present only from time of first relapse, no. | 7 |
| Skin, no. | 1 |
| Soft tissue, no. | 3 |
| Meningosis alone, no. | 5 |
| Meningosis + other site, no. | 2 |
| Prior autologous transplantation, no. (%) | |
| Yes | 10 (9.6) |
| No | 93 (90.4) |
| No. of chemotherapy cycles before HCT§, no. (%) | |
| 2 cycles | 36 (35) |
| 3 cycles | 24 (23.3) |
| 4 cycles | 15 (14.6) |
| More than 4 cycles | 28 (27.2) |
| Median (range) | 3 (2-9) |
| Charlson Comorbidity Index score, no. (%) | |
| 0 | 42 (40.8) |
| 1 | 38 (36.8) |
| 2 | 13 (12.6) |
| 3 | 3 (2.9) |
| Greater than 3 | 3 (2.9) |
| NE | 4 (3.9) |
| Median time from diagnosis to transplantation, | |
| d (range) | 215 (73-1187) |
| Donor type, no. (%) | |
| Fully matched family donor | 39 (37.9) |
| Partially matched family donor | 2 (1.9) |
| Fully matched unrelated donor | 49 (47.6) |
| Partially matched unrelated donor | 13 (12.6) |
| Donor sex match, no. (%) | |
| Match | 59 (57.5) |
| Patient male/donor female | 9 (8.7) |
| Patient female/donor male | 24 (23.3) |
| NE | 11 (10.7) |
| CMV status, no. (%) | |
| Patient-/donor- | 21 (20.4) |
| Patient+/donor- | 34 (33.0) |
| Patient-/donor+ | 10 (9.7) |
| Patient+/donor+ | 31 (30.1) |
| NE | 7 (6.8) |
| Stem cell source, no. (%) | |
| Mobilized PBSCs | 93 (90.3) |
| Bone marrow | 10 (9.7) |
| Median no. CD34+ cells within the graft,∥× 106/kg (range) | 8.2 (2.2-23) |
. | Values . |
|---|---|
| No. patients | 103 |
| Sex, no. (%) | |
| Female | 49 (47.6) |
| Male | 54 (52.4) |
| Median age, y (range) | 51.8 (18.5-68.2) |
| Diagnosis, no. (%) | |
| De novo AML | 76 (73.8) |
| AML secondary to MDS | 21 (20.4) |
| AML secondary to other malignancies | 6 (5.8) |
| Cytogenetic subgroups, no. (%)* | |
| Good | 5 (4.9) |
| Intermediate | 49 (47.6) |
| Poor | 47 (45.6) |
| Unknown | 2 (1.9) |
| Stage at transplantation, no. (%) | |
| Primary induction failure (PIF)† | 37 (35.9) |
| Relapse after CR1 < 6 mo (ER) | 53 (51.5) |
| Untreated/Ld AraC, no. | 29 |
| In aplasia, no. | 3 |
| Refractory,‡ no. | 20 |
| In CR2, no. | 1 |
| Refractory relapse after > 6 mo in CR,‡ no. (%) | 8 (7.8) |
| 2nd relapse | 5 (4.8) |
| Untreated, no. | 4 |
| Refractory, no. | 1 |
| Median marrow blasts, % (range) | 30 (0-90) |
| Extramedullary disease | |
| Total, no. (%) | 11 (10.6) |
| Present from time of diagnosis, no. | 4 |
| Present only from time of first relapse, no. | 7 |
| Skin, no. | 1 |
| Soft tissue, no. | 3 |
| Meningosis alone, no. | 5 |
| Meningosis + other site, no. | 2 |
| Prior autologous transplantation, no. (%) | |
| Yes | 10 (9.6) |
| No | 93 (90.4) |
| No. of chemotherapy cycles before HCT§, no. (%) | |
| 2 cycles | 36 (35) |
| 3 cycles | 24 (23.3) |
| 4 cycles | 15 (14.6) |
| More than 4 cycles | 28 (27.2) |
| Median (range) | 3 (2-9) |
| Charlson Comorbidity Index score, no. (%) | |
| 0 | 42 (40.8) |
| 1 | 38 (36.8) |
| 2 | 13 (12.6) |
| 3 | 3 (2.9) |
| Greater than 3 | 3 (2.9) |
| NE | 4 (3.9) |
| Median time from diagnosis to transplantation, | |
| d (range) | 215 (73-1187) |
| Donor type, no. (%) | |
| Fully matched family donor | 39 (37.9) |
| Partially matched family donor | 2 (1.9) |
