Abstract
Telomerase activity has been suggested to be critically involved in hematopoietic stem cell (HSC) self-renewal. However, it has been unclear whether human HSCs have telomerase activity and how telomerase activity is regulated within the HSC and progenitor pool. Here, we isolated living cord-blood (CB) CD34+ cells with up-regulated human telomerase reverse transcriptase (hTERT) expression by using an hTERT-reporting adenoviral vector encoding destabilized green fluorescent protein (dGFP) driven by the hTERT promoter, and functionally characterized them in comparison with control vector–transduced CD34+ cells expressing GFP. Following a 2-day serum-free transduction protocol, cells were sorted into a dGFP+ and a GFP+ fraction. Cell-cycle analysis revealed that the dGFP+ cells had a greater proportion of cells in S/G2/M phase compared with the GFP+ cells, (56% ± 1.8% vs 35% ± 4.3%; P < .001) and fewer cells in G0 phase (8.1% ± 3.0% vs 20% ± 4.7%; P < .01) However, the colony-forming and short-term nonobese diabetic/severe combined immunodeficient (NOD/SCID) B2m–/– mice bone marrow–repopulating capacities were similar between the dGFP+ and the GFP+ cells. Interestingly, the dGFP+ cells had a 6-fold lower repopulating capacity in NOD/SCID mice compared with the GFP+ cells and lacked secondary NOD/SCID B2m–/– mice bone marrow–repopulating capacity. Thus, up-regulation of hTERT expression within the CB HSC pool is accompanied by decreased self-renewal capacity.
Introduction
Hematopoietic stem cells (HSCs) have the ability to self-renew and differentiate into all hematopoietic lineages.1 The mechanisms of HSC self-renewal are poorly understood, and among the factors involved, the telomerase enzyme complex has been suggested to play a critical role. The telomerase enzyme complex consists of 2 essential components, the catalytic protein component human telomerase reverse transcriptase (hTERT) and the telomerase RNA component (TERC) that provides the RNA template; both components work in a synchronized manner to add the TTAGGG sequence to the telomeres.2 This telomere maintenance function protects the ends of the chromosomes against chromosome fusions, degradation, and recombination events that would otherwise lead to genetic instability, causing cell death or occasionally the development of malignancy.3-6 In addition, telomerase activity is suggested to be directly involved in cell proliferation processes.6,7 The activity of the telomerase complex is predominantly restricted at the transcriptional level of the hTERT gene, while TERC is more abundantly expressed in most tissues.8,9
Compelling evidence exists that telomerase, as a cell-autonomous mechanism, can prevent or postpone fatal telomere shortening during stem cell self-renewal or extensive cell proliferation of its progenitors.10 In support of the hypothesis that telomerase is important in HSC self-renewal, murine candidate HSCs were demonstrated to have high telomerase activity compared to mature progenitor cells,11 telomere length in HSCs was shortened following serial transplantations,12 and HSCs from telomerase-null mice showed early exhaustion upon serial transplantations.13 However, the translation of such knowledge to the understanding of telomerase function in primitive human hematopoietic cells is complicated by species differences. First, telomeres in inbred mouse strains are significantly longer compared with those in humans.12 Second, loss of telomerase activity is reasonably well tolerated in mice, with mice deficient in TERC showing compromised proliferative capacity in primitive hematopoietic progenitors only in late generations.14 In contrast, a 50% reduction of telomerase activity in humans can manifest with severe bone marrow hypocellularity.15 Mutations in hTERC have been linked to the rare autosomal dominant form of dyskeratosis congenita disorder, and both hTERC and hTERT mutations are found in sporadic cases of aplastic anemia, diseases that if not treated frequently, lead to a bone marrow failure syndrome.16-22 Thus, telomerase activity in human primitive hematopoietic cells appears to be differently and tightly regulated compared with that in murine HSCs and progenitors. Experimental studies on healthy human bulk progenitor cell populations have demonstrated low telomerase activity in CD34+CD38– cells, while higher levels were observed in CD34+CD38+ cells with increased activity upon proliferation of such cells.23-25 However, since repopulating HSCs represent a minor fraction of the CD34+CD38– cells,26 and candidate human HSCs currently assessed as NOD/SCID mice bone marrow repopulation cells (SRCs) contain heterogeneous subpopulations with distinct engraftment and differentiation potentials,27-32 it has not been clear from these studies whether the rare SRCs have telomerase activity and how the telomerase activity is regulated in the SRC pool. A main challenge then is to develop strategies to report telomerase activity at the single-cell level and to directly assess these cells for repopulating versus progenitor activity. Therefore, it has not been possible to assess whether telomerase activity is directly required by repopulating HSCs or by their downstream progenitors.
In this study, we used adenoviral reporter vectors for isolation of CB CD34+ cells with up-regulated hTERT expression at the single-cell level. This strategy allowed functional characterization of hTERT-expressing cells independent of cell phenotype. The adenoviral vectors used in this study were based on a recently developed fiber retargeted adenoviral vector system with Ad35 tropism (Ad5F35 vectors).33,34 These vectors use ubiquitously expressed CD46 antigen for cellular entry.35 We have recently demonstrated that such vectors allow efficient gene transfer into nondividing SRCs.36
Our data demonstrate that the hTERT reporter vector allowed isolation of primitive hematopoietic progenitor cells with up-regulated hTERT expression. In the SRC pool, hTERT expression was up-regulated in proliferating short-term SRCs. hTERT expression remained relatively high in committed colony-forming progenitor cells, but was down-regulated in mature myeloid cells. Our data suggest that self-renewing HSCs are not among those CD34+ cells expressing the highest levels of telomerase.
Materials and methods
Adenoviral vectors
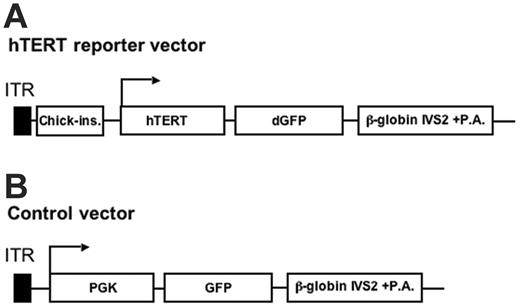
The generation of the adenoviral hTERT reporter vector (Figure 1A) encoding dGFP37 with a half-life of 2 hours under the control of the hTERT promoter has been recently decribed.38 The previously described Ad5F35-GFP vector encoding green fluorescent protein (GFP) under the control of the phosphoglycerate kinase-1 (PGK1) promoter33,36 was used as the control vector for this study (Figure 1B). These vectors were purified and quantified for infectious units (IUs) and physical particles as previously described.33,36 The functional titers of the virus batches used in this study ranged between 5 × 1010 and 3 × 1011 IU/mL with a functional–to–physical-particle ratio of 1:10.
Illustration of the hTERT reporter and control adenoviral vector. (A) The hTERT reporter adenoviral vector. In this vector, the dGFP expression cassette driven by the hTERT promoter (–576 to +30 of the hTERT gene sequence) was engineered in the E1 region. The expression cassette was shielded by the chicken β-like globin gene insulator for ensuring specificity of the hTERT promoter.38 (B) The control Ad5F35-GFP vector encoding GFP under the control of the constitutively active PGK1 promoter has been described previously.33,36 Both vectors are fiber-retargeted Ad5F35 vectors.
Illustration of the hTERT reporter and control adenoviral vector. (A) The hTERT reporter adenoviral vector. In this vector, the dGFP expression cassette driven by the hTERT promoter (–576 to +30 of the hTERT gene sequence) was engineered in the E1 region. The expression cassette was shielded by the chicken β-like globin gene insulator for ensuring specificity of the hTERT promoter.38 (B) The control Ad5F35-GFP vector encoding GFP under the control of the constitutively active PGK1 promoter has been described previously.33,36 Both vectors are fiber-retargeted Ad5F35 vectors.
Cell culture, adenoviral vector transduction, flow cytometric analysis, and cell sorting
CB CD34+ cells were isolated by using a CD34+ cell isolation kit (Miltenyi Biotech, Bergisch Gladbach, Germany) and stored in liquid N2 as previously described.36 Prior to transduction, CD34+ cells were thawed and cultured in serum-free X-vivo 15 medium (Bio Whittaker, Walkersville, MD) containing 1% bovine serum albumin (BSA; Stem Cell Technologies, Vancouver, BC, Canada), 2 mM L-glutamine, 100 μM 2-mercaptoethanol, 100 U/mL penicillin, and 100 μg/mL streptomycin in 24-well plates at a cell density of 1.0 × 105 cells per mL medium and well. The medium was supplemented with either thrombopoietin (TPO) alone at 50 ng/mL (surviving conditions) or a cytokine cocktail consisting of TPO (50 ng/mL), stem-cell factor (SCF) at 100 ng/mL, and Flt-3 ligand (FL) at 50 ng/mL (proliferating conditions). Following overnight incubation, the viral vectors were added. Unbound viruses were washed away 3 hours later and cells were resuspended in fresh medium supplemented with the same cytokine(s) as before. As a control for a telomerase-negative cell population, cord-blood (CB) mononuclear cells (MNCs) were depleted from T cells by using anti-CD3 monoclonal antibodies (mAbs) conjugated to Dynabeads according to manufacturer's instructions (Dynal Biotech ASA, Oslo, Norway). The T-cell–depleted MNCs were cultured and transduced as described for CD34+ cells, except that BSA was exchanged to 20% fetal calf serum (FCS) and that cytokines were exchanged to 100 ng/mL granulocyte macrophage-colony stimulating factor (GM-CSF), 20 ng/mL interleukin-3 (IL-3), and 20 ng/mL SCF. K562 cells were cultured and transduced in Dulbecco modified Eagle medium (DMEM) (Invitrogen, Carlsbad, CA) with 10% FCS at a cell density of 1.0 × 105 cells per mL medium and well. All transductions in this study were performed at a multiplicity of infection (MOI) of 100.
CD34+ cells were analyzed at 48 hours after transduction for dGFP or GFP expression in combination with staining for the CD34 antigen as described previously.36 In addition, T-cell–depleted MNCs were stained with PE-conjugated anti-CD33/15 mAbs and APC-conjugated anti-CD34 mAb, and the dGFP and GFP expression were assessed in 7-aminoactinomycin D (7-AAD) negatively stained myeloid cells33,39 with a CD34–CD33/15+ phenotype (Figure 2A). All sortings for dGFP+ or GFP+ cells were performed with CD34+ cells cultured with the support of TPO alone. Since more than 99% of the cells were CD34+ at this time point, staining for CD34 was omitted. The purity of sorted GFP+ cells was more than 98% as assessed by re-analysis in all experiments.
Quantification of hTERT mRNA expression by real-time PCR
RNA was isolated from 5.0 × 104 mock-transduced CD34+ cells cultured under surviving or proliferating conditions or from sorted CD34–CD15/33+ cells using the RNeasy Mini kit (Qiagen, Valencia, CA). In the same manner, RNA from 5.0 × 104 sorted dGFP+ or GFP+ CD34+ cells cultured under surviving conditions was isolated. In addition, RNA from transduced unsorted CD34+ cell populations was extracted. Both sorted dGFP+ or GFP+ cells and unsorted mock-transduced cells were harvested at 2 days after transduction. In addition, RNA from 5.0 × 104 K562 cells was isolated at days 2, 6, and 10 after transduction. cDNA was transcribed (Superscript III; GibcoBRL, Carlsbad, CA), and hTERT mRNA levels were quantified using Taqman primers and probes (Hs00 162 669_m1) from Applied Biosystems (Foster City, CA) in an ABI Prism 7700 Sequence Detection System, according to the manufacturer's instruction. In each polymerase chain reaction (PCR), cDNA derived from 1250 cells was used. hTERT mRNA levels were presented as 2-cycle threshold (CT), where the CT is the numbers of Taqman PCR cycles at which the PCR product reaches a defined threshold.
Telomeric amplification protocol assay
The telomerase activity was semiquantitatively assessed by using the fluorescence based TRAPEZE XL Telomerase detection kit (Chemicon International, Temecula, CA). In brief, 1.0 × 105 cells derived from the same cell fractions as for the hTERT mRNA level analysis were pelleted and frozen at –80°C. Telomerase activity was quantified as previously described in a Fluostar plate reader by using FLUO32 software (BMG Labtechnologies, Durham, NC) according to the manufacturer's instructions.38 Data shown in Figure 2D and 2G is the FL emission signal for each sample after subtraction of the FL signal from the minus telomerase control (ΔFL) divided by the R emission signal after subtracting the minus Taq polymerase control (ΔR).
The hTERT reporter vector reported hTERT expression in hematopoietic progenitor cells via dGFP expression. (A) The hTERT reporter vector allowed barely detectable levels of dGFP expression in the telomerase-negative CB CD34–CD15/33+ cells. (B) In contrast, nearly all K562 cells became dGFP+ following the hTERT reporter vector transduction. (C) hTERT expression in K562 cells was assessed using real-time PCR. No significant differences in endogenous hTERT expression were observed between the hTERT reporter–transduced K562 cells compared with the control vector, or the mock-transduced cells at the indicated days after transduction. Data shown are the averages and standard deviations of hTERT mRNA levels from 3 independent experiments. (D) No significant differences in telomerase activity in the K562 cells were observed between the same 3 groups as analyzed in panel C. Data shown are the averages and standard deviations of telomerase activity. (E) A substantial fraction of the CD34+ cells expressed dGFP+, both under survival conditions (TPO alone; T) and proliferating conditions (TPO, SCF, and FL; TSF). (F) hTERT mRNA expression was assessed using real-time RT-PCR on the indicated cell populations as described in “Materials and methods.” Data shown are the averages and standard deviations of hTERT mRNA levels from 1250 cells from 5 independent experiments. (G) CD34–CD15/33+ and transduced CD34+ cells were assessed for telomerase activity using the TRAP assay. Data shown are the averages and standard deviations of telomerase activity of cell extracts from 1000 cells per assay (n = 3). HI indicates heat-inactivated sample; ND, not detectable. For significance tests, paired t tests were used.
The hTERT reporter vector reported hTERT expression in hematopoietic progenitor cells via dGFP expression. (A) The hTERT reporter vector allowed barely detectable levels of dGFP expression in the telomerase-negative CB CD34–CD15/33+ cells. (B) In contrast, nearly all K562 cells became dGFP+ following the hTERT reporter vector transduction. (C) hTERT expression in K562 cells was assessed using real-time PCR. No significant differences in endogenous hTERT expression were observed between the hTERT reporter–transduced K562 cells compared with the control vector, or the mock-transduced cells at the indicated days after transduction. Data shown are the averages and standard deviations of hTERT mRNA levels from 3 independent experiments. (D) No significant differences in telomerase activity in the K562 cells were observed between the same 3 groups as analyzed in panel C. Data shown are the averages and standard deviations of telomerase activity. (E) A substantial fraction of the CD34+ cells expressed dGFP+, both under survival conditions (TPO alone; T) and proliferating conditions (TPO, SCF, and FL; TSF). (F) hTERT mRNA expression was assessed using real-time RT-PCR on the indicated cell populations as described in “Materials and methods.” Data shown are the averages and standard deviations of hTERT mRNA levels from 1250 cells from 5 independent experiments. (G) CD34–CD15/33+ and transduced CD34+ cells were assessed for telomerase activity using the TRAP assay. Data shown are the averages and standard deviations of telomerase activity of cell extracts from 1000 cells per assay (n = 3). HI indicates heat-inactivated sample; ND, not detectable. For significance tests, paired t tests were used.
Cell-cycle analysis
Colony assay
For quantifying the frequency of committed progenitor cells with colony-forming capacity, 500 mock-transduced cells, sorted dGFP+, and GFP+ cells were plated in triplicate in 1 mL methylcellulose medium (MethoCult H4230; Stem Cell Technologies) supplemented with human SCF (25 ng/mL), GM-CSF (50 ng/mL), IL-3 (25 ng/mL), and erythropoietin (5 U/mL). In the same manner, sorted CD34–CD33/15+ cells were plated at 5000 cells/mL methylcellulose medium. The formation of burst-forming unit-erythroid (BFU-E), colony-forming unit-granulocyte-macrophage (CFU-GM), and CFU-granulocyte, erythrocyte, macrophage, and megakaryocyte (CFU-GEMM) were counted after 14 days of culture.
NOD/SCID mice transplantation assay
Detailed procedures for housing, transplantation, and assessment of human-cell engraftment in NOD/SCID and NOD/SCID B2m–/– mice have been described previously.36,42 In brief, following a sublethal irradiation of 3.5 Gy (350 rad), 8- to 10-week-old animals were given transplants of 1.0 × 105 sorted dGFP+, GFP+, or mock-transduced cells via tail-vein injection. At 6 weeks after transplantation, mouse bone marrow cells were prepared from both femurs and tibias, depleted for red cells with NH4Cl, and stained with APC-conjugated anti–human CD45 mAb together with PE-conjugated anti-CD15 and anti-CD33 mAbs, anti-CD19 mAb, anti-CD34 mAb, or isotype-matched control mAbs. For secondary transplantations in NOD/SCID B2m–/– recipients, 20 to 50 × 106 total bone marrow cells from each primary NOD/SCID B2m–/– recipient were individually transplanted into secondary recipients by tail-vein injections, and human-cell engraftment was assessed 6 weeks later as for the primary recipients. All mAbs used in this study were obtained from Becton Dickinson Immunocytometry Systems (San Jose, CA).
Results
Isolation of CB CD34+ cells with up-regulated hTERT expression by using the hTERT reporting adenoviral vector
In order to assess hTERT expression in single living CB CD34+ cells, we developed an hTERT reporting adenoviral vector, encoding dGFP37 under the control of the hTERT promoter (hereafter referred to as the hTERT reporter vector; Figure 1). This vector is an Ad5F35 vector and uses ubiquitously expressed CD46 as a cellular receptor.35 By using the hTERT reporter vector, we have shown that hTERT expression and telomerase activity in a variety of cancer cell lines can be specifically reported at the single-cell level via dGFP expression without affecting endogenous hTERT expression and telomerase activity.38 For assessing potential nonspecific dGFP expression following the hTERT reporter vector infection of primary hematopoietic cells, T-cell–depleted MNCs were transduced. As a reference for the transduction efficiency, the control Ad5F35-GFP vector encoding GFP under the control of the PGK1 promoter was used. As assessed in mature myeloid cells with a CD34–CD15/33+ phenotype, 1.5% ± 1.0% (n = 5) of the cells expressed barely detectable levels of dGFP following the hTERT reporter vector transduction (Figure 2A). In contrast, 74% ± 15% (n = 5) of the cells expressed GFP following transduction with the control vector, demonstrating a high gene transfer efficiency by the Ad5F35 vectors into this cell type. However, following infection of K562 cells with the hTERT reporter or the control vector, nearly all cells became dGFP+ or GFP+ (Figure 2B). In addition, K562 cells and sorted CD34–CD15/33+ cells were assessed for hTERT mRNA expression using real-time RT-PCR and telomerase activity using the Telomeric amplification protocol (TRAP) assay. In accordance with the lack of dGFP expression following the hTERT reporter vector transduction, no detectable levels of hTERT mRNA and telomerase activity could be measured in CD34–CD15/33+ cells; whereas high levels of hTERT mRNA and telomerase activity were detected in K562 cells (Figure 2C,D,F,G). Thus, the hTERT reporter vector is silent in primary hematopoietic cells without hTERT expression/telomerase activity. To directly address whether endogenous hTERT expression might be negatively influenced by the dGFP expression, hTERT expression and telomerase activity in transduced K562 cells were measured at 2, 6, and 10 days after infection. dGFP expression was lost at around day 7 and GFP expression at around day 10. Importantly, no significant difference in hTERT expression and telomerase activity was observed between the hTERT reporter vector, the control vector, or the mock-transduced K562 cells, which indicate that the hTERT reporter vector–mediated dGFP expression does not affect endogenous hTERT expression and telomerase activity (Figure 2C-D).
The CB CD34+ cells were cultured with early acting cytokines to support the survival and proliferation of primitive cells. This was achieved in serum-free medium supplemented with either TPO alone (surviving conditions) or with a cytokine cocktail of TPO, SCF, and FL (proliferating conditions).43,44 Following transduction with the control vector, 47% ± 6.7% (n = 6) of the CB CD34+ cells cultured in surviving conditions expressed GFP, while 17% ± 4.3% (n = 6) of the cells expressed dGFP following the hTERT reporter vector transduction (Figure 2E). Under proliferating conditions, 76% ± 1.4% and 32% ± 5.7% (n = 3) of the CB CD34+ cells expressed GFP or dGFP following the control and the hTERT reporter vector transduction, respectively.
In addition, a 5-fold increase in hTERT reporter vector viral particles only slightly increased the frequency of dGFP-expressing cells (data not shown), indicating that the different frequencies of dGFP- and GFP-expressing cells observed between the 2 vectors were not due to the use of different MOIs. Instead, these data suggest that around one third of successfully transduced CB CD34+ cells allowed dGFP expression following the hTERT reporter vector infection. Sorted dGFP+ cells were analyzed for hTERT mRNA levels compared with mock-transduced CD34+ cells and GFP+ cells sorted following the control vector infection. This was done at 2 days after transduction, when the adenoviral vector mediated transgene-expression peaks.36 The dGFP+ cells showed significantly higher levels of endogenous hTERT expression compared with the GFP+ cells, the unsorted hTERT reporter vector–transduced cells, and the mock-transduced cells (P < .05, n = 5; Figure 2F). Furthermore, similar levels of endogenous hTERT expression were observed between the mock-transduced CD34+ cells and the unsorted hTERT reporter vector– or the control vector–transduced CD34+ cells (Figure 2F). These data suggest that the endogenous hTERT expression is not negatively influenced by the reporter dGFP expression in CD34+ cells. Furthermore, mock-transduced CD34+ cells cultured under proliferating conditions showed significantly higher levels of hTERT expression compared with the CD34+ cells cultured under surviving conditions (P < .01, n = 5; Figure 2F). We also measured telomerase activity in the same cell fractions. A 2-fold higher telomerase activity was observed in CD34+ cells cultured under proliferating conditions compared with CD34+ cells cultured under surviving conditions (P < .05, n = 4; Figure 2G). Collectively, these data suggest that endogenous hTERT expression among CD34+ cells is heterogeneous and the hTERT reporter vector–mediated expression of dGFP allowed detection of CB CD34+ cells with up-regulated hTERT expression and telomerase activity. These data are in agreement with previous reports demonstrating that hTERT expression and telomerase activity are differentiation stage and culture condition dependent in primitive hematopoietic cells.8,23,25
CB CD34+ cells with up-regulated hTERT expression are actively cycling
The cell-cycle–dependent regulation of hTERT expression and telomerase activity has been debated.7,45-48 In cultured CD34+ cells, single cytokines were unable to up-regulate telomerase activity.23,25 For assessing the cell-cycle status of CB CD34+ cells with up-regulated hTERT expression at minimal cytokine stimulation, the dGFP+ cells or GFP+ cells were sorted following transduction under survival conditions and stained for the Ki-67 antigen and 7-AAD.40,41 Data presented in Figure 3 show that the sorted dGFP+ cells contained a significantly higher proportion of cells in the S/G2/M phase compared with the GFP+ cells (56% ± 1.8% vs 35% ± 4.3%, n = 4; P < .001), and fewer cells with relatively higher levels of Ki-67 staining in G0 phase (8.1% ± 3.0% vs 20% ± 4.7%, n = 4; P < .01), while the frequency of the G1 phase cells was similar between the 2 groups. These data suggest that hTERT expression in CD34+ cells is cell-cycle dependent. However, the finding that a substantial fraction of the sorted telomerase-negative CD34–CD15/33+ cells were actively cycling (Figure 3) suggests that the differentiation stage of a particular cell may play a more predominant role in determining its telomerase activity than its cell-cycle status.
Cell-cycle analysis using Ki-67 and 7-AAD. CD34+ cells cultured and transduced under survival conditions were sorted based on dGFP+ or GFP+ expression and assessed for cell-cycle status by Ki-67 and 7-AAD staining. The mock-transduced CD34+ cells and sorted mature myeloid CD34–CD15/33+ cells were assessed in the same manner. The percentages of cells within each cell-cycle stage are depicted in density plots. Data presented are from a representative experiment out of 4 independent experiments.
Cell-cycle analysis using Ki-67 and 7-AAD. CD34+ cells cultured and transduced under survival conditions were sorted based on dGFP+ or GFP+ expression and assessed for cell-cycle status by Ki-67 and 7-AAD staining. The mock-transduced CD34+ cells and sorted mature myeloid CD34–CD15/33+ cells were assessed in the same manner. The percentages of cells within each cell-cycle stage are depicted in density plots. Data presented are from a representative experiment out of 4 independent experiments.
CB CD34+ cells with up-regulated hTERT expression contained similar frequency of colony-forming progenitor cells as the CD34+ bulk cells. Sorted dGFP+ (▪), GFP+ cells (▦), and mock-transduced cells (□) were plated into methylcellulose medium supplemented with cytokines at 500 cells/mL/plate, as described in “Materials and methods.” Similarly, the sorted CD34–CD15/33+ cells were plated at 5000 cells/mL/plate (▨). The content of BFU-E, CFU-GM, and CFU-GEMM cells was scored 2 weeks after plating according to standard criteria. The averages and standard deviations of colony numbers per plate from 3 independent experiments are shown.
CB CD34+ cells with up-regulated hTERT expression contained similar frequency of colony-forming progenitor cells as the CD34+ bulk cells. Sorted dGFP+ (▪), GFP+ cells (▦), and mock-transduced cells (□) were plated into methylcellulose medium supplemented with cytokines at 500 cells/mL/plate, as described in “Materials and methods.” Similarly, the sorted CD34–CD15/33+ cells were plated at 5000 cells/mL/plate (▨). The content of BFU-E, CFU-GM, and CFU-GEMM cells was scored 2 weeks after plating according to standard criteria. The averages and standard deviations of colony numbers per plate from 3 independent experiments are shown.
CB CD34+ cells with up-regulated hTERT expression contain similar frequency of committed progenitor cells as bulk CD34+ cells
A substantial subpopulation of CB CD34+ cells has the capacity to form colonies when plated in methylcellulose medium supplemented by appropriate cytokines. Previous studies have not been able to assess whether telomerase activity is preferentially high in certain types of committed colony-forming progenitor cells mainly due to an inability in isolating live cells with hTERT expression. Two weeks following plating of mock-transduced, sorted dGFP+ and GFP+ cells, we observed similar numbers and frequencies of BFU-E, CFU-GM, and CFU-GEMM (Figure 4). In addition, the telomerase-negative CD34–CD15/33+ cells were nearly devoid of colony-forming cells (Figure 4). The relatively low numbers of CFU-Cs following plating of 10-fold more CD34–CD15/33+ cells compared with the CD34+ cells could be due to sorting impurity. Therefore, CD34+ cells with up-regulated hTERT expression contain similar numbers and proportions of myeloid colony-forming progenitor cells as bulk CD34+ cells. These data show that hTERT expression is up-regulated in committed progenitors.
CB CD34+ cells with up-regulated hTERT expression contained short-term multipotent SRCs. Sorted dGFP+, GFP+, and mock-transduced cells were transplanted into NOD/SCID B2m–/– or NOD/SCID mice via tail-vein injections at 1.0 × 105 cells per animal. Human-cell engraftment in mouse bone marrow was assessed 6 weeks later. (A) The gating for assessing human-cell engraftment (hu-CD45+ cells), myeloid differentiation (CD33/15+ cells), lymphoid differentiation (CD19+ cells), and regeneration of primitive CD34+ cells from a representative animal is shown. (B) The percentages of hu-CD45+ cells in the bone marrow of primary NOD/SCID B2m–/– mice recipients following transplantation of dGFP+, GFP+, or mock-transduced cells are shown. (C) The percentages of hu-CD45+ cells in the bone marrow of NOD/SCID mice recipients following transplantation of dGFP+, GFP+, or mock-transduced cells are shown. (D) The percentages of hu-CD45+ cells in the bone marrow of secondary NOD/SCIDB2m–/– recipients following transplantation with total bone marrow cells from primary NOD/SCIDB2m–/– mice recipients are shown. In panels B-D, each dot represents one mouse and each figure is a summary of 2 independent experiments. The horizontal bars show the average levels of hu-CD45+ cells.
CB CD34+ cells with up-regulated hTERT expression contained short-term multipotent SRCs. Sorted dGFP+, GFP+, and mock-transduced cells were transplanted into NOD/SCID B2m–/– or NOD/SCID mice via tail-vein injections at 1.0 × 105 cells per animal. Human-cell engraftment in mouse bone marrow was assessed 6 weeks later. (A) The gating for assessing human-cell engraftment (hu-CD45+ cells), myeloid differentiation (CD33/15+ cells), lymphoid differentiation (CD19+ cells), and regeneration of primitive CD34+ cells from a representative animal is shown. (B) The percentages of hu-CD45+ cells in the bone marrow of primary NOD/SCID B2m–/– mice recipients following transplantation of dGFP+, GFP+, or mock-transduced cells are shown. (C) The percentages of hu-CD45+ cells in the bone marrow of NOD/SCID mice recipients following transplantation of dGFP+, GFP+, or mock-transduced cells are shown. (D) The percentages of hu-CD45+ cells in the bone marrow of secondary NOD/SCIDB2m–/– recipients following transplantation with total bone marrow cells from primary NOD/SCIDB2m–/– mice recipients are shown. In panels B-D, each dot represents one mouse and each figure is a summary of 2 independent experiments. The horizontal bars show the average levels of hu-CD45+ cells.
CB CD34+ cells with up-regulated hTERT expression were depleted of long-term self-renewing SRCs, but retained short-term multipotent SRCs
Among CD34+ cells, rare but heterogeneous SRCs with distinct self-renewal and differentiation capacities represent the candidate human HSCs.27-32 It has been unknown whether hTERT is expressed in the candidate human HSC compartment. By transplanting sorted dGFP+ and GFP+ cells (cultured and transduced under surviving conditions) into NOD/SCID B2m–/– and NOD/SCID mice, we assessed the SRC composition in CB CD34+ cells with up-regulated hTERT expression. The mock-transduced cells were transplanted as an additional control for bulk CD34+ cells. In the primary NOD/SCID B2m–/– mice recipients, similar levels of human-cell engraftment in mice bone marrow were observed between the dGFP+ and the GFP+ cells (34% ± 18% and 35% ± 11%, respectively; Figure 5B) and no lineage skewing could be observed between the 3 groups (Figure 5A; Table 1). Compared with the NOD/SCID B2m–/– mice, the NOD/SCID mice have been shown to allow engraftment of fewer short-term SRCs.29,49 Furthermore, the NOD/SCID mice repopulating cells are predominantly in a quiescent cell-cycle state in contrast to the NOD/SCID B2m–/– repopulating cells that can also be actively cycling.29 Therefore, we asked whether the CB CD34+ cells with up-regulated hTERT expression were hampered in NOD/SCID mice repopulating capacity. The human-cell engraftment was on average 6-fold lower in the NOD/SCID mice that received dGFP+ cell transplants compared with those that received transplants of the GFP+ cells (3.0% ± 2.9% vs 18% ± 15%, respectively; P < .001; Figure 5C); nevertheless, a similar lineage distribution was observed between the 2 groups (Table 2). These data are in agreement with the observation that actively cycling cells are hampered in NOD/SCID mice repopulating capacity,50 and GFP+ cells following transduction with the control Ad5F35-GFP vector contain nondividing NOD/SCID mice repopulating cells.36 To address whether the similar engraftment levels between the dGFP+ and the GFP+ cells in the primary NOD/SCID B2m–/– mice were due to short-term SRCs with limited self-renewing capacity among the dGFP+ cells, unfractioned bone marrow cells from each primary recipient were individually transplanted into secondary NOD/SCID B2m–/– mice recipients. Interestingly, none of 8 secondary recipients in the dGFP+ group was positive for human-cell engraftment in their bone marrow. In contrast, 3 of 7 and 3 of 5 secondary recipients were positive for human cell engraftment in the GFP+ group and the mock-transduced control group, respectively (Figure 5D). Therefore, within the SRC compartment, up-regulation of hTERT expression is accompanied by a transition from more primitive self-renewing SRCs to short-term, yet multipotent, SRCs with limited self-renewal capacity.
CB CD34+ cells with up-regulated hTERT expression allowed multilineage differentiation in NOD/SCID B2m-/- mice bone marrow
Group . | No. mice in group . | CD33/15+, % . | CD19+, % . | CD34+, % . |
|---|---|---|---|---|
| dGFP+ | 9 | 20 ± 12 | 85 ± 6.9 | 12 ± 3.8 |
| GFP+ | 8 | 16 ± 8.0 | 84 ± 10 | 17 ± 11 |
| Mock | 5 | 18 ± 4.9 | 85 ± 4.0 | 16 ± 6.1 |
Group . | No. mice in group . | CD33/15+, % . | CD19+, % . | CD34+, % . |
|---|---|---|---|---|
| dGFP+ | 9 | 20 ± 12 | 85 ± 6.9 | 12 ± 3.8 |
| GFP+ | 8 | 16 ± 8.0 | 84 ± 10 | 17 ± 11 |
| Mock | 5 | 18 ± 4.9 | 85 ± 4.0 | 16 ± 6.1 |
Sublethally irradiated NOD/SCID B2m-/- mice received transplants of sorted dGFP+, GFP+, or mock-transduced cells at 1 × 105 cells per animal via tail-vein injection. At 6 weeks after transplantation, the human-cell engraftment and lineage differentiation were assessed in mice bone marrow. Data shown are the averages and standard deviations of the percentages of myeloid, B lymphoid, and CD34+ cells in the CD45+ human cells.
CB CD34+ cells with up-regulated hTERT expression allowed multilineage differentiation in NOD/SCID mice bone marrow
Group . | No. mice in group . | CD33/15+, % . | CD19+, % . | CD34+, % . |
|---|---|---|---|---|
| dGFP+ | 11 | 36 ± 15 | 54 ± 16 | 8.9 ± 5.0 |
| GFP+ | 8 | 28 ± 4.4 | 67 ± 6.6 | 13 ± 5.0 |
| Mock | 8 | 32 ± 12 | 65 ± 13 | 14 ± 5.0 |
Group . | No. mice in group . | CD33/15+, % . | CD19+, % . | CD34+, % . |
|---|---|---|---|---|
| dGFP+ | 11 | 36 ± 15 | 54 ± 16 | 8.9 ± 5.0 |
| GFP+ | 8 | 28 ± 4.4 | 67 ± 6.6 | 13 ± 5.0 |
| Mock | 8 | 32 ± 12 | 65 ± 13 | 14 ± 5.0 |
Sublethally irradiated NOD/SCID mice received transplants of sorted dGFP+, GFP+, or mock-transduced cells at 1 × 105 cells per animal via tail-vein injection. At 6 weeks after transplantation, the human-cell engraftment and lineage differentiation were assessed in mice bone marrow. Data shown are the averages and standard deviations of the percentages of myeloid, B lymphoid, and CD34+ cells in the CD45+ human cells.
Discussion
Previous studies on the role of telomerase activity in human primitive hematopoietic cells were predominantly based on measurement of telomerase activity on phenotypically defined CD34+ or CD34+CD38– cells by using the TRAP assay.23,25,51,52 These studies did not directly link hTERT expression and telomerase activity to any functionally distinct subpopulation of CD34+ cells. Importantly, phenotype-independent characterization of CD34+ cells following ex vivo culture is desirable, since the CD34+CD38– phenotype no longer corresponds to the SRC frequency in ex vivo expanded cells.53 Based on the findings that Ad5F35 tropism retargeted adenoviral vectors allowed efficient gene transfer into nondividing SRCs,36 we have developed a hTERT reporting adenoviral vector for the identification and isolation of single living CB CD34+ cells with high levels of hTERT expression. We show that following transduction of CB CD34+ cells with the hTERT reporting adenoviral vector, around one-third of the successfully transduced cells expressed dGFP, suggesting a heterogeneity in hTERT expression among CB CD34+ cells. In the subsequent experiments, we addressed whether CB CD34+ cells with up-regulated hTERT expression could be defined to distinct subpopulations regarding HSC/progenitor functionality and cell-cycle status. Our data demonstrate that CB CD34+ cells with up-regulated hTERT expression contain actively cycling short-term multipotent SRCs and committed colony-forming progenitor cells. No self-renewing SRCs were detected.
The strict balance between self-renewal and differentiation of HSCs is a tightly regulated process, and telomerase activity has been suggested to play a critical role in HSC maintenance.10,54 Telomerase activity is widely believed to be important for the telomere maintenance of stem cells and their immediate successors as these cells undergo extensive cell proliferation. Telomerase deficiency due to genetic mutations in the TERC or hTERT gene results in bone marrow failure in humans.15-22 However, due to our inability to prospectively isolate primitive hematopoietic cells with telomerase activity, human primitive hematopoietic cells have not been functionally characterized in the context of telomerase activity.
In this study, the hTERT expression and telomerase activity were stringently reported in single living CB CD34+ cells by using adenoviral vector–mediated dGFP expression under the control of the hTERT promoter. The hTERT reporter vector allowed dGFP expression in CB CD34+ cells with up-regulated hTERT expression and telomerase activity, but was silent in hTERT and telomerase–negative CD34–CD15/33+ mature myeloid cells. The specificity of the hTERT reporter vector in reporting hTERT expression was demonstrated, as significantly higher levels of hTERT mRNA expression was observed in sorted dGFP+ cells following the hTERT reporter vector infection compared with mock-transduced cells and sorted GFP+ cells infected with the control vector. The lack of detectable differences in telomerase activity under survival conditions between these cell populations could be due to the fact that the TRAP assay only allows semiquantitative assessment of telomerase activity. Alternatively, the half-life of the telomerase complex may be longer than the population doubling time of the CD34+ cells, or that other factors than hTERT expression may influence the telomerase activity measured by the TRAP assay. The dGFP reporter has a rather short half-life of 2 hours; as such, the levels of dGFP expression will likely reflect the dynamic change of hTERT expression during CD34+ cell proliferation and differentiation. In this study, we used a short 2-day transduction protocol under low cytokine stimulatory conditions, which has been shown unable to up-regulate telomerase activity, while telomerase activity was up-regulated during 3 to 4 days of ex vivo culture under strong stimulatory conditions.23,25
The functional properties of the dGFP+ CD34+ cells were assessed at the committed progenitor-cell and candidate-HSC level. Compared with the GFP+ and mock-transduced cells, the dGFP+ cells contained similar frequencies of myeloid colony-forming cells. Candidate human HSCs are currently assessed as SRCs. Clonal tracking and cell-fractionation studies have revealed that SRCs are heterogeneous regarding engraftment kinetics and duration, lineage differential potential, and ability to repopulate secondary recipients.27-32 Short-term SRCs, mostly actively cycling, predominantly repopulate NOD/SCID B2m–/– mice, and can also repopulate NOD/SCID mice when their natural killer cell activity is suppressed.27,29 In contrast, long-term SRCs, mostly at quiescent cell-cycle status, can repopulate NOD/SCID and NOD/SCID B2m–/– mice at equal efficiency.27,29,50 Interestingly, a similar repopulating capacity was found in the NOD/SCID B2m–/– mice bone marrow between the dGFP+ and the GFP+ cells, but a 6-fold lower repopulation capacity in NOD/SCID mice bone marrow was observed for the dGFP+ cells compared with the GFP+ cells. In both NOD/SCID and NOD/SCID B2m–/– mice primary recipients, multilineage engraftment was achieved with the dGFP+ cells. These data suggest that within the SRC compartment, the hTERT expression is up-regulated in a transition from more primitive SRCs to short-term SRCs. In support of this hypothesis, the dGFP+ cells lacked secondary SRC capacity in NOD/SCID B2m–/– mice, whereas secondary SRC capacity was demonstrated for the GFP+ cells.
Previously, a “cell-cycle model” for telomerase activity in human hematopoietic progenitor cells was proposed, which suggests that telomerase activity is repressed in freshly isolated CD34+CD38– cells or nondividing CD34+ cells; is activated on cell proliferation, expansion, and cell-cycle entry, and maintained during progression into the progenitor compartment (CD34+CD38+); and is repressed again in terminally differentiated cells, which have been generally believed to be silent in cell cycle.23,25,55 Our cell-cycle analysis data indeed support the “cell-cycle model.” However, the finding that a substantial fraction of the mature myeloid (CD34–CD15/33+) telomerase-negative cells were actively cycling demonstrates that the telomerase activity also is differentiation stage dependent. Therefore, hTERT expression in the hematopoietic system has both a hierarchical and a cell-cycle–dependent component.
It is possible that the HSCs, mainly residing in quiescence, are devoid of hTERT expression, but it cannot be excluded that the quiescent long-term SRCs have the capacity to up-regulate hTERT expression during their self-renewing divisions or that hTERT expression in these cells is below the detection threshold under our conditions.
It could also be argued that dGFP expression might negatively influence endogenous hTERT expression in a small subpopulation of CD34+ cells expressing the highest levels of endogenous hTERT, which might contain the long-term SRCs. Although the mean level of hTERT expression in CD34+ cells was not affected by dGFP expression and SRCs reside in the CD34+CD38– cell population with low telomerase activity,23 this possibility cannot be entirely excluded. However, our findings from the cell lines with different levels of endogenous hTERT expression argue against this possibility. As shown in Figure 2, endogenous hTERT expression in K562 cells was not negatively affected by dGFP expression following infection with the hTERT reporter vector, although K562 cells expressed remarkably higher levels of hTERT compared with CD34+ cells. In addition, our recent study demonstrated that endogenous hTERT expression in A549, HeLa, and HL-60 cells was not negatively influenced by the hTERT reporter vector–mediated dGFP expression, although A549 cells and HL-60 cells exhibited much higher levels of hTERT expression compared with HeLa cells.38 Furthermore, our cell-cycle analysis revealed that the dGFP+ cells were more active in cell cycle, which suggests that this cell fraction contains fewer long-term SRCs, since long-term SRCs are predominantly quiescent.29,50
Our study reconciles the previous discrepancy between the observations suggesting low to undetectable levels of telomerase activity in primitive CD34+CD38– cells and the patient data indicating that a dysfunction of telomerase activity leads to a bone marrow failure syndrome as a likely result of a HSC deficiency.16-22 Our data indicate that a telomerase deficiency could strongly impact short-term HSCs and possibly committed progenitors. We hypothesize that a compromised short-term proliferation capacity would force enhanced recruitment of long-term HSCs into proliferation, and would, over time, lead to exhaustion of the long-term HSCs, resulting in a bone marrow failure syndrome in patients. Our study also is in agreement with the observations that following allogeneic stem cell transplantation in patients, accelerated telomere shortening in donor-derived cells is observed in the first year when short-term HSCs are forced into extensive proliferation, while homeostasis of telomere length was achieved later.56,57
In summary, by detecting single living CB CD34+ cells with up-regulated hTERT expression, we have prospectively isolated highly proliferative short-term multipotent SRCs and committed progenitor cells. The utility of such cells in clinical stem-cell transplantation applications may reduce early posttransplantation complications following high-dose chemotherapy. Human primitive hematopoietic cells have been traditionally isolated based on cell-surface marker expression. To our knowledge, this is the first study that has prospectively separated 2 functionally distinct SRC populations based on a cell-intrinsic marker. As we show here and previously,36 fiber-retargeted Ad5F35 adenoviral vectors allow efficiently gene transfer into nondividing long-term primitive SRCs. We speculate that various subpopulations of the candidate human HSC compartment can be identified and characterized by using Ad5F35 vector–mediated reporting and manipulation of gene expression. The use of nonintegrating adenoviral vectors would also reduce insertional mutagenesis–related effects associated with retroviral protocols.58,59
Prepublished online as Blood First Edition Paper, April 6, 2006; DOI 10.1182/blood-2005-09-008904.
Supported by the Swedish Cancer Society, Swedish Children's Cancer Foundation, the Märit and Hans Rausing charitable foundation, the Royal Physiografic Society in Lund, the Siv-Inger & Per-Erik Anderssons Minnesfond, Funds of Lund University Hospital, the Gunnar Nilsson Cancer Foundation, and the Erik Philip-Sörensen Foundation. The Lund Strategic Research Center for Stem Cell Biology and Cell Therapy is supported by a Center of Excellence grant from the Swedish Foundation for Strategic Research.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We would like to thank Camilla Björklöv, Lillian Wittman, and Eva Gynnstam for expert assistance with the animal experiments, and Anna Fossum and Dr Zhi Ma for excellent flow cytometric cell sorting. We are indebted to Marianne Nilsson and the staff at the department of Obstetrics and Gynecology, Helsingborg, for collecting umbilical cord-blood samples. The authors thank all members of Karlsson laboratory for interaction and support, and Keith Humphries for insightful discussions.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal