Abstract
Cell-free hemoglobin's (CFH) high affinity for nitric oxide (NO) could limit CFH's use as an oxygen-carrying blood replacement fluid because it scavenges NO, causing vasoconstriction and hypertension. However, the extent to which perivascular NO levels change following intravascular administration of hemoglobin (Hb) with different molecular dimensions correlates with vasoconstrictive responses in the microcirculation is unknown. The study objective was to determine vasoconstrictive effects following bolus infusions of (1) αα cross-linked Hb; (2) polymerized bovine Hb; or (3) polyethylene glycol-decorated Hb (PEG-Hb), by measurements of in vivo microvessel diameter, blood flow, perivascular NO concentration, and systemic hemodynamic parameters. All CFHs caused reductions in perivascular NO levels, not correlated to microvascular responses. PEG-Hb (largest molecular volume) maintained blood flow, while the others caused vasoconstriction and reduced perfusion. All solutions increased mean arterial pressure due to vasoconstriction and blood volume expansion, except for PEG-Hb, which increased blood pressure due to blood volume expansion and maintenance of cardiac output. In conclusion, perivascular NO reduction is similar for all Hb solutions because NO binding affinities are similar; however, effects on vascular resistance are related to the type of molecular modification, molecular volume, and oxygen affinity.
Introduction
Cell-free hemoglobin (Hb) of oxygen carriers (CFHs) has a very high affinity for nitric oxide (NO), which limits NO bioavailability, an effect that is hypothesized to promote systemic hypertension and microvascular vasoconstriction. Since CFHs are being developed to be used in critical conditions such as resuscitation from shock and the treatment of acute anemia, such a response would greatly limit their efficacy. Preventing vasoactivity and hypertension as a result of NO scavenging has been a major focus in the development and design of Hb-based blood replacement fluids.1
The NO affinity of chemically modified Hbs (polythylene glycol [PEG]-decorated and polymerized) is the same in vitro despite causing variable levels of hypertension in vivo.2,3 Hb extravasation into the vessel wall is presumed to cause NO scavenging.4,5 Polymerization of the protein that limits extravasation has in some cases elicited6,7 or prevented8 hypertension. In general, large conjugated or polymerized Hbs with increased effective molecular radii appear to be inversely correlated with the hypertensive response.9 Shielding of the Hb molecule by surface decoration or by encapsulation into vesicles increases the diffusion distance between Hb and the endothelium and may be another mechanism for reducing or eliminating the hypertensive response in vivo.9-11
Presence of CFH in the plasma layer between red blood cells (RBCs) and the endothelium should reduce perivascular NO levels compared with RBCs alone and alter the diffusion field of NO toward the intravascular space lowering NO availability to smooth muscle causing varying degrees of systemic hypertension and microvascular vasoconstriction. In this study using the hamster window chamber, perivascular NO levels were measured after administration of CFH to determine the extent of changes in microvascular tone, perivascular NO concentration, and mean arterial pressure (MAP) after introducing different concentrations and types of modified CFH. Direct comparison of these data will allow a better understanding of whether the change in NO levels by CFH consistently leads to hypertension and vasoconstriction
Materials and methods
Animal preparation
Investigations were performed in 50- to 65-g male Golden Syrian hamsters (Charles River Laboratories, Boston, MA). Animal handling and care were provided following the procedures outlined in the Guide for the Care and Use of Laboratory Animals.12 The local Animal Subjects Committee approved this study. The hamster window chamber model is widely used for microvascular studies in the unanesthetized state, and the complete surgical technique for its preparation has been previously described in detail.13,14 This model allows the study of an intact subcutaneous tissue and a single thin retractor muscle free from surgical manipulation and exposure to ambient atmospheric conditions.
Systemic and laboratory parameters
MAP and heart rate (HR) were monitored continuously (MP 150; Biopac System, Santa Barbara, CA) except when the catheters were used to take samples for laboratory parameters. An arterial blood sample, taken in a heparinized microcapillary tube (40 μL), was centrifuged to determine hematocrit (HCT). The plasma from this sample was used to determine plasma Hb concentration (B-Hemoglobin; HemoCue, Mission Viejo, CA).
Microhemodynamics
Arteriolar and venular blood flow velocities were measured online by using the photodiode cross-correlation method15 (Photo Diode/Velocity Tracker 102B; Vista Electronics, San Diego, CA). Measured centerline velocity (V) was corrected according to vessel size to obtain mean RBC velocity.16 Video image-shearing was used to measure vessel diameter (D) (Image Shearing Monitor; Vista Electronics).17 Blood flow (Q) was calculated as Q = V · π(D/2).2 Changes in arteriolar and venular diameter from baseline were used as indicators of changes in vascular tone.
Experimental design
The unanesthetized animal was placed into a restraining tube with free access to wet food pellets during the experiment. Animals had 30 minutes to adjust to the tube environment prior to measuring baseline parameters (MAP, HR, blood gases, HCT). The tube containing the conscious animal was then affixed to the microscopic stage of an intravital microscope (BX51WI, 40 × objective, NA 0.7 SW; Olympus, Central Valley, PA). The tissue image was projected onto a CCD camera (4815-2000; COHU, San Diego, CA) connected to a videocassette recorder and viewed on a monitor. Arterioles and venules, chosen by their visual acuity (2-3 each type), were characterized by their blood flow velocity and diameter. There was no selection bias, since vessels were chosen from baseline observations and the same vessels were followed throughout the experiment.
NO electrodes
Perivascular NO levels were measured using amperiometric bipolymer-coated (Nafion and o-phenylenediamine) carbon fiber microelectrodes.18 Electrodes are fabricated by sequential dipping and drying in Nafion (5% in aliphatic alcohols; Sigma-Aldrich, St Louis, MO). Electrodes were additionally coated with 5 mM o-phenylenediamine dihydrocholoride (1,2-benzenediame solution), which selectively repels ascorbic acid and dopamine.19 Current generated was measured with a potentiostat and an electrometer-amplifier (Keithley 610C, Cleveland, OH). Electrode selectivity to ascorbic acid was measured by its response to a 30-mM solution equilibrated with 100% argon and compared with the magnitude of the response to 1 nM NO. Only electrodes having less than a 2% response to ascorbic acid and an NO sensitivity of 7 nM were used in this study. Stability of the electrodes was reconfirmed after the measurements by repeating the calibration procedure. Electrodes outside the defined characteristics or that had changed more than 5% caused both the data and electrode to be discarded from the study.
Perivascular NO measurements
The window chamber cover glass was removed after completion of microhemodynamic measurements following treatment, and the tissue was superfused (∼ 5 mL/min) with physiological Krebs salt solution. The heated solution was equilibrated with 95% N2 and 5% CO2, which maintained the suffusate at pH 7.4. It was dripped onto the tissue to maintain it at 35°C to 36°C and to minimize O2 delivery to the tissue.20 NO measurements began 20 minutes after removal of the window to allow tissue to stabilize and microvascular hemodynamics to return to baseline levels.21 Perivascular NO measurements were made by penetrating the tissue with the NO electrode using a micromanipulator to position the tip as close as possible to the microvessel without touching the wall. Care was taken not to touch the vessel wall since this stimulation causes transient and sustained increases in NO concentration.18,22 Changes in perivascular NO concentration were used to estimate the extent to which CFH eliminates active NO. Perivascular placement of the electrode provides the best estimate of the NO level in the vascular smooth muscle.
Nitric oxide synthase (NOS) inhibition
The LNAME study group was used to determine the lower level of perivascular NO in this model. Animals were treated with N-omega-nitro-l-arginine methyl ester hydrochloride (LNAME; Sigma-Aldrich, St Louis, MO) dissolved in saline and introduced via a continuous infusion from the venous catheter (30 mg/kg, 20 μL/min). Systemic parameters, microvascular hemodynamics, and perivascular NO concentration were evaluated 30 minutes later. A pilot study showed that this dosage sustained a moderate level of arteriolar vasoconstriction with an increase in blood pressure for 2 hours. Normal perivascular NO concentration was measured in another group that served as the control for the LNAME group.
Scavenging by cell-free hemoglobin protocol
The level of Hb NO scavenging was estimated by measuring perivascular NO concentration after introduction of CFH into the circulation. Animals were infused with a bolus of CFH equivalent to 10% of their blood volume (estimated as 7% of the body weight) via the jugular vein, at a rate of 0.2 mL/min. Systemic parameters, microvascular hemodynamics, and perivascular NO concentration were evaluated 30 minutes after initiation of treatment.
The physical and oxygen transport properties of the solutions are summarized in Table 1.23 The methemoglobin (MetHb) levels were measured spectrophotometrically (Lamda 20; Perkin-Elmer, Foster City, CA) to insure that they were less than 5%. Experimental groups were as follows: (1) 0.9% NaCl (saline): saline infusion was the no-treatment group used to assess the effect of the experimental protocol; (2) αα cross-linked Hb (ααHb): human Hb cross-linked between the α chains with 3,5-bis(dibromosalicyl)fumarate under deoxy conditions and purified by ion exchange chromatography following previously described techniques.25 This intramolecularly cross-linked conjugated Hb is a homogenous molecular species with an apparent molecular weight and volume comparable with unmodified Hb. It is the smallest molecular volume CFH in this study and is known to cause hypertension.26 (3) Polyethylene glycol-conjugated Hb (Mal-PEG Hb [MP4]; Sangart, San Diego, CA). This derivative of Hb carries an average of 6 copies of PEG-5K. Although the calculated molecular weight (MW) is only about 95 kDa, the molecular volume (hydrodynamic volume) is comparable with that of a polymer with 4 Hb tetramers (MW, 256 kDa). The large molecular volume is due to the surface decoration by PEG; it has the largest effective molecular volume of the CFHs studied in this investigation. (4) Polymerized bovine Hb (PBH, Oxyglobin; Biopure, Boston, MA): PBH is generated by oligomerization of bovine Hb using glutaraldehyde, which is then purified by size exclusion chromatography. It has an apparent molecular weight of 180 kDa and accordingly should be a mixture of trimers and tetramers of bovine Hb. The molecular volume of PBH is intermediate to that of ααHb and MP4. PBH (13.0 g/dL) was diluted with 0.9% NaCl to obtain a 4% solution (PBH4).
The CFH samples were diluted to Hb content of 4 g/dL to match the concentration of MP4. The polymerized bovine Hb, PBH, was also used without dilution to study the concentration dependency of the response.
Cardiac index (CI)
Cardiac output (CO) was measured by a modified thermodilution technique27 with the thermocouple implanted in the carotid artery and the indicator injected via a jugular catheter. The probe and central catheter were placed surgically 1 day prior to measurements. On the day of the experiment, the femoral artery was catheterized to monitor blood pressure (FAP). Baseline measurements were made after recovery from anesthesia and when FAP was stable for 30 minutes (approximately 2 hours). CO and FAP were again assessed 30 minutes after treatment with CFHs. CI is CO divided by body weight. Peripheral vascular resistance (PVR) was estimated as FAP divided by CI.
Data analysis
All values are shown as mean ± standard deviation unless otherwise noted; n and N denote the number of animals and vessels studied, respectively. Statistics were performed using Prism version 4.0 for Windows (GraphPad, San Diego, CA). Differences within groups were first tested with one-way analysis of variance (ANOVA) for repeated measures and for multiple comparisons between groups. Bonferroni post hoc test was used if significance was obtained. Changes were considered statistically significant if P was less than .05.
Results
Microvascular studies
All animals tolerated and completed the experimental protocols without any adverse events. A total of 37 animals was included in the microcirculation study and distributed into 7 experimental groups as follows: control (n = 6), LNAME (n = 5), saline (n = 6), ααHb (n = 5), MP4 (n = 5), PBH4 (n = 5), and PBH (n = 5).
The systemic parameters for these animals (n = 37) at baseline were (1) MAP, 103 ± 9 mmHg; (2) HR, 417 ± 48 bpm; and (3) HCT, 47.1% ± 2.1%. Microvessels chosen for these studies were characterized by vessel diameter and blood flow at baseline. There were no statistical differences among the vessels chosen for the different experimental groups. The average vessel diameter and RBC velocity of the vessels studied for all experimental groups were (n = 200): 57.8 ± 17.5 μm and 4.7 ± 2.4 mm/sec, respectively, for arterioles, and (n = 193): 68.6 ± 19.6 μm and 1.7 ± 0.9 mm/sec, respectively, for venules. NO levels in the control animals were 194 ± 88 and 189 ± 72 nM for arterioles and venules, respectively.
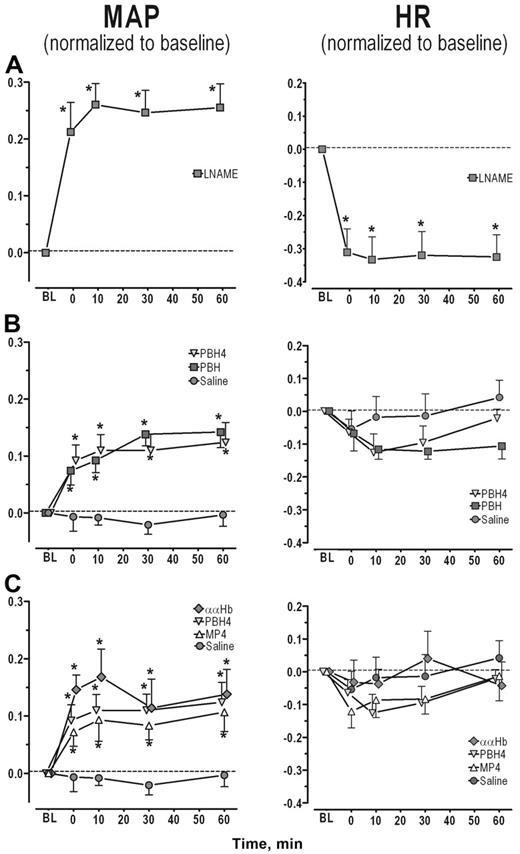
Nitric oxide synthase inhibition with LNAME treatment. LNAME treatment led to a significant and sustained increase in MAP with a concomitant decrease in HR (Figure 1A, P < .05). Both arteriolar and venular diameters and blood flows were significantly reduced from baseline (Figure 2A left and center panel, P < .05). As expected, NO levels were statistically reduced in both arterioles and venules relative to the control experimental group (Figure 2A right panel, P < .001).
Scavenging of NO by CFH. The 5 experimental groups were treated with the hypervolemic infusion protocol: ααHb, MP4, PBH4, or PBH with saline as the comparison group. All animals completed the protocol without adverse response. There were no statistically significant changes in HCT before and after administration of the CFH (Table 2). Comparison between the 4% CFH experimental groups found MP4 plasma Hb levels to be significantly lower relative to the ααHb (P = .012) and PBH4 (P = .057) experimental groups.
Infusion of saline did not affect MAP and the slight decrease in HR was not statistically significant (Figure 1B circle). Arteriolar and venular diameters and flows before and after saline infusion were not statistically different from baseline (Figure 2B left and center panels). Similarly periarteriolar and venular NO levels were not different from levels obtained in the control group (Figure 2A-B right panel).
Plasma hemoglobin concentration: PBH and PBH4. MAP increased with PBH and PBH4 when compared with baseline and with the saline-treated group (Figure 1B, P < .001). HR tended to fall with the initial rise in MAP for both PBH and PBH4, but these changes were not consistent enough to be statistically different from saline (Figure 1B). The arteriolar vasoconstriction obtained with PBH and PBH4 was statistically different from saline (Figure 2B left panel, P < .001). Venular diameter was unaffected by the CFH. Arteriolar and venular blood flows were statistically reduced for both PBH and PBH4 compared with saline (Figure 2B center panel). Periarteriolar and venular NO concentrations for both PBH and PBH4 were significantly reduced compared with saline (Figure 2B right panel, P < .001). In addition to the increase in plasma CFH concentration between the PBH and PBH4, changes in systemic and microvascular parameters were similar.
Molecular configuration: ααHb, MP4, and PBH4. Systemic and microhemodynamic changes for this study group are shown in Figures 1C and 2C. Infusion of CFHs increased MAP in each experimental group for the duration of the study (statistically significantly different from saline, P < .05). Hypertension due to ααHb peaked at 10 minutes, but there were no statistical differences among CFHs. The initial reduction in HR was not statistically different from saline. Arteriolar tone in the MP4 and saline groups was the same. Arterioles constricted with both ααHb and PBH4 relative to saline and MP4 (P < .001). Venular diameters did not differ from saline statistically. Blood flow was statistically increased in MP4 arterioles compared with saline (P < .05), while venular flows were not different. Blood flow was statistically reduced in arterioles and venules in the ααHb and PBH4 groups relative to saline and MP4 (P < .05). Periarteriolar and venular NO levels for all the CFH solutions were reduced relative to saline (P < .001) but were not statistically different from each other.
Changes in MAP and HR for each study group. (A) NOS inhibition. Both the increase in MAP and the decrease in HR were statistically significant from baseline at all time points. (B) CFH concentration. MAP was statistically increased by PBH and PBH4 compared with saline at all time points, but increases were not statistically different from each other. (C) CFH molecular configuration. MAP was statistically increased by all CFHs compared with saline (P < .05), but no statistical differences were obtained between the groups at each time point. All data are shown as mean ± SEM. Parameters are presented as change relative to baseline, thus no change from baseline would be denoted as 0, while 0.1 would mean a 10% increase from baseline. MAP changes within each experimental group were all statistically significantly different from baseline except for saline (P < .05; this significance is not denoted in the graphs). The asterisk is used to denote the statistical significance at each time point between the experimental groups (P < .05).
Changes in MAP and HR for each study group. (A) NOS inhibition. Both the increase in MAP and the decrease in HR were statistically significant from baseline at all time points. (B) CFH concentration. MAP was statistically increased by PBH and PBH4 compared with saline at all time points, but increases were not statistically different from each other. (C) CFH molecular configuration. MAP was statistically increased by all CFHs compared with saline (P < .05), but no statistical differences were obtained between the groups at each time point. All data are shown as mean ± SEM. Parameters are presented as change relative to baseline, thus no change from baseline would be denoted as 0, while 0.1 would mean a 10% increase from baseline. MAP changes within each experimental group were all statistically significantly different from baseline except for saline (P < .05; this significance is not denoted in the graphs). The asterisk is used to denote the statistical significance at each time point between the experimental groups (P < .05).
Systemic studies: cardiac index and vascular resistance
CO was measured for all CFHs (n = 4 per group). Baseline CI and FAP were 265 ± 59 mL per kg-min and 99 ± 9 mmHg, respectively (Table 3).
Effect of concentration. CI was statistically reduced relative to saline for both concentrations. Changes in FAP for PBH4 and PBH were no different from saline. However, estimated PVR calculated using FAP was significantly increased for PBH relative to saline.
Effects of molecular configuration. CI was statistically lowered for ααHb and PBH4 relative to saline and MP4, which were not different from each other. There were no changes in FAP among the CFH groups and saline. The large reduction in CI for ααHb caused a significant increase in estimated PVR relative to the other groups.
Discussion
The principal finding of this study is that the intravascular introduction of the 3 CFHs causes an increase in mean arterial pressure and a reduction in perivascular NO levels; however, the reduction in perivascular NO levels did not result in proportional levels of microvascular vasoconstriction. Since these CFHs are different in their molecular configurations, it follows that molecular structure of Hb plays a role in eliciting the vasoconstriction. Results summarized in Figure 3A-B show that the reduction of perivascular NO is similar for all CFHs, therefore vascular responses are likely to involve other factors besides NO scavenging. The pressor effect of CFHs in vivo is well documented but there is only scattered information regarding concomitant effects on the microvascular tone and perivascular NO levels. This study also shows that systemic blood pressure and vascular tone in the tissue of the hamster window chamber model are not directly correlated.
NOS inhibition
Blocking NO synthesis with LNAME treatment increased MAP, decreased HR, and caused vasoconstriction. Perivascular NO levels did not fall to zero as predicted if all sources of NOS were completely blocked by LNAME. A possible interpretation of this finding is that the route of LNAME is diffusion limited, and it may not reach the entire interstitial tissue and therefore unable to block all sources of NO. Therefore the measurement of perivascular NO level represents a steady state of flux of NO from interstitial sources. Additionally, sources of nitrates may undergo reduction, producing NO. In pilot studies, the LNAME dosage was escalated (up to 50 mg/kg), but this did not lead to higher MAP nor was additional vasoconstriction observed in this tissue. Similar changes in blood flow and vasoconstriction were previously obtained with this animal model.28 It is likely that other homeostatic mechanisms become activated, leading to redistribution of LNAME, and therefore a zero perivascular NO level cannot be achieved in vivo only by NOS inhibition.
Response to Hb plasma concentration
Increasing plasma Hb concentration by about 3-fold (PBH4 vs PBH) resulted in the same degree of change in MAP, microvessel tone, tissue perfusion, and perivascular NO levels. This finding demonstrates that a plasma Hb content of 0.5 g/dL (approximately 78 μM) is sufficient to have scavenged all intravascular NO, presumably depleting all endothelial sources; thus, increasing plasma Hb content did not further reduce perivascular NO levels. Therefore, only at very low plasma Hb concentrations may it be possible to detect a concentration-dependent relationship between Hb and perivascular NO levels similar to those obtained in vitro.29 Our finding does not preclude that some differences between CFHs and the scavenging rate of NO exists and suggests that in vivo this difference would be detectable at Hb concentrations that are too low for using these materials as physiological oxygen carriers. The difference in concentration was discernable in the increase in peripheral vascular resistance. In vivo studies in cats show that an increase in MAP after tetrameric Hb transfusion was blocked by prior LNAME treatment, attributing the pressor response to NO scavenging.6
Response at the microvascular level: vessel diameter, blood flow, and perivascular NO levels. (A) NOS inhibition. Significant vasoconstriction, loss of perfusion, and reduced perivascular NO levels were obtained (P < .05). (B) CFH concentration. The microvascular response was not a function of the CFH concentration. Significant decrease in arteriolar diameter, microvascular blood flow, and perivascular NO were obtained with both PBH and PBH4 (P < .05). (C) CFH molecular configuration. The smaller CFH molecules (ααHb and PBH4) caused arteriolar constriction leading to reduced microvascular perfusion compared with baseline and MP4. MP4 was not vasoconstrictive and had increased arteriolar blood flow. All CFHs reduced perivascular NO to similar levels relative to saline (P < .05).  and ▪ denote the arterioles and venules, respectively. Blood flow data are shown as mean ± SEM. Parameters are present as relative to baseline, thus no change from baseline would be denoted as 1, while 1.1 would mean a 10% increase from baseline. * and ** denote a statistical significant difference relative to saline and MP4, respectively.
and ▪ denote the arterioles and venules, respectively. Blood flow data are shown as mean ± SEM. Parameters are present as relative to baseline, thus no change from baseline would be denoted as 1, while 1.1 would mean a 10% increase from baseline. * and ** denote a statistical significant difference relative to saline and MP4, respectively.
Response at the microvascular level: vessel diameter, blood flow, and perivascular NO levels. (A) NOS inhibition. Significant vasoconstriction, loss of perfusion, and reduced perivascular NO levels were obtained (P < .05). (B) CFH concentration. The microvascular response was not a function of the CFH concentration. Significant decrease in arteriolar diameter, microvascular blood flow, and perivascular NO were obtained with both PBH and PBH4 (P < .05). (C) CFH molecular configuration. The smaller CFH molecules (ααHb and PBH4) caused arteriolar constriction leading to reduced microvascular perfusion compared with baseline and MP4. MP4 was not vasoconstrictive and had increased arteriolar blood flow. All CFHs reduced perivascular NO to similar levels relative to saline (P < .05).  and ▪ denote the arterioles and venules, respectively. Blood flow data are shown as mean ± SEM. Parameters are present as relative to baseline, thus no change from baseline would be denoted as 1, while 1.1 would mean a 10% increase from baseline. * and ** denote a statistical significant difference relative to saline and MP4, respectively.
and ▪ denote the arterioles and venules, respectively. Blood flow data are shown as mean ± SEM. Parameters are present as relative to baseline, thus no change from baseline would be denoted as 1, while 1.1 would mean a 10% increase from baseline. * and ** denote a statistical significant difference relative to saline and MP4, respectively.
Hb molecular configurations and oxygen transport characteristics
The molecular configurations of the 3 CFHs used in this study are different and should influence the rate at which these molecules extravasate the circulation, leading to the hypothesis that increasing molecular volume of these CFHs would decrease their rate of exit from the circulation by kidney filtration and extravasation across the vascular wall. The presence of the Hb molecule in the vessel wall, as a consequence of extravasation, has been proposed to be an additional NO sink, limiting NO diffusion from endothelial cells to smooth muscle causing vasoconstriction. Studies have shown a strong correlation between molecular size, vasoconstriction, and hypertension.9 Infusion of CFHs with molecular diameters ranging from 7 to 68 nm showed that the constriction of resistance arterioles was proportional to the level of hypertension using the hamster window model. Recently, studies with cross-linked tetrameric Hb (similar in size to ααHb used in the current study) found that CFH in the plasma does not scavenge sufficient NO in the plasma space to significantly affect baseline tone in vascular beds with tight endothelial junctions found in the cranial circulation but does produce substantial constriction in beds with porous endothelium (kidney and small intestine),6 supporting the hypothesis that vasoconstriction is due to extravasation of the CFH molecule. CFH has been shown to increase vascular permeability and induce vascular leaks in the intestine,30 further reducing NO availability to smooth muscle. The hypothesis that low-molecular-weight components cause the pressor response was also explored with a dialyzed polymerized CFH (polymers > 300 kDa, molecular radius estimated at 25 nm), and results suggest that exchange transfusion did not promote an increase in arterial pressure.31 In the present investigation, the range of molecular radii studied is narrow, therefore we cannot conclude that vasoconstriction found with ααHb and PBH4 was not in part due to extravasation.
Another possible mechanism to explain vasoconstriction is the rate of oxygen delivery to the vessel wall.32,33 The cardiovasculature delivers oxygen to tissue to match oxygen demand, and excessive delivery of oxygen or hyperoxia leads to autoregulatory arteriolar vasoconstriction to prevent tissue overoxygenation. CFH in plasma increases oxygen delivery due to facilitated diffusion, which is directly proportional to the magnitude of the diffusion constant of the Hb molecule, which is inversely proportional to its molecular radius. The molecular radius of ααHb is about 3 nm, while that of MP4 is 9 nm, thus the flux of oxygen due to facilitated diffusion in the plasma layer in the presence of ααHb is 3-fold that of MP4. This effect is confirmed by studies using an artificial capillary system comparing CFHs with RBCs, which showed that unmodified Hb and ααHb (small molecules) have markedly increased O2 release relative to larger chemically modified Hb molecules regardless of their oxygen affinity (p50).34 Therefore, the decreased diffusivity of MP4 compared with smaller CFH molecular configurations offsets the greater O2 availability to the vessel wall due to facilitated diffusion. This process is further enhanced by the higher oxygen affinity of MP4 that restricts oxygen release to locations with large oxygen gradients. Thus the combination of low diffusivity and increased oxygen affinity (lower p50) of MP4 reduces hyperoxia-driven vasoconstriction, and, as a result, microvascular hemodynamics is maintained. It should be noted that hyperoxygenation of the microvascular wall is not necessarily related to an increase in pO2 in this tissue. Facilitated diffusion increases oxygen delivery to the vascular wall, while the driving pO2 gradient is the same because CFH creates an additional oxygen flux due to the diffusion of oxygen bound to molecular hemoglobin. Lowering NO availability in the microcirculation was shown to increase the sensitivity of the microvasculature to respond to the vasoconstrictive effect of hyperoxygenation.35 Therefore, in conditions where NO is uniformly scavenged, vasoconstriction should be lessened with the compound that delivers less oxygen to the vascular wall.
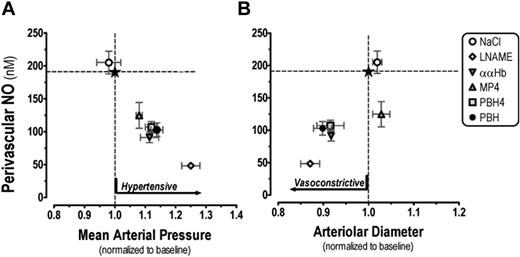
Changes to MAP and arteriolar diameter as a function of perivascular NO levels. Reduction of arteriolar perivascular NO by the introduction of CFH caused a concomitant pressure response, while the change in vascular resistance at the microvascular level represented by arteriolar vasoconstriction was not present with the MP4 experimental group (A). MP4 reduced perivascular NO and increased MAP similarly in magnitude to the other CFHs, however the slight arteriolar dilation caused a statistically significant increase in arteriolar blood flow (B). Data are presented as mean ± SEM. Mean arteriolar pressure is 30 minutes after the introduction of CFH. The normal levels for each parameter are marked with dotted lines; the intersection, by a star.
Changes to MAP and arteriolar diameter as a function of perivascular NO levels. Reduction of arteriolar perivascular NO by the introduction of CFH caused a concomitant pressure response, while the change in vascular resistance at the microvascular level represented by arteriolar vasoconstriction was not present with the MP4 experimental group (A). MP4 reduced perivascular NO and increased MAP similarly in magnitude to the other CFHs, however the slight arteriolar dilation caused a statistically significant increase in arteriolar blood flow (B). Data are presented as mean ± SEM. Mean arteriolar pressure is 30 minutes after the introduction of CFH. The normal levels for each parameter are marked with dotted lines; the intersection, by a star.
MP4 with colloid osmotic pressure of approximately 50 mmHg may have increased the blood oncotic pressure by increasing vascular volume via auto transfusion36 as shown by the reduction in plasma Hb in the MP4 group (Table 2). Furthermore, MP4 is the least likely molecule to extravasate due to its large molecular size compared with the other CFH solutions. Thus, the pressor response by MP4 may be attributed to an increase in blood volume that also leads to an increase in microvascular blood flow. There were no measurable differences between the systemic hematocrit in the MP4 group that may be due to the low resolution of the measurement technique.
Cardiac index and vascular resistance
MP4 has the same effect as saline on the CI, while PBH, PBH4, and ααHb lowered CI to 78%, 78%, and 84% of baseline, respectively. This finding further differentiates MP4 as a nonvasoactive material as shown by estimates of PVR calculated from femoral artery pressure (FAP) (Table 3, column 3). Unexpectedly, the hypertension found using the MAP was not present in these experiments where the experimental procedure allowed measuring FAP but not MAP. These small animals have substantial pressure drops between the carotid and the femoral arteries due to the small caliber of the arterial vessels, as shown by the consistent trend in the data despite its large dispersion. Using data on cardiac index and MAP measurements, we again calculated PVR (Table 3, columns 4 and 5). Despite the large uncertainty in the measurements due to the magnitude of the standard deviations, there is a trend consistent with calculation of PVR, showing that CFHs, with the exception of MP4, significantly increase PVR.
Volume expansion should increase in blood pressure for all materials except saline, with the increased vascular resistance being an additional factor for PBH, PBH4, and ααHb. The differences in increased volume were not significant between materials. Increase in blood pressure is not necessarily pathological unless it is develops from an increased vascular resistance, in which case it decreases perfusion and functional capillary density (FCD).
The microvascular results in this study in the hamster window chamber model provide only a limited view of the microvascular response to CFHs. This model allows for detailed and precise location of the measurement of perivascular NO with concomitant determination of changes in vascular tone and blood flow. Lifting of the glass window and superfusion of the tissue to perform the NO measurements could change the microvascular environment. These effects were minimized by stabilizing the vascular tone and blood flow back to baseline levels prior to obtaining measurements. The baseline NO levels are in a similar range but are not as high as recently reported from measurements in the exteriorized rat mesentery and small intestine,37 which were in the 300-nM range. No differences in arteriolar and venular NO levels are similar to the finding by other studies.37,38 However, it is unknown if similar responses are mirrored elsewhere, particularly in major organs. It is likely that the limited field of view may in part explain why the increase in MAP with the lack of vasoconstriction is possible. Our previous studies with this experimental model and CFHs introduced in an extreme exchange transfusion protocol found that the pressure drop in the microvascular network was located in different segments of the system.39 A greater pressure drop and corresponding vasoconstriction prior to the microcirculation was found for PBH and ααHb, while for MP4 more of the systemic pressure was transmitted to the microcirculation. Maintenance of microvascular perfusion pressure by MP4 should be related to the lack of vasoconstriction observed in the present study. Some CFHs have been shown to decrease cardiac output in hemorrhagic shock resuscitation studies,40-43 except for MP4, which recovered and sustained normal cardiac output.44 Blood volume contracted and expanded in a moderate exchange transfusion study with ααHb and a bovine Hb modified with PEG, respectively.45
Mechanistically, the behavior of PEG-Hb must be related to its unique ability of maintaining a significantly higher level of functional capillary density in extreme hemodilution and hemorrhagic shock resuscitation,46 an effect not attainable with other CFHs. This result was obtained in experiments in the hamster window preparation, and thus there is some uncertainty as to whether it is common to other organs and tissues. However, the increase in blood flow in the absence of vasodilation suggests that similar events may take place in the heart, leading to increased perfusion pressure, improved capillary perfusion, and cardiac function.
In summary, the results of this study show that perivascular NO scavenging is the same for all 3 molecular configurations of Hb when introduced into the circulation at the same concentration; however, the microvascular effects of these solutions are different. This difference should be related to properties of the molecule other than NO binding. Among these properties might be their molecular volume, suggesting that extravasation and facilitated diffusion-related mechanisms are factors that determine the effects on microvascular function. Small molecules are vasoactive and raise blood pressure, while MP4, with the largest molecular volume of all the CFHs studied here, does not cause vasoconstriction, but rather increased microvascular blood flow. MP4 also elicits a blood pressure rise; an effect that may be in part due to an increase in blood volume in combination with the maintenance of cardiac output, a consequence of its higher COP, as supported by the estimated blood volume expansion based on the changes in total Hb and no changes of peripheral vascular resistance. Conversely, the remainder of CFHs increased blood pressure and significantly decreased cardiac output, due to a significant increase in vascular resistance. In summary, our findings support the concept that vasoconstriction does not directly correlate with NO scavenging by Hb-based oxygen carriers and provide evidence suggesting factors such as molecular dimension, retention time, and gas-carrying properties are more likely to regulate vascular tone and perfusion.
Prepublished online as Blood First Edition Paper, July 20, 2006; DOI 10.1182/blood-2006-02-005272.
Supported in part by National Institutes Health (NIH) HL076182 (A.G.T, P.C.), HL071064 (S.A.A., B.N.M.), HL076163 (R.M.W.), HL064395 (M.I.), and HL062318 (M.I.) and US Army PR023085 (A.G.T., S.A.A., B.N.M.). MP4 was provided by Sangart (San Diego, CA) through collaborative research partnership funding from NIH R24 HL064395.
R.M.W. has declared a financial interest in Sangart, whose potential product was studied in the present work. S.A.A., M.I., B.N.M., and R.M.W. hold patents related to the work that is described in the present study. A.G.T. and P.C. declare no competing financial interests.
A.G.T. designed and performed research, analyzed data, and wrote the paper; P.C. performed research, analyzed data, and contributed to the writing of the paper; B.N.M. provided vital materials; S.A.A. provided vital materials and contributed to the writing of the paper; R.M.W. provided vital materials and contributed to the writing of the paper; and M.I. analyzed data and contributed to the writing of the paper.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
We acknowledge the excellent technical assistance with the animal preparation and experiments of Patricia Nance, Cynthia Walser, and Froilan Barra. We also thank Dr Peter C. Y. Chen for his assistance with the statistical analysis.
One of the authors (R.M.W.) has declared a financial interest in a company whose potential product was studied in the present work. Several of the authors (S.A.A., M.I., B.N.M., and R.M.W.) hold patents related to the work that is described in the present study.