| Fully matched unrelated donor | 49 (47.6) |
| Partially matched unrelated donor | 13 (12.6) |
| Donor sex match, no. (%) | |
| Match | 59 (57.5) |
| Patient male/donor female | 9 (8.7) |
| Patient female/donor male | 24 (23.3) |
| NE | 11 (10.7) |
| CMV status, no. (%) | |
| Patient-/donor- | 21 (20.4) |
| Patient+/donor- | 34 (33.0) |
| Patient-/donor+ | 10 (9.7) |
| Patient+/donor+ | 31 (30.1) |
| NE | 7 (6.8) |
| Stem cell source, no. (%) | |
| Mobilized PBSCs | 93 (90.3) |
| Bone marrow | 10 (9.7) |
| Median no. CD34+ cells within the graft,∥× 106/kg (range) | 8.2 (2.2-23) |
Ld indicates low dose; HCT, hematopoietic cell transplantation; NE, not evaluable.
According to the SWOG/ECOG criteria.34
As defined by persistent leukemia after at least 2 courses of induction chemotherapy.
As defined by persistent leukemia after at least 1 course of combination chemotherapy containing high-dose AraC for reinduction.
Myeloablative conditioning for autologous SCT was counted as 1 cycle.
Analysis restricted to PBSC transplantations.
Donors other than HLA-identical siblings were used in 62 cases (60.2%). Transplants consisted of mobilized PBSCs in 93 cases; 10 patients received BM according to the donor's preference. In addition to the comorbidities included in the Charlson Comorbidity Index, other concomitant diseases were severe obesity (n = 4), systemic neurologic disorders (Guillain-Barré syndrome, multiple sclerosis with tetraplegia, n = 1 each), hereditary thrombophilia (n = 1), heavy nicotine abuse (n = 5), and active infections without organ dysfunction (tuberculosis, toxoplasmosis, n = 1 each). A history of documented fungal infection was reported in 31 cases (systemic aspergillosis, n = 4; aspergillus pneumonia, n = 25; systemic candidosis, n = 2); 3 had recently resolved viral pneumonia.
Neutropenia, engraftment, and chimerism
At start of FLAMSA chemotherapy, 36 (35%) of the patients presented with neutrophil counts below 500/μL as a consequence of leukemic marrow infiltration or prior cytotoxic treatment. Sixty-seven (65%) patients showed more than 500 neutrophils/μL at start of treatment. With FLAMSA-RIC, these patients uniformly developed pancytopenia. Neutrophil count reached values below 500/μL at a median of 8 days (range, 2-12 days) before transplantation. After SCT, 100 patients engrafted, whereas 3 were not evaluable for engraftment because of death in aplasia. No primary graft failure was observed. The median time to neutrophil and thrombocyte engraftment was 14 days (range, 9-40 days) and 18 days (range, 6-90 days). Altogether, the overall duration of neutropenia in 65 patients who had more than 500 neutrophils/μL at start of the FLAMSA regimen and achieved engraftment after transplantation was 22 days (range, 17-39 days).
Donor chimerism was analyzed in BM and in the peripheral blood CD3+ cells. At day +30, 94% of patients had greater than 90% donor cells in both compartments, with the percentage of patients with 100% donor chimerism being higher in BM as compared with peripheral T cells (82.4% versus 55.0%). At day 90, median donor chimerism was 100% in both compartments.
Graft-versus-host disease
After transplantation, acute GvHD developed in 63 of 100 evaluable patients, reaching grade I in 35, grade II in 13, grade III in 11, and grade IV in 4 patients. The median time of onset was day +19 (range, 7-62). Skin, liver, and gut were affected in 57, 10, and 20 patients. Transplantation from donors other than HLA-identical siblings (72% versus 43%; P = .006), and from a female donor to a male recipient (88% versus 49%; P = .021), was associated with a higher risk of acute GvHD.
Chronic GvHD developed in 24 (32.5%) of patients surviving more than 100 days, including those patients who developed cGvHD following prophylactic DLT. The median interval from transplantation to onset of de novo cGvHD was 181 days (range, 91-546 days). Nineteen patients had limited disease; 5 had extensive disease. Among PBSC recipients, cGvHD showed a borderline association with higher doses of transfused CD34+ cells (40% versus 19%; P = .05).
Infections and toxicity
Twenty-six patients developed pneumonia, caused by fungi (n = 12), bacteria (n = 2), virus (n = 8), or of unknown origin (n = 4), leading to septic shock in 4 cases. Bacteremia was detected in 42 patients, causing symptoms of sepsis or septic shock in 16 cases. Infection of the central venous catheter was the source in 17 patients. Viral (n = 7), bacterial (n = 3), or fungal (n = 2) gastroenteritis, herpes zoster (n = 5), HSV-associated stomatitis (n = 4), viral cystitis (n = 4), and bacterial soft tissue infections (n = 3) were other frequently reported infectious complications. Side effects not related to infections or GvHD were classified using WHO criteria. Twenty-four patients developed no or maximum grade I toxicity. In contrast, grades III and IV toxicity was seen in 54 patients, with some patients having more than one severe organ damage (Table 2).
Organ toxicity according to WHO criteria
. | Grade III, no. events . | Grade IV, no. events . |
|---|---|---|
| Mucositis | 12 | 0 |
| Cardiac | 5 | 2 |
| Renal | 13 | 5 |
| Liver | 7 | 3 |
| Lung | 4 | 0 |
| CNS | 2 | 2 |
| Hemorrhagic cystitis | 5 | 0 |
| Thromboembolic | 2 | 1 |
| Gastrointestinal | 5 | 0 |
| Hemorrhage | 4 | 1 |
| Microangiopathy | 5 | 0 |
. | Grade III, no. events . | Grade IV, no. events . |
|---|---|---|
| Mucositis | 12 | 0 |
| Cardiac | 5 | 2 |
| Renal | 13 | 5 |
| Liver | 7 | 3 |
| Lung | 4 | 0 |
| CNS | 2 | 2 |
| Hemorrhagic cystitis | 5 | 0 |
| Thromboembolic | 2 | 1 |
| Gastrointestinal | 5 | 0 |
| Hemorrhage | 4 | 1 |
| Microangiopathy | 5 | 0 |
Data include only side effects not related to infections or GvHD.
Disease response, relapse, and outcome
CR rate at day +30 was 91.2% (94 of 103 patients, including all with extramedullary disease). Six patients reconstituted with blasts and were regarded as primary treatment failures, and 3 patients were not evaluable because of early death. After a median follow-up of 25 months (range, 3-68 months), 35 patients (37% of initial responders) experienced leukemia relapse at a median of 4 months (range, 1.3-26.6 months) after transplantation. It was hematologic in 31 cases and extramedullary in 4 cases. In addition, one patient developed cytogenetic relapse. In 33 cases (94%), relapse occurred within the first year after transplantation.
At time of last follow-up, 46 patients were alive. Thirty-six patients (35%) had died from persistent (n = 6) or relapsed (n = 30) leukemia at a median of 5.5 months (range, 1-31 months) from transplantation. Estimated cumulative incidence of death from leukemia at 1 and 2 years from transplantation was 28.7% (95% CI, 20.7%-39.6%) and 39.3% (95% CI, 29.9%-51.5%), respectively. A history of more than 2 cycles of chemotherapy prior to conditioning for allogeneic SCT (P = .002; HR, 7.4) and transplantation from an HLA-identical donor (P = .008; HR, 3.85) were associated with increased risk of death from leukemia. Twenty-one patients (20%) had died of causes other than leukemia, including preexisting comorbidity, between day 0 and day +736 (median, day +99). Causes of death were infections (n = 8, including the 3 cases of sepsis in aplasia), GvHD alone (n = 4) or in combination with infections (n = 7), venoocclusive disease (n = 1), and hemorrhage (n = 1). Estimated nonrelapse mortality rate at 100 days, 1 year, and 2 years from transplantation was 10.6% (95% CI, 6.1%-18,7%), 17.2% (95% CI, 11.1%-26.4%), and 22.2% (95% CI, 14.8%-33.1%), respectively. In a univariate analysis, risk for NRM was higher in male patients receiving a graft from a female donor (P = .046) and in both female and male patients after unrelated transplantation (P = .044). However, both factors were not significant in a multivariate analysis.
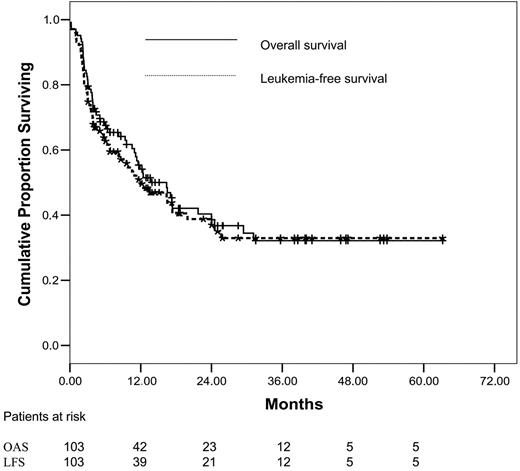
As of May 4, 2005, median survival of the entire cohort was 16.4 months (95% CI, 11-21.5 months). At 1, 2, and 4 years after transplantation, estimated OS was 54%, 40%, and 32%. The respective leukemia-free survival rates were 50%, 39%, and 32% (Figure 1). Among patients without extramedullary disease, OS at 1, 2, and 4 years was 55%, 46%, and 38%; LFS was 51%, 45%, and 38%. In comparison to the entire cohort, patients with primary induction failure (n = 37) showed a significantly better outcome, achieving a 2-year OS and LFS of 62.5% and 62% (Figure 2).
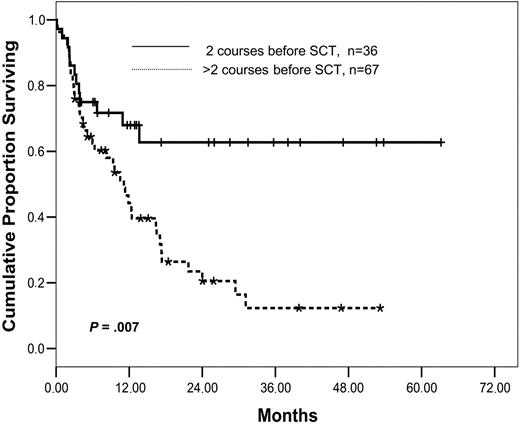
Risk factors for survival are presented in Table 3. Five pretransplantation variables were associated with better outcome in univariate analysis: the stage at transplantation (PIF versus others), a lower number of treatment courses prior to transplantation (2 versus more than 2), a BM infiltration by less than 50% of blasts, less than the median of 215 days' time from diagnosis to transplantation, and a higher number of CD34+ cells in the graft. Using a Cox regression model for multivariate analysis, 2 courses of prior treatment (P = .007; HR, 3.01 for OS; P = .002; HR, 3.25 for LFS) (Figure 3) and a higher content of CD34+ cells in the graft (P = .047; HR, 2.01 for OS; P = .002; HR, 1.8 for LFS) were the only significant pretransplantation factors. In contrast, age, sex, cytogenetic subgroups, and the Charlson Comorbidity Index score were not predictive for outcome. Transplantation from an unrelated donor was associated with a reduced risk of death from leukemia, suggesting a superior GvL effect in the unrelated setting. However, because of an increased risk of nonrelapse mortality after an unrelated donor transplantation, this did not translate into a superior overall survival. Although the number of patients with extramedullary disease was too small to reach statistical significance in survival analysis (P = .1), the prognosis of these patients was dismal: 7 of 11 patients experienced leukemic relapse, 4 of them before day +100. Six died of leukemia, 4 of treatment related-causes, and 1 is currently under therapy for early relapse.
Risk factors for outcome
. | Overall survival . | . | Leukemia-free survival . | . | ||
|---|---|---|---|---|---|---|
. | Univariate . | Multivariate (HR) . | Univariate . | Multivariate (HR) . | ||
| Stage at SCT, PIF vs other | .017 | NS | .006 | NS | ||
| No. of chemotherapy cycles before SCT, 2 vs more than 2 | .008 | .007 (3.01) | .008 | .002 (3.25) | ||
| BM infiltration by leukemic blasts at SCT, less than or greater than 50% | .07 | NS | .09 | NS | ||
| Time from diagnosis to SCT, less than or greater than median | .011 | NS | .008 | NS | ||
| CD34+ cell counts in the graft, less than or greater than median | .028 | .047 (2.00) | .02 | .05 (1.8) | ||
. | Overall survival . | . | Leukemia-free survival . | . | ||
|---|---|---|---|---|---|---|
. | Univariate . | Multivariate (HR) . | Univariate . | Multivariate (HR) . | ||
| Stage at SCT, PIF vs other | .017 | NS | .006 | NS | ||
| No. of chemotherapy cycles before SCT, 2 vs more than 2 | .008 | .007 (3.01) | .008 | .002 (3.25) | ||
| BM infiltration by leukemic blasts at SCT, less than or greater than 50% | .07 | NS | .09 | NS | ||
| Time from diagnosis to SCT, less than or greater than median | .011 | NS | .008 | NS | ||
| CD34+ cell counts in the graft, less than or greater than median | .028 | .047 (2.00) | .02 | .05 (1.8) | ||
HR indicates hazard ratio; NS, not significant.
Overall survival (solid curve) and leukemia-free survival (dashed curve) in patients with primary induction failure (n = 37).
Overall survival (solid curve) and leukemia-free survival (dashed curve) in patients with primary induction failure (n = 37).
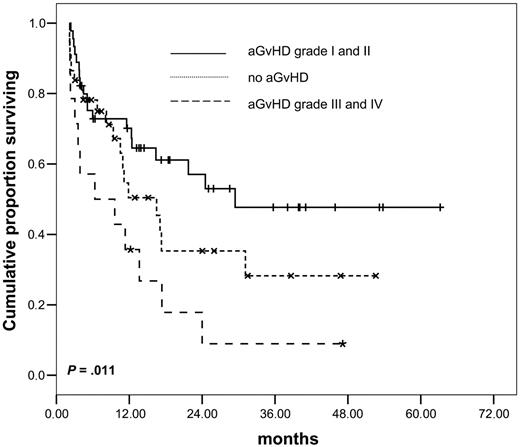
Development of acute GvHD had a significant influence on outcome: in a landmark analysis at day 62 (ie, the latest interval after transplantation at which aGvHD was developed), patients with mild forms (grades I and II) had a better OS and LFS as compared with patients without aGvHD. However, aGvHD of grades III and IV was associated with high NRM and was deleterious for outcome (P = .011 for OS and P = .024 for LFS, log rank, multigroup comparison; Figure 4). In contrast, chronic GvHD did not show a significant influence on survival.
Prophylactic DLT
Prophylactic donor lymphocyte transfusion (pDLT) was given in the centers of Munich and Wiesbaden only. Seventeen (23%) of the 73 patients treated in these 2 centers fulfilled the criteria for pDLT, that is, being alive and without ongoing infection at least 4 months after transplantation and being free of immunosuppressive medication for at least 30 days without developing GvHD. Although pDLT could be given from day +120, it had to be postponed in most patients, before all requirements were fulfilled. Therefore, median time from transplantation to first pDLT was 159 days (range, 120-284 days). Two patients received 1 transfusion, 6 received 2, and 9 received 3 transfusions in escalating doses, containing a median of 1 × 106,1 × 107, and 2 × 107 CD3+ cells/kg at pDLT 1, 2, and 3. Reasons for giving fewer than 3 transfusions were development of GvHD or relapse.
Overall survival as of number of chemotherapy cycles prior to conditioning for SCT.P = .007.
Overall survival as of number of chemotherapy cycles prior to conditioning for SCT.P = .007.
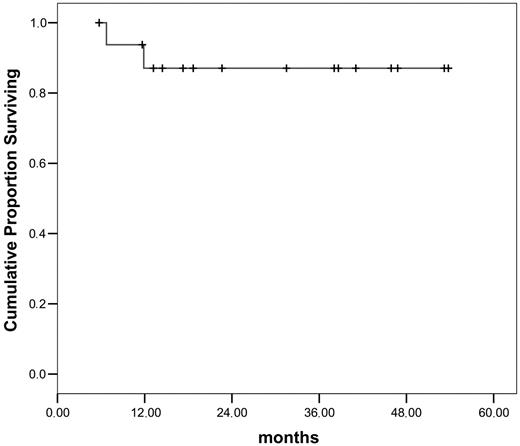
So far, leukemic relapse following pDLT was observed in 5 of 17 patients. Two of these patients died free of blasts under reinduction treatment, 2 achieved a stable secondary CR following adoptive immunotherapy, and 1 patient received a second transplant and is in hematologic remission with persisting minimal residual disease. Hence, 14 patients are alive in continuous CR after pDLT. With a median follow-up of 31.5 months (range, 15.5-56 months) in this small cohort, OS at 3 years from transplantation is 87% (Figure 5). GvHD was the main complication of pDLT: aGvHD grade III developed in 2 and cGvHD in 5 patients. No patient with GvHD after pDLT experienced leukemic relapse. Reasons not to give pDLT to 38 patients alive at day +120 in the 2 centers included cGvHD (n = 16), continued immunosuppression (n = 2), refusal by donor or patient (n = 2), infections (n = 1), reduced performance status (n = 2), a history of aGvHD grade IV after transplantation (n = 2), and relapse (n = 13).
Discussion
On the basis of a variety of multicenter trials in AML, a widely accepted definition of refractoriness to conventional chemotherapy has been established.24-26 Accordingly, refractory AML is defined by either primary failure to achieve a complete remission after 2 cycles of induction therapy, relapse following a first CR of fewer than 6 months, second or higher relapse, or relapsed disease not responding to intensive salvage therapy. For these patients, the chance to achieve a CR with standard treatment, including the use of high-dose AraC, is 10% to 20% at best, and overall survival at 1 year is less than 10% with a median survival of 4 months only. In case of failing one reinduction attempt, the probability of CR is below 1%.33 In the light of these poor results with conventional therapy, allogeneic SCT is the recommended treatment for refractory AML.34-37 However, results are limited by a high relapse incidence and a high nonrelapse mortality.38-41 To improve the dismal outcome of patients with refractory AML, the sequential application of cytoreductive chemotherapy using the FLAMSA regimen, followed by reduced-intensity conditioning for allogeneic SCT, and prophylactic donor lymphocyte transfusion in appropriate patients was evaluated in the study presented here.
Influence of acute GvHD on overall survival, multigroup comparison.P = .011.
The FLAMSA-RIC protocol was intended to increase the safety and maintain the antileukemic efficacy of allogeneic SCT by separating the 2 main aspects of the preparative regimen; that is, cytoreduction on one hand and immunosuppressive conditioning for transplantation on the other hand. FLAMSA chemotherapy, although immunosuppressive by itself, mainly aimed at targeting rapidly proliferating malignant cells, to reduce the leukemia burden before RIC and SCT. Chemotherapy was followed by 3 days of rest, allowing time, in particular to the intestinal mucosa, to recover from acute toxicity. RIC using 4 Gy TBI, cyclophosphamide, and ATG (the latter 2 drugs used in reduced dosages in patients with a genoidentical donor, and in standard dosages in patients with an unrelated or mismatched donor) was thought to provide additional immunosuppression to safely allow engraftment. In addition, total body irradiation may have an antileukemic effect on less rapidly proliferating or even quiescent leukemic cells. This concept proved to be highly effective, as indicated by an OS and LFS at 2 years from transplantation of 40% and 39% for the entire cohort, and 46% and 45% in patients without extramedullary disease. In patients with primary induction failure, results were most encouraging, showing a 2-year OS and LFS of 62.5% and 62%, respectively. These results compare favorably to previous studies on the treatment of refractory AML using standard conditioning regimens for SCT, wherein OS rates from 20% to 30% have been reported.38,40-43 The current data are of particular value, when considering the characteristics of our study population: beside the refractoriness of the leukemia, 3 other factors contributed to an increased overall risk for treatment failure in these patients. First, the median age was 51.8 years—that is, considerably higher than in the studies mentioned above, which had evaluated the effect of allogeneic SCT for advanced or refractory AML in patients of a median age between 28 and 37 years. Second, this was a heavily pretreated group of patients, with two thirds of the patients having received more than 2 courses of chemotherapy, including autologous transplantation in 10% of the patients. Third, comorbidity was considerable, as shown by 58% of the patients having CCI scores of 1 or more, as compared with 12% to 22% in the myeloablative and 44% to 47% in the nonmyeloablative groups from Seattle, based on which the score was adapted for patients receiving SCT.28,29
A formal comparison of our results to other recent conditioning regimens of similar intensity is difficult because of the heterogeneity of included patients. In addition, most published studies do not allow to assess the proportion of patients with refractory AML as defined in our analysis. Bertz et al18 showed 19 elderly patients receiving a RIC transplant for AML in untreated, refractory, or chemo-sensitive relapse, MDS–refractory anemia, refractory anemia with excess of blasts in transformation, sAML/MDS, and osteomyelofibrosis. Although no subgroup analysis was presented for refractory patients, the median OAS of 663+ days in 5 patients with PIF suggests a promising activity of the proposed conditioning regimen, comprising fludarabine, melphalan, and carmustine. In contrast, in another report on SCT in elderly patients, 7 of 10 patients fulfilling the criteria for refractory disease chosen by us had died after 1 year from unrelated donor transplant.17 Recently, 2 very promising studies reported on impressively low NRM rates after conditioning with fludarabine, in combination with either intravenous busulfan21 or 8 Gy TBI.22 However, although both groups achieved excellent results in patients who received transplants in remission, the outcome of patients with active disease was inferior.
Despite these encouraging overall results, the relapse rate in our cohort was still considerable, Thirty-three patients relapsed within the first years after transplantation, resembling the cumulative 1-year relapse incidence of 51% to 63% that has been reported from the studies using standard conditioning regimens. However, major progress was made in terms of nonrelapse mortality. In a large retrospective analysis by the IBMTR, NRM at 2 years from standard intensity conditioning transplant reached 90% in patients with advanced leukemia who were older than 45.39 NRM rates between 44% and 70% have been reported by others.44,45 In contrast, 1-year NRM was only 17.2% in our study. Of course, it must be considered that advances in supportive care and HLA matching have reduced NRM of SCT over time, thereby making historical comparisons questionable. Nevertheless, in the light of the high risk for NRM within our cohort, the current results are promising and suggest that our approach is associated with a considerable reduction of NRM without loss of antileukemic activity, even in highly aggressive disease.
In a multivariate analysis of potential risk factors for outcome, a lower number of cycles of chemotherapy given before transplantation was the pretransplantation variable showing the strongest association with better OS and LFS (P = .007 and P = .002, respectively). A similar observation has been reported earlier by the IBMTR.38 Interestingly, in our analysis, this difference could not be attributed to a higher NRM, as one would have expected, given the higher cumulative organ toxicity in heavily pretreated patients. In contrast, the risk of death from leukemia was markedly increased in this subgroup. This seems remarkable, because patients were included into the analysis regardless of their response to reinduction therapy. In addition, the stage of the disease at transplantation was not different among patients pretreated with 2 versus more courses of chemotherapy. The observation might be a hint for a selection of chemoresistant leukemic cells during repeated reinduction attempts and favors an earlier timing of allogeneic SCT, once AML has appeared to be refractory to chemotherapy. It is also in line with previous investigations by the Seattle group, demonstrating that untreated first relapse might be the preferred time to undertake allogeneic SCT for AML.46 In addition, reinduction attempts, including administration of high-dose AraC, have a high mortality rate up to 26% in refractory patients,33,35,47 a fact that would also argue against this approach in patients with PIF or early relapse.
Beside the antileukemic effect of the conditioning regimen, the therapeutic efficacy of allogeneic SCT mainly relies on the GvL reaction, which is based on the interaction of host antigen-presenting cells and immunocompetent cells of the donor. Although the relevance of the GvL effect in rapidly proliferating diseases such as refractory AML is not fully established, a certain role is supported within our analysis by the increased risk of leukemic death in patients with an HLA-identical donor (although this did not translate into a better overall survival because of higher NRM) and by the superior outcome of patients with mild aGvHD as compared with those who did not develop GvHD. Prophylactic transfusion of donor lymphocytes might be a way to additionally exploit the GvL effect in high-risk patients with AML. The clinical results obtained after pDLT in a subgroup of our study population are encouraging. Nevertheless, one must keep in mind that by using very strict inclusion criteria for pDLT (ie, being alive and in remission at day +120, without GvHD, off immunosuppressive therapy, and free of severe infections), we might have selected a favorable subset of patients who would have had an excellent outcome anyway. Therefore, the impact of pDLT in this clinical setting cannot be determined on the basis of our data. Keeping this, as well as the induction of GvHD following pDLT in 7 of our 17 patients, in mind, a careful evaluation in controlled clinical trials will be necessary, before a role of pDLT can be established.
In conclusion, the current data suggest that the chosen sequential strategy of intensive chemotherapy, followed after 3 days of rest by reduced-intensity conditioning for allogeneic SCT, and pDLT might represent a step forward in the treatment of refractory AML. Because the protocol has been evaluated in a precisely defined cohort of patients, a formal comparison with future strategies in this clinical setting should be possible. The superior outcome of patients with PIF and the advantage of patients with less prior chemotherapy suggest that allogeneic SCT should be considered early in the course of a patient with AML not responding to conventional chemotherapy. Nevertheless, the still considerable incidence of relapse and the unsatisfactory results in patients with extramedullary disease leave room for further improvement.
Prepublished online as Blood First Edition Paper, March 21, 2006; DOI 10.1182/blood-2005-10-4165.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank all the nurses on the various transplantation units and outpatient clinics for their dedicated work and Simona Iacobelli, Rome, for statistical advice. In addition, the help of Elke Dammann, Hannover, Karin Davis, Wiesbaden, and Christiane Lallinger, Munich, for the data collection process is highly appreciated.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